Abstract
Key message
Hydroxy fatty acids produced in plant seed oil are important industrial material. This review focuses on the use of metabolic engineering approaches for the production of hydroxy fatty acids in transgenic plants.
Abstract
Vegetable oil is not only edible but can also be used for industrial purposes. The industrial demand for vegetable oil will increase with the continued depletion of fossil fuels and ensuing environmental issues such as climate change, caused by increased carbon dioxide in the air. Some plants accumulate high levels of unusual fatty acids in their seeds, and these fatty acids (FAs) have properties that make them suitable for industrial applications. Hydroxy fatty acids (HFAs) are some of the most important of these industrial FAs. Castor oil is the conventional source of HFA. However, due to the presence of toxin ricin in its seeds, castor is not cultivated on a large scale. Lesquerella is another HFA accumulator and is currently being developed as a new crop for a safe source of HFAs. The mechanisms of HFA synthesis and accumulation have been extensively studied using castor genes and the model plant Arabidopsis. HFAs accumulated to 17 % in the seed oil of Arabidopsis expressing a FA hydroxylase gene from castor (RcFAH12), but its seed oil content and plant growth decreased. When RcFAH12 gene was coexpressed with additional castor gene(s) in Arabidopsis, ~30 % HFAs were accumulated and the seed oil content and plant growth was almost restored to the wild-type level. Further advancement of our understanding of pathways, genes and regulatory mechanisms underlying synthesis and accumulation of HFAs is essential to developing and implementing effective genetic approaches for enhancing HFA production in oilseeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vegetable oil is stored in plants as triacylglycerol (TAG), which can be obtained from most seeds and some fruits (mesocarp), including the olive (Olea europaea), avocado (Persea americana), and oil palm (Syagrus cocoides) (Huang et al. 2009). The perennial shrub jojoba (Simmondsia chinensis) is one of the few plants that stores wax esters in its seeds (Ohlrogge et al. 1978). Fatty acids (FAs) from vegetable oils provide humans and animals with both chemical energy and essential nutrients, such as linoleic acid (18:2) and linolenic acid (18:3). Vegetable oils are mainly used as food, but some are used for other purposes, including livestock feed and industrial applications. The ratio between food, feed, and industrial uses of global vegetable oils and animal fats was 80:6:14, but has recently changed to 74:6:20, mainly due to higher biodiesel production (Gunstone 2008), which is predicted to increase. The global production of vegetable-oil-based biodiesel dramatically increased 125 % in 4 years, from 10.4 billion liters in 2007 and 23.4 billion liters in 2011 (http://www.eia.gov/cfapps/ipdbproject/iedindex3.cfm?tid=79&pid=81&aid=1&cid=ww,&syid=2007&eyid=2011&unit=TBPD). The global production of vegetable oils and animal fats was 100–110 megatonne (Mt) in 2000 (Gunstone and Hamilton 2001), whereas the production of major vegetable oils alone was 169.23 Mt in 2013/14 (United States Department of Agriculture 2014).
The industrial uses of vegetable oils can be classified into two groups, biofuels and industrial chemical feedstocks. Biofuels, obtained by the methylation of the FAs from vegetable oils, are used for vehicle transportation and for aviation fuels (Sorda et al. 2010; Fairley 2011). Due to the chemical structures and physical properties of some vegetable FAs similar to those of petroleum (Metzger 2009), theoretically vegetable oil-derived FAs can replace all petrochemical feedstocks. Vegetable-oil-based biodiesel is a renewable and environmentally friendly alternative fuel (Demirbas 2009; Fairley 2011). Chemical feedstock FAs are used as additives or raw materials for numerous industrial products, including lubricants, inks, plastics, soaps, cosmetics, paints, coatings, and adhesives.
Most of the FAs in vegetable oils are composed of palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), 18:2, and 18:3, the so-called “common” FAs. Some plants produce uncommon FAs with unique physical and chemical properties suitable for specific industrial applications, they are often called “unusual FAs” (UFAs; Smith 1971). UFAs can be classified based on the following criteria: (1) carbon chain length; (2) functional groups; (3) numbers and positions of unsaturated bonds; and (4) the types of unsaturated bonds. Information on the UFAs found in plants are given in Table 1. For example, lauric acid (12:0) is a medium-chain FA, shorter than the common FAs (C16–18). Erucic acid (22:1Δ13) is a very-long-chain FA. Ricinoleic acid (18:1-OH) and lesquerolic acid (20:1-OH) contain a hydroxyl group, and vernolic acid (12,13-epoxy-18:1Δ9) has an epoxy group. Petroselinic acid (18:1Δ6) is a monounsaturated FA but the position of the double bond differs from that of oleic acid. α-Eleostearic acid (18:3Δ9,11t,13t) and calendic acid (18:3Δ8t,10t,12) are conjugated FAs that contain conjugated double bonds, rather than methylene-interrupted double bonds. Crepenynic acid (18:2Δ9,12a) contains a triple bond and sterculic acid (9,10-methylene-18:1Δ9) is a cyclic FA that contains a cyclopropane ring. Hydroxy FAs (HFAs) are among the most important industrially useful FAs and can be widely used in industry as chemical feedstock.
To produce industrial oils at lower cost, vegetable oils should contain a uniformed FA at high concentration for ease of purification and processing. However, most vegetable oils are mixtures of TAGs containing at least three of these five common FAs. Moreover, most unusual and novel FAs are produced in species not yet developed as crops. Thus, additional knowledge and tools are required to engineer desirable FAs in existing oilseeds. Recent research efforts have been focused on production of industrial FAs in transgenic plants using biotechnology. In this review, we discuss mostly the current metabolic engineering of plant oils for the production of HFAs as well as future perspectives.
HFA-producing plants: castor and lesquerella
Castor oil contains up to 90 % 18:1-OH (Table 1), so it is a valuable commercial source of HFAs. Castor oil and its derivatives are widely used in industrial products such as anticorrosion coatings, nylon, plastics, paints, cosmetics, soaps, detergents, and lubricants, and as an additive in aircraft fuel (Caupin 1997). Castor is mainly cultivated in tropical–subtropical regions. India is currently the foremost producers of castor seed, followed by China. Although castor oil is useful for industry, several factors limit castor oil production. First, castor seeds contain the toxin ricin and potent allergenic 2S albumins, which entail safety concerns. Second, only a few cultivars suitable for mechanical harvesting have been developed, limiting large-scale of commercial production. Third, the supply of castor oil fluctuates because of the economic and political instability of the countries where castor oil is produced (Holic et al. 2012). Improving agronomic traits of castor cultivar using biotechnology has been investigated, but due to the low transformation efficiency in castor, limited successes have been reported (Malathi et al. 2006; Sujatha and Sailaja 2005; Sailaja et al. 2008; Sujatha et al. 2009).
Lesquerella (Physaria fendleri), which belongs to the mustard family (Brassicaceae), produces 60 % 20:1-OH (Table 1) in its seed oil and is a potential crop for the semi-arid regions of North America (Dierig et al. 2011). Lesquerella does not contain the toxin ricin, so it is considered a safe source of HFAs. Considerable efforts have been made to improve the agronomic traits of lesquerella through breeding (Isbell et al. 2008; Dierig et al. 1993, 2011). Some lesquerella lines with 33 % have been developed (Dierig et al. 2006), which is comparable to most Brassicaceae oilseed crops. However, there are still several agronomic traits can be improved to make lesquerella a commercially viable crop, such as seed yield, herbicide tolerance and auto-fertility (Dierig et al. 2011). Recently, a highly efficient and stable transformation system was established (Chen 2011), which provides means to improve this crop through genetic engineering.
Biosynthesis pathways of TAG and HFA
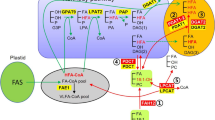
Biosynthesis of TAG follows de novo synthesis of FA in the plastid and TAG assembly in the endoplasmic reticulum (ER) (Chapman and Ohlrogge 2012; Li-Beisson et al. 2013). Newly synthesized common FAs such as 18:1, 16:0 and 18:0 are released from the plastid and then converted to acyl-coenzyme As (CoAs). The acyl-CoAs can be incorporated into TAG through glycerol-3-phosphate (G3P) pathway also known as the Kennedy pathway (Weiss and Kennedy 1956; Weiss et al. 1960; Somerville et al. 2000). As exemplified in Fig. 1, the reactions in Kennedy pathway are catalyzed sequentially by several enzymes, including glycerol-3-phosphate acyltransferase (GPAT), lysophosphatidic acid acyltransferase (LPAT), and diacylglycerol acyltransferase (DGAT). First, acyl-CoA is incorporated into the sn-1 position of G3P by GPAT, and the subsequent product, lysophosphatidic acid (LPA), is converted into phosphatidic acid (PA) by LPAT. PA is dephosphorylated and converted to diacylglycerol (DAG) by PA phosphatase (PAP). Finally, TAG is synthesized through the acylation of the sn-3 position of DAG by DGAT. Since PA and DAG are also precursors for synthesis of membrane glycerolipids, GPAT and LPAT overlap the pathway for membrane lipids in all cells. In addition to Kennedy pathway, the newly synthesized acyl-CoAs can also be incorporated directly into membrane lipid phospatidylcholine (PC) by the acyl editing reactions (Lands 1965; Li-Beisson et al. 2013). These acyl editing reactions can be catalyzed either by forward and reverse reactions of lyso-PC acyltransferase (LPCAT) to yield acyl-CoA, or by a phospholipase A–type activity to yield a free FA that then is activated to acyl-CoA (Fig. 1). Since PC is the substrate for many FA-modifying enzymes (desaturase, hydroxylase, etc.), rapid de-acylation and re-acylation of PC results in an acyl-CoA pool enriched with modified FAs (mFA), which are then utilized for TAG synthesis. In fact, TAG assembly may not be as simple as the sequential acylation of glycerol with GPAT, LPAT, and DGAT by the Kennedy pathway. Phospholipid:DAG acyltransferase (PDAT) has been identified that syntheses TAG by transacylation of the sn-2 FA from PC onto sn-3 position of DAG, with lyso-PC as a co-product (Dahlqvist et al. 2000) (Fig. 1). Besides, metabolic labeling experiments indicate that there are at least two pools of DAG, de novo DAG and PC-derived DAG, and the relative proportion of each DAG utilized for TAG synthesis is likely to be species-dependent (Bates et al. 2009; Bates and Browse 2011). The de novo synthesis of DAG involves Kennedy pathway as described above. The PC-derived DAG utilizes de novo DAG to first synthesize PC. Removal of the headgroup of PC by PC:DAG phosphocholine transferase (PDCT) generates PC-derived DAG (Hu et al. 2012) (Fig. 1). Alternatively, PC-derived DAG can be produced by the reverse action of CDP-choline:DAG cholinephosphotransferase (CPT) (Slack et al. 1983). As FA in PC can be modified, PC-derived DAG also provides a means to increase the presence of mFA in TAG.
Schematic diagram of the predicted pathways in the synthesis and accumulation of HFAs in the castor oil endosperm. FAs are synthesized in the plastid and C16–18 FAs are released into the cytosol. The biosynthesis of TAG and PC is initiated from G3P in the ER, but little TAG is synthesized directly from de novo DAG. PC and PC-derived HFA-DAG are converted simultaneously from de novo DAG and HFA-PC, respectively, by PDCT. HFA is synthesized from 18:1 incorporated in the sn-2 position of PC by FAH12. HFAs are removed from HFA-PC by LPCAT or PLA2/LACS and released into the cytosol as HFA-CoA, which is used to synthesize HFA-TAG. HFAs in HFA-PC are used directly for the synthesis of HFA-TAG without acyl editing by PDAT. LPC, a byproduct of acyl editing or TAG synthesis by PDAT, is reconverted into PC with acyl-CoA (mainly 18:1-CoA) by LPCAT. The HFA-TAG synthesized by PDAT or DGAT is stored in oil bodies budded from the ER. The white boxes near the arrows indicate the enzymes that catalyze the conversion between the molecules connected by the arrow. Dashed arrows demonstrate the reactions that are least likely to occur. ACBP acyl-CoA-binding protein, DAG diacylglycerol, DGAT diacylglycerol acyltransferase, FAH12 oleate Δ12-hydroxylase, G3P glycerol-3-phosphate, GPAT glycerol-3-phosphate acyltransferase, LACS long-chain acyl-CoA synthetase, LPA lysophosphatidic acid, LPAT lysophosphatidic acid acyltransferase, LPC lysophosphatidylcholine, LPCAT lysophosphatidylcholine acyltransferase, PA phosphatidic acid; PAP phosphatidic acid phosphatase, PC phosphatidyl choline, PDAT phosphatidylcholinediacylglycerol acyltransferase, PDCT phosphatidylcholinediacylglycerol cholinephosphotransferase, CPTr CDP-choline:DAG cholinephosphotransferase reverse reaction, PLA 2 phospholipase A2, TAG triacylglycerol
Castor oil contains 90 % 18:1-OH (Table 1), over 70 % of the TAGs have all three sn positions esterified with 18:1-OH (Lin et al. 2003). The gene responsible for the synthesis of 18:1-OH in castor is oleate Δ12-hydroxylase (RcFAH12; van de Loo et al. 1995). RcFAH12 and the gene encoding microsomal oleate Δ12-desaturase (FA desaturase 2; FAD2) from Arabidopsis share about 67 % amino acid identity (van de Loo et al. 1995). FAH12 is known to be one of the various divergent forms of FAD2, together with the genes encoding FA conjugase, FA acetylenase, and FA epoxygenase (Shanklin and Cahoon 1998; Lee et al. 1998; Cahoon et al. 1999; Dyer et al. 2002; Cahoon and Kinney 2004). FAD2 catalyzes the synthesis of 18:2 by forming a double bond between the 12th and 13th carbons of 18:1 esterified on the sn-2 position of phosphatidylcholine (PC; Okuley et al. 1994). Likewise, FAH12 catalyzes the synthesis of 18:1-OH by adding a hydroxyl group to the 12th carbon of 18:1, also esterified on the sn-2 position of PC (Galliard and Stumpf 1966; Bafor et al. 1991). In castor, 18:1-OH is released from 18:1-OH-PC and activated to 18:1-OH-coenzyme A (CoA) (Moreau and Stumpf 1981). Since 18:1-OH-PC only accumulates to about 5 % of FA in PC (Ståhl et al. 1995), this requires that at least 90 % of seed 18:1 fluxes into the sn-2 position of PC is efficiently removed from PC and redistributed into the sn-1, sn-2, and sn-3 positions of TAG. Acyl editing and Kennedy pathway were suggested to be major routes of TAG synthesis in castor (Bafor et al. 1991), but other routs mediated through PC, such as PDAT (Dahlqvist et al. 2000), PDCT (Hu et al. 2012) cannot be excluded (Fig. 1).
Lesquerella oil contains ~60 % of 20:1-OH, almost all of the 20:1-OH are esterified on the sn-1 and sn-3 positions of TAG (Hayes and Kleiman 1996). Biochemical studies indicated that in lesquerella, 18:1-OH was initially formed by hydroxylation of 18:1 at sn-2-PC, then the 18:1-OH was released and activated to 18:1-OH-CoA; the newly formed 18:1-OH-CoA was rapidly elongated to 20:1-OH-CoA which is further utilized for TAG assembly (Engeseth and Stymne 1996; Reed et al. 1997). Kennedy pathway could be a major mechanism to incorporate 20:1-OH into lesquerella TAG. The lack of HFA at the sn-2 of TAG could be explained by LPATs in lesquerella discriminating 20:1-OH substrate. The lesquerella FAH12 gene responsible for hydroxylation of 18:1-PC to form 18:1-OH-PC was isolated and characterized (Broun et al. 1998). The gene encoding the condensing enzyme from lesquerella, PfKCS3, has also been isolated, and its activity has been shown to specifically catalyze elongation of 18:1-OH-CoA (Moon et al. 2001). In addition to 18:1-OH and 20:1-OH, lesquerella seed accumulates a low level of densipolic acid (12-hydroxy-octadec-cis-9,15-enoic acid: 18:2-OH) and auricolic acid (14-hydroxy-eicos-cis-11,17-enoic acid: 20:2-OH), which are formed by involving a microsomal ∆15 desaturase (Engeseth and Stymne 1996; Reed et al. 1997).
Expression of various FHA12 genes in Arabidopsis and other Brassicaceae plants
In a transgenic Arabidopsis (Col-0) expressing seed-specific RcFAH12, four types of HFAs accumulated, 18:1-OH (7.8 %), 18:2-OH (6.6 %), 20:1-OH (2.5 %), and 20:2-OH (0.4 %). These HFAs comprised a total 17 % of the seed oil (Broun and Somerville 1997). Arabidopsis seed oils contain large proportions of 18:3 and 11-eicosenoic acid (20:1Δ11), each at ~15 % in seed oil (Rossak et al. 2001; Guo et al. 2009). To generate seed oil with uniform HFA, such as 18:1-OH, various Arabidopsis mutants defecting at FA desaturation (fad3), elongation (fae1), or both steps (fad2/fae1, fad3/fae1) were used as a background plants for expressing RcFAH12. When RcFAH12 was expressed in fad3, 18:1-OH accumulated at 16.2 % (total 18.7 %), in fae1 at 13–15 % (total 17 %), in fad2/fae1at 8.7 % (total 19.2 %), and in fad3/fae1 at 7.0 % (total 7.0 %) (Smith et al. 2003; Lu et al. 2006).
According to Burgal et al. (2008), the RcFAH12-transgenic Arabidopsis lines that accumulated >20 % HFAs in their seed oil were invariably unstable, and the progeny seeds did not germinate well. These results suggest that high levels of HFAs may damage the metabolism and physiology of seeds which leads to the loss of oil content, seed weight and seed vigor. When RcFHA12 is expressed alone, the highest and inheritable level of HFA in seed oil is 17 % observed by multiple groups (Broun and Somerville 1997; Smith et al. 2003; Kumar et al. 2006; Lu et al. 2006). Two transgenic Arabidopsis lines expressing RcFAH12 in fae1, CL37 and CL7, showing both stable and the highest level of 17 ± 1 % HFAs in seed oil were selected from over 50 lines (Lu et al. 2006; Burgal et al. 2008). These lines have been used as a background plants for testing additional genes contributing to HFAs accumulation in the seed oils (Burgal et al. 2008; van Erp et al. 2011; Kim et al. 2011; Hu et al. 2012).
RcFAH12 were also introduced into Brassicaceae oilseeds Brassica napus and Camelina. The resulting transgenic plants showed 15.6 or 15 % HFA content in seed oil of B. napus (Broun et al. 1998) or Camelina (Lu and Kang 2008), respectively. These levels are similar to those of transgenic Arabidopsis seeds expressing RcFAH12.
Based on RcFAH12 sequence similarity, a lesquerella 12-hydroxylase gene, PfFAH12, was isolated and characterized (Broun et al. 1998). Unlike RcFAH12 which is a strict hydroxylase, PfFAH12 is a bifunctional oleate 12-hydroxylase/desaturase (Broun et al. 1998). Transgenic Arabidopsis or B. napus expressing PfFAH12 showed accumulation of HFA at 16.5 or 9.9 %, respectively (Broun et al. 1998). To examine whether the hydroxylases from other Physaria species were also bifunctional, a gene encoding oleate 12-hydroxylase was amplified by PCR from Physaria lindheimeri, a species that accumulates over 80 % 20:1-OH in its seed oil (Dauk et al. 2007). Molecular and biochemical analysis revealed that PlFAH12 was primarily a fatty acid hydroxylase and should not be considered as bifunctional (Dauk et al. 2007). Transgenic Arabidopsis expressing PlFAH12 accumulated 18 % HFA in seed oil (Dauk et al. 2007).
Besides RcFAH12, PfFAH12, and PlFAH12, Arabidopsis has also been transformed with the FAH12 gene from fungi. The fungal pathogen Claviceps purpurea, which causes ergot disease in wheat and barley, forms a specialized structure called the “sclerotium”, which accumulates high levels of HFA (Mantle and Nisbet 1976). When the FAH12 gene from C. purpurea was introduced in Arabidopsis Col-0 and fad2/fae1 mutant, HFAs accounted for 19.65 and 25 % of the seed oils, respectively (Meesapyodsuk and Qiu 2008).
Barriers of producing HFAs in transgenic plants
Inhibition of HFA synthesis in developing seeds
As described, transgenic experiments with various FAH12 have consistently failed to achieve high levels of HFAs. Besides, transgenic Arabidopsis expressing FAH12 alone was often accompanied by reduced oil content, seed weight, and seed vigor (Dauk et al. 2007; Burgal et al. 2008; Bates and Browse 2011; van Erp et al. 2011). It was hypothesized that Arabidopsis may not have an efficient system to incorporate HFA into TAG, the buildup HFA might be targeted for ß-oxidation or a futile cycle of synthesis and degradation, leading to limited accumulation of HFA in seed oil and reduction of total oil per seed (van de Loo et al. 1995; Broun and Somerville 1997; Moire et al. 2004; Bates and Browse 2011). Similar phenomenon were reported for transgenic plants expressing other UFA such as laurate-FA (Eccleston and Ohlrogge 1998) and epoxy-FA (Li et al. 2012). However, there were evidences suggested that acetyl-CoA carboxylase (ACCase) activity could be down-regulated by FA feedback inhibition and consequently, the rate of fatty acid synthesis decreased (Shintani and Ohlrogge 1995; Andre et al. 2012). Recent experiments demonstrated that the major factor limiting oil content in transgenic Arabidopsis expressing RcFAH12 was due to inefficient utilization of HFAs during TAG assembly on the ER, which may induce an unknown pathway that activates post-translational down-regulation of ACCase in plastid leading to reduced FA and TAG synthesis (Bates et al. 2014). Therefore, any mechanism that helps on efficient channeling of HFAs into TAG may limit the buildup of HFA and increase HFA-TAG content in seed oil.
Metabolic bottlenecks in HFA flux
Since PC is the site for production of UFA, introduction of transgenes that produce UFA into non-UFA accumulators typically cause accumulation of higher UFA on PC in the host plants than in the native source (Singh et al. 2001; Cahoon et al., 2006; van Erp et al. 2011; Bates and Browse 2012; Snapp et al. 2014; Bates et al. 2014). When host transgenic plants fail to efficiently utilize the PC-UFA, bottlenecks could occur in any pathways that direct UFA into TAG assembly, such as removal of UFA from PC, channeling UFA into acyl-CoA pool, synthesis of de novo DAG and PC-derived DAG, and final acylation by DGAT and/or PDAT (Bates and Browse 2012). Metabolic labeling experiments have demonstrated one such bottleneck in transgenic Arabidopsis expressing RcFAH12 that was at HFA flux through PC-derived DAG (Bates and Browse 2011). Although HFA-containing de novo DAG was synthesized, it was not efficiently utilized for TAG synthesis, or incorporated to PC and appeared to be turned over (Bates and Browse 2011). Even only 17 % HFA accumulated in transgenic Arabidopsis expressing RcFAH12, large proportion of HFA were found at the sn-2 position of TAG (van Erp et al. 2011). It was suggested that Arabidopsis may mostly utilize PC-derived DAG for TAG synthesis, the accumulation of HFA mostly at the sn-2 position of TAG was attributed to the substantial flux of HFA-containing PC-derived DAG out of PC after production of HFA by RcFAH12 (Bates and Browse 2012). Therefore, the bottleneck in conversion of HFA-containing de novo DAG to PC may limit the accumulation of HFA at the sn-1 position of TAG, and potentially lead to reduction in oil accumulation (Bates and Browse 2011).
Overcoming the barriers
Increase of HFAs in seed oils using additional genes
DGAT DGAT catalyzes the final and rate-limiting step of TAG biosynthesis (Ichihara et al. 1988; Fig. 1). It is generally accepted that depending on the plant species, DGAT1 or DGAT2 is the major enzyme responsible for the accumulation of seed TAG. DGAT1 and DGAT2 are localized in the endoplasmic reticulum (ER; Zou et al. 1999; Lardizabal et al. 2001; Shockey et al. 2006). Arabidopsis DGAT1 is strongly involved in regulating the seed oil content in plants that do not produce UFAs (Zou et al. 1999; Jako et al. 2001; Zheng et al. 2008). DGAT2 from some UFA-producing plants plays an important role in the accumulation of UFAs in TAG (Kroon et al. 2006). Consistent with these results, when castor DGAT2 was expressed in CL37, HFAs accumulation boosted from 17 to 30 % of the seed oil (Burgal et al. 2008). In addition, Arabidopsis double-transgenic lines expressing RcFAH12/RcDGAT2 increased seed weight and oil content. These seeds germinated well and their subsequent plant growth and seed production were also normal (Burgal et al. 2008).
PDAT PDAT catalyzes the transfer of the FA at the sn-2 position of PC to the sn-3 position of DAG (Dahlqvist et al. 2000). Since the FA at the sn-2 position of PC can be hydroxylated, the resulting HFA can be removed and incorporated into TAG by PDAT (Banas et al. 2000; Fig. 1). The role of castor PDAT in HFA-containing TAG synthesis has been well studied by two groups (van Erp et al. 2011; Kim et al. 2011), both of whom found that castor PDATs were comprised of three members, and one of them, RcPDAT1A (van Erp et al. 2011), the same as RcPDAT1-2 (Kim et al. 2011), was highly expressed in developing castor seeds. Since castor accumulates 90 % HFA in seed TAG, it has been proposed that the enzyme encoded by RcPDAT1A (or RcPDAT1-2) could be important for HFA-TAG synthesis. Indeed, when RcPDAT1A or RcPDAT1-2 was expressed in CL37, the HFAs content increased from 17 to 25–28 % in the seed oil (van Erp et al. 2011; Kim et al. 2011). Besides, CL37 seeds expressing RcPDAT1A (or RcPDAT1-2) showed increased seed weight and had normal seed germination and plant growth phenotypes (van Erp et al. 2011; Kim et al. 2011). To determine if RcPDAT1A (or RcPDAT1-2) and RcDGAT2 have an additive effect on HFA levels, both groups introduced RcDGAT2 to CL37 expressing RcPDAT1A (or RcPDAT1-2). A small increase of HFA content was observed in the seed oil of triple transgenic CL37 RcPDAT1A RcDGAT2 homozygous lines (van Erp et al. 2011). In addition, the triple transgenic seeds showed a 19.6 % increase in the mass of HFAs per seed (van Erp et al. 2011).
PDCT PDCT catalyzes PC and DAG conversion by headgroup exchange between PC and DAG (Lu et al. 2009; Hu et al. 2012; Fig. 1). Arabidopsis PDCT (AtPDCT) plays a major role in regulating the degree of unsaturation in seed oils (Lu et al. 2009). AtPDCT can convert sn-2-PUFA-PC and sn-2-18:1-DAG to sn-2-18:1-PC and sn-2-PUFA-DAG, respectively (Bates et al. 2012), i.e., PDCT catalyzes the reaction that exchanges the head group of unsaturated PC and de novo DAG, thus producing PC-derived DAG. It was assumed that PDCT from seed of HFA-producing plant can convert HFA-PC into HFA-DAG, subsequently HFA-DAG can be converted to HFA-TAG (Fig. 1). A castor PDCT gene was isolated and coexpressed in CL37. The resulted transgenic Arabidopsis had increased HFA content from 17 to 23 % in seed oil (Hu et al. 2012). Additional expression of RcDGAT2 further enhanced the HFA content to 28 % (Hu et al. 2012). The authors noted that coexpression of AtPDCT did not increase HFA in CL37, indicating that RcPDCT had evolved to effectively convert HFA-PC to HFA-DAG.
3-Ketoacyl-CoA synthase (KCS) The KCS3 from P. fendleri (PfKCS3) encodes the enzyme responsible for the specific elongation of C18-HFAs to C20-HFAs in lesquerella (Moon et al. 2001). Interestingly, when the PfKCS3 gene was coexpressed with the RcFAH12 gene in Camelina, the proportion of HFAs, especially C20-HFAs, in the transgenic Camelina seed oil increased 1.4-fold (from 13.8 to 19.4 %), and the proportion of HFAs in the polar lipids decreased from 5 to 1.5 % (Snapp et al. 2014). Furthermore, the oil content and germination rate of Camelina seeds expressing PfKCS3/RcFAH12 were restored to levels similar to those of nontransgenic Camelina. Hence, the HFA content per seed of Camelina expressing PfKCS3-RcFAH12 was 150 % greater than that of Camelina expressing RcFAH12 alone. It was speculated that C20-HFAs elongated by PfKCS3 may not be effectively incorporated into the sn-2 position of PC, because no C20-HFAs were detected in the polar lipids. Therefore C20-HFAs would be more readily incorporated into TAG through Kennedy pathway (Snapp et al. 2014).
As described above, at least 4 different genes, RcDGAT2, RcPDAT1A (or RcPDAT1-2), RcPDCT, and PfKCS3, have been demonstrated to be effective in channeling of HFAs into TAG which may limit the buildup of HFA and restore HFA-TAG content in seed oil.
On-going or under-explored candidate genes involved in HFA-TAG synthesis
LPCAT This enzyme plays a role in regulating the degree of unsaturation in seed oils. Hence, the proportion of PUFAs in the seed oil was reduced in the Arabidopsis lpcat1/2 double mutant (Bates et al. 2012), and the LPCAT-overexpressing line contained a higher proportion of PUFAs in the seed oil than the wild type (Wang et al. 2012). The constant deacylation and reacylation is called acyl editing or the Lands cycle (Stymne and Stobart 1984; Lager et al. 2013; Lands 1965). In Arabidopsis, two LPCAT genes, LPCAT1 and LPCAT2, are involved in acyl editing (Bates et al. 2012). In the developing silique of the Arabidopsis dgat1 mutant, PDAT1 was upregulated to compensate for the lack of DGAT activity and was mainly responsible for the synthesis of TAG. The expression of LPCAT2, but not LPCAT1, was also upregulated and was chiefly responsible for the reacylation of the LPC produced by PDAT1 (Xu et al. 2012). Recent studies have revealed the role of LPCATs in transferring polyunsaturated acyl and ricinoleoyl groups from PC directly into the acyl-CoA pool by acyl exchange. (Xu et al. 2012; Bates et al. 2012; Wang et al. 2012; Lager et al. 2013). They suggest that LPCATs are responsible for incorporation of newly synthesized FA into PC (forward reaction), and transferring polyunsaturated FA and HFAs produced on PC back to the acyl-CoA pool (reverse reaction). When reverse reactions were measured, a ricinoleoyl group at the sn-2 position of PC was removed three- to sixfold faster than an oleoyl group by seven LPCATs from five species tested, including Arabidopsis, castor and lesquerella. These results suggest an important role for LPCATs in removing ricinoleoyl groups from the sn-2 position of PC to an acyl-CoA pool (Fig. 1). The acyl-CoA pool enriched with HFA can facilitate production of HFA-TAG through the Kennedy pathway. The annotated castor LPCAT genes 30170.m014002 and 30174.m008937 show broadly equivalent expression in the whole-plant tissues, rather than seed-specific or seed-abundant expression (Brown et al. 2012). The Arabidopsis genes corresponding to these genes were confirmed to be lysophosphatidyl ethanolamine acyltransferase (LPEAT) genes (Stålberg et al. 2009). 30190.m01126, annotated as the membrane-bound O-acyltransferase (MBOAT) gene of castor, is significantly expressed in the endosperm (Brown et al. 2012), and was confirmed to have an LPCAT function (Arroyo-Caro et al. 2013a; Lager et al. 2013).
Phospholipase A 2 (PLA 2 ) PLA2. hydrolyzes phospholipids at the sn-2 position and releases free FAs. One of the routes of the released FAs is to enter the acyl-CoA pool for TAG assembly (Fig. 1). Thus, PLA2 together with LPCAT serve as FA editing enzymes for modifying the composition of acyl-CoA pool. The role of PLA2 in castor oil biosynthesis has been long proposed (Bafor et al. 1991), but the gene encoding PLA2 has been elusive. Recently, a lecithin:cholesterol acyltransferase (LCAT)-like PLA with high PLA2 activity was identified in Arabidopsis (Chen et al. 2012). Its ortholog in lesquerella was found highly expressed in developing seeds. Coexpression of C. purpurea FAH12 and the PfLCAT-PLA gene in yeast revealed that PfLCAT-PLA facilitates the channeling of HFA from PC to TAG (Chen et al. 2014). Identification of PLA 2 and more LCAT-PLA genes and overexpression of them in transgenic oilseeds would help to elucidate the role of PLA2 or LCAT-PLA in HFA-TAG synthesis in plants.
CPT CDP-choline:DAG cholinephosphotransferase (CPT) catalyzes the condensation reaction of CDP-choline with DAG to generate PC. However, the reaction was found to be reversible (Slack et al. 1983; 1985; Dewey et al. 1994). Since PC is the substrate for FA modification, such as hydroxylation and desaturation. The reverse reaction of CPT provides a mechanism for supplying mFA-DAG, which can then be utilized for mFA-TAG synthesis (Fig. 1). Thus, CPT could contribute to PC-derived DAG formation, in addition to PDCT, for TAG assembly. A castor CPT gene (RcCPT) has been identified, ubiquitously expressed in all organs examined (Brown et al. 2012). Compared with RcCPT, the expression of RcPDCT was two-fold higher in the endosperm than in the leaves or male flowers, and was higher than the expression of RcCPT (Brown et al. 2012). The role RcCPT in castor oil biosynthesis remains to be investigated. Identification of CPTs favoring production of PC-derived HFA-DAG would provide as new target for increased HFA in transgenic plants.
Long-chain Acyl-CoA synthetase (LACS) Nine LACS genes have been identified in Arabidopsis (Shockey et al. 2002). AtLACS1 and AtLACS2 are involved in the wax and cutin synthesis (Schnurr et al. 2004; Lü et al. 2009). AtLACS6 and AtLACS7 are involved in the β-oxidation of fatty acid (Fulda et al. 2002). AtLACS9 is localized on the outer plastidial envelope and activates free fatty acid released from acyl-ACP by fatty acyl-ACP thioesterase. AtLACS8 was predicted to encode the plastidial LACS like AtLACS9 (Schnurr et al. 2002). Due to functionally redundancy of these family members, research has not identified a single member responsible for seed oil synthesis (Schnurr et al. 2002; Zhao et al. 2010). Surprisingly, AtLACS9 and AtLACS8, which were found to be involved in seed oil synthesis together with AtLACS1, which is also associated with cuticular lipid synthesis (Zhao et al. 2010). Even though both genes are expressed seed-abundantly, AtLACS8 localizes in the ER, unlike AtLACS9 (Zhao et al. 2010). Putative seven LACS genes are found in castor (Brown et al. 2012). LACS localized in the ER from castor seed is predicted to contribute to convert HFA free fatty acid into HFA-CoA form, which is used as a constituent of HFA-TAG (Fig. 1). Functional studies need to be carried out to determine its role in castor TAG synthesis.
Acyl-CoA binding protein (ACBP) ACBP binds acyl-CoA and is associated with the stability of acyl-CoA in the cytosol, the transport of acyl-CoA, and the prevention of acyl-CoA hydrolysis by acyl-CoA hydrolase (Xiao and Chye 2009). Overexpression of ACBP in plants resulted in modifications of FA composition in seed oil (Enikeev and Mishutina 2005; Yurchenko et al. 2009). Overexpression of a B. napus ACBP6 gene (BnACBP6) increased 18:2 and 18:3 at expense of 20:1 and saturated FA in transgenic Arabidopsis seed oil (Yurchenko et al. 2009). The results of in vitro LPCAT assays suggested that ACBP may enhance the incorporation of 18:1 into PC by promoting acyl exchange between PC and acyl-CoA catalysed by LPCAT (Yurchenko et al. 2009). An increased rate of 18:1 esterification to PC creates more substrate for FAD2 desaturase, which may result in a greater production of 18:2, which may be further desaturated to 18:3 by FAD3. A castor ACBP gene (RcACBP6), 29827.m002594, corresponding to AtACBP6 and BnACBP6 has been identified and found to be highly expressed in the endosperm (Brown et al. 2012). Whether RcACBP6 plays a similar role to BnACBP6 remains to be investigated. Overexpression of RcACBP6 would induce HFA group of HFA-PC to release into cytosol and bind HFA-CoA, and consequently increasing the level of HFAs in seed oil.
LPAT LPAT acylates the sn-2 position of the LPA with acyl-CoA and yields PA. The LPAT enzyme belongs to a multigene family and is considered one of the most stringent acyltransferases with respect to substrate discrimination (Frentzen 1998). Thus, identification of a LPAT that participates in the channeling of HFA-CoA to TAG is a key to the successful genetic engineering of HFA-producing crops using this enzyme. Five isoforms of LPAT exist in Arabidopsis and AtLPAT2 is known to be involved in TAG synthesis in developing seeds (Kim et al. 2005). Castor has nine LPATs, RcLPAT2 and RcLPATB are expressed in developing seeds (Arroyo-Caro et al. 2013b). In vitro enzyme assays indicate that RcLPAT2 had a preference for acylating 18:1-OH-CoA when 18:1-OH-LPA was used as an acyl acceptor, however when 18:1-LPA was used as the acyl-acceptor, RcLPAT2 preferred 18:1-CoA as a substrate (Arroyo-Caro et al. 2013b). The results indicate that RcLPAT2 might participate in both TAG and membrane glycerolipid synthesis in castor. RcLPATB, having no orthologue in Arabidopsis, is highly homologous to the LPAT genes from meadowfoam (Limnanthes spp.) or coconut (Cocos nucifera), whose oils are enriched with UFAs, 20:1Δ5 or 12:0, at the sn-2 positions of their TAGs (Table 1). Enzymes encoded by these genes exhibited a strong preference for the utilization of 20:1Δ5 or 12:0, respectively, in agreement to microsomal activities recorded in their seeds (Frentzen 1998). However, enzyme assays indicated that RcLPATB prefers saturated fatty acids (12:0–16:0) as substrates independently of the acyl group in the acceptor molecule, and has a broad specificity on other acyl-CoAs including 18:1-OH and 18:1 (Arroyo-Caro et al. 2013b). Although these in vitro data cannot infer definitely whether RcLPAT2 and/or RcPLATB play important roles in castor TAG assembly in vivo, it is possible to test their functions by seed specific expression of these genes in transgenic oilseeds.
GPAT GPAT catalyzes the first reaction in the Kennedy pathway by acylation of FA into the sn-1 position of G3P. One plastidial GPAT and nine nonplastidial GPATs have been identified in Arabidopsis (Nishida et al. 1993; Zheng et al. 2003; Li et al. 2007; Gidda et al. 2009). Most GPATs are involved in the synthesis of cutin and suberin (Li et al. 2007; Beisson et al. 2007; Yang et al. 2010). AtGPAT9 is located in the ER and is predicted to be associated with TAG synthesis in seeds (Gidda et al. 2009). A tung tree (Vernicia fordii) GPAT9 (VfGPAT9) was found colocalized in the same ER subdomain with VfDGAT2 and interacted with VfDGAT2 (Gidda et al. 2011). Castor GPAT9 (30122.m000357), which is highly homologous to VfGPAT9, also localizes in the ER (Brown et al. 2012). Therefore, the RcGPAT9 may potentially contribute to HFA-TAG synthesis.
Conclusion and future perspectives
It has been nearly two decades since the identification of the key gene RcFAH12. Research efforts have made significant progress toward production of HFAs in transgenic oilseeds through discovery and overexpression of additional genes. The maximum percentage of HFA in transgenic seed oil has progressed up to 30 % (Burgal et al. 2008). However, this increase is not yet sufficient for economically sustainable production of HFAs from transgenic oilseed crops. Obviously, there are additional candidate genes waiting to be discovered. These include underexplored genes, or isoforms from known gene families that have high selectivity to HFAs and specifically favors channeling and incorporation them into TAG. Genomics has provided tools for generating genome-wide database useful for uncovering castor genes (Brown et al. 2012; Troncoso-Ponce et al. 2011). Establishment of new seed transcriptomes from other HFA accumulators, such lesquerella and Physaria lindheimeri, which accumulates 60 and 80 % of 20:1-OH, respectively, should provide additional valuable resources for discovering new target genes. Choice of host crop that has a high rate of acyl editing to remove HFA from PC and also primarily utilizes Kennedy pathway for TAG assembly has been suggested to be ideal for transgenic production of HFA (Bates and Browse 2012). Identification of such crops requires determination of the relative fluxes through various TAG assembly pathways. Lesquerella is under development as an industrial crop for production of a safe source of HFA. Its seed oil already contains 55–60 % 20:1-OH, mostly at the sn-1 and the sn-3 positions of TAG (Hayes and Kleiman 1996). Identification of a LPAT highly selective to 20:1-OH-CoA is the key to increase HFA level over 80 % of seed oil. Knowledge and information obtained from studies of HFA synthesis may improve our understanding of the mechanism underlying other UFA production in oilseed crops, which will advance the development of plant-based petrochemical feedstocks.
Author contribution statement
The preparation of this manuscript was a collaboration of KRL, GQC, and HUK.
References
Amri IN (2011) The Lauric (Coconut and Palm Kernel) Oils. In: Gunstone FD (ed) Vegetable oils in food technology: composition, properties and uses, 2nd edn. Wiley-Blackwell, Oxford, pp 169–198
Andre C, Haslam RP, Shanklin J (2012) Feedback regulation of plastidic acetyl-CoA carboxylase by 18:1-acyl carrier protein in Brassica napus. Proc Natl Acad Sci USA 109:10107–10112
Armougom R, Grondin I, Smadja J (1998) Fatty acid composition of lipid extracts of some tropical cucurbit seeds. Ocl Ol Corps Gras Lipids 5:323–328
Arroyo-Caro JM, Chileh T, Alonso DL, García-Maroto F (2013a) Molecular characterization of a lysophosphatidylcholine acyltransferase gene belonging to the MBOAT family in Ricinus communis L. Lipids 48:663–674
Arroyo-Caro JM, Chileh T, Kazachkov M, Zou J, Alonso DL, García-Maroto F (2013b) The multigene family of lysophosphatidate acyltransferase (LPAT)-related enzymes in Ricinus communis. Cloning and molecular characterization of two LPAT genes that are expressed in castor seeds. Plant Sci 199–200:29–40
Badami RC, Patil KB (1980) Structure and occurrence of unusual fatty acids in minor seed oils. Prog Lipid Res 19:119–153
Bafor M, Smith MA, Jonsson L, Stobart K, Stymne S (1991) Ricinoleic acid biosynthesis and triacylglycerol assembly in microsomal preparations from developing castor-bean (Ricinus communis) endosperm. Biochem J 280:507–514
Banas A, Johansson I, Stymne S (1992) Plant microsomal phospholipases exhibit preference for phosphatidylcholine with oxygenated acyl groups. Plant Sci 84:137–144
Banas A, Dahlqvist A, Ståhl U, Lenman M, Stymne S (2000) The involvement of phospholipid: diacylglycerol acyltransferases in triacylglycerol production. Biochem Soc Trans 28:703–705
Bao XM, Katz S, Pollard M, Ohlrogge J (2002) Carbocyclic fatty acids in plants: biochemical and molecular genetic characterization of cyclopropane fatty acid synthesis of Sterculia foetida. Proc Natl Acad Sci USA 99:7172–7177
Bates PD, Browse J (2011) The pathway of triacylglycerol synthesis through phosphatidylcholine in Arabidopsis produces a bottleneck for the accumulation of unusual FAs in transgenic seeds. Plant J 68:387–399
Bates PD, Browse J (2012) The significance of different diacylgycerol synthesis pathways on plant oil composition and bioengineering. Front Plant Sci. 2(3):147. doi:10.3389/fpls.2012.00147
Bates PD, Durrett TP, Ohlrogge JB, Pollard M (2009) Analysis of acyl fluxes through multiple pathways of triacylglycerol synthesis in developing soybean embryos. Plant Physiol 150:55–72
Bates PD, Fatihi A, Snapp AR, Carlsson AS, Browse J, Lu C (2012) Acyl editing and headgroup exchange are the major mechanisms that direct polyunsaturated fatty acid flux into triacylglycerols. Plant Physiol 160:1530–1539
Bates PD, Johnson SR, Cao X, Li J, Nam J-W, Jaworski JG, Ohlrogge JB, Browse J (2014) FA synthesis is inhibited by inefficient utilization of unusual FAs for glycerolipid assembly. Proc Natl Acad Sci USA 111:1204–1209
Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB (2007) The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19:351–368
Broun P, Somerville C (1997) Accumulation of ricinoleic, lesquerolic, and densipolic acids in seeds of transgenic Arabidopsis plants that express a fatty acyl hydroxylase cDNA from castor bean. Plant Physiol 113:933–942
Broun P, Boddupalli S, Somerville C (1998) A bifunctional oleate 12-hydroxylase: desaturase from Lesquerella fendleri. Plant J 13:201–210
Brown AP, Kroon JTM, Swarbreck D, Febrer M, Larson TR, Graham IA, Caccamo M, Slabas AR (2012) Tissue-specific whole transcriptome sequencing in castor, directed at understanding triacylglycerol lipid biosynthetic pathways. PLoS One 7:e30100
Burgal J, Shockey J, Lu C, Dyer J, Larson T, Graham I, Browse J (2008) Metabolic engineering of hydroxy fatty acid production in plants: RcDGAT2 drives dramatic increases in ricinoleate levels in seed oil. Plant Biotechnol J 6:819–831
Cahoon EB, Kinney AJ (2004) Dimorphecolic acid is synthesized by the coordinate activities of two divergent Δ12-oleic acid desaturase. J Biol Chem 279:12495–12502
Cahoon EB, Carlson TJ, Ripp KG, Schweiger BJ, Cook GA, Hall SE, Kinney AJ (1999) Biosynthetic origin of conjugated double bonds: production of fatty acid components of high-value drying oils in transgenic soybean embryos. Proc Natl Acad Sci USA 96:12935–12940
Cahoon EB, Ripp KG, Hall SE, Kinney AJ (2001) Formation of conjugated Δ8, Δ10-double bonds by Δ12-oleic acid desaturase-related enzymes. J Biol Chem 276:2637–2643
Cahoon EB, Schnurr JA, Huffman EA, Minto RE (2003) Fungal responsive fatty acid acetylenases occur widely in evolutionarily distant plant families. Plant J 34:671–683
Cahoon EB, Dietrich CR, Meyer K, Damude HG, Dyer JM, Kinney AJ (2006) Conjugated fatty acids accumulate to high levels in phospholipids of metabolically engineered soybean and Arabidopsis seeds. Phytochemistry 67:1166–1176
Caupin HJ (1997) Products from castor oil: Past, present, and future. In: Gunstone FD, Padley FB (eds) Lipid technologies and applications. Marcel Dekker, New York, pp 787–795
Chapman KD, Ohlrogge JB (2012) Compartmentation of triacylglycerol accumulation in plants. J Biol Chem 287:2288–2294
Chen GQ (2011) Effective reduction of chimeric tissue in transgenics for the stable genetic transformation of Lesquerella fendleri. HortScience 46:86–90
Chen G, Greer MS, Lager I, Lindberg Yilmaz J, Mietkiewska E, Carlsson AS, Stymne S, Weselake RJ (2012) Identification and characterization of an LCAT-like Arabidopsis thaliana gene encoding a novel phospholipase A. FEBS Lett 586:373–377
Chen G, Tian B, Greer MS, Caldo KM, Singer S, Mietkiewska E, Dyer J, Smith M, Qiu X, Stymne S, Weselake RJ (2014) Characterization of a LCAT-like phospholipase A from hydroxy-fatty acid producing Lesquerella fendleri. In: 21st international symposium on plant lipids. Guelph, Ontario, Canada, pp 24
Dahlqvist A, Ståhl U, Lenman M, Banas A, Lee M, Sandager L, Ronne H, Stymne S (2000) Phospholipid: diacylglycerol acyltransferase: an enzyme that catalyzes the acyl-CoA-independent formation of triacylglycerol in yeast and plants. Proc Natl Acad Sci USA 97:6487–6492
Dauk M, Lam P, Kunst L, Smith MA (2007) A FAD2 homologue from Lesquerella lindheimeri has predominantly fatty acid hydroxylase activity. Plant Sci 173:43–49
Demirbas A (2009) Political, economic and environmental impacts of biofuels: a review. Appl Energy 86:S108–S117
Dewey RE, Wilson RF, Novitzky WP, Goode JH (1994) The AAPT1 gene of soybean complements a cholinephosphotransferase-deficient mutant of yeast. Plant Cell 6:1495–1507
Dierig DA, Thompson AE, Nakayama FS (1993) Lesquerella commercialization efforts in the United States. Ind Crop Prod 1:289–293
Dierig DA, Dahlquist GH, Tomasi PM (2006) Registration of WCL-LO3 high oil Lesquerella fendleri germplasm. Crop Sci 46:1832–1833
Dierig DA, Wang G, McCloskey WB, Thorp KR, Isbell TA, Ray DT, Foster MA (2011) Lesquerella: new crop development and commercialization in the U.S. Ind Crop Prod 34:1381–1385
Dyer JM, Chapital DC, Kuan JC, Mullen RT, Turner C, McKeon TA, Pepperman AB (2002) Molecular analysis of a bifunctional fatty acid conjugase/desaturase from tung. Implications for the evolution of plant fatty acid diversity. Plant Physiol 130:2027–2038
Eccleston VS, Ohlrogge JB (1998) Expression of lauroyl-acyl carrier protein thioesterase in Brassica napus seeds induces pathways for both FA oxidation and biosynthesis and implies a set point for triacylglycerol accumulation. Plant Cell 10:613–621
Engeseth N, Stymne S (1996) Desaturation of oxygenated fatty acids in Lesquerella and other oil seeds. Planta 198:238–245
Enikeev AG, Mishutina UO (2005) Physiological effects of rapeseed transformation with the acb gene as affected by the genetic vector structure. Rus J Plant Physiol 52:668–671
Fairley P (2011) Next generation biofuels. Nature 474:S2–S5
Frentzen M (1998) Acyltransferases from basic science to modified seed oils. Eur J Lipid Sci Technol 100:161–166
Fulda M, Shockey J, Werber M, Wolter FP, Heinz E (2002) Two long-chain acyl-CoA synthetases from Arabidopsis thaliana involved in peroxisomal fatty acid beta-oxidation. Plant J 32:93–103
Galliard T, Stumpf PK (1966) Fat metabolism in higher plants. 30 Enzymatic synthesis of ricinoleic acid by a microsomal preparation from developing Ricinus communis seeds. J Biol Chem 241:5806–5812
Gidda SK, Shockey JM, Rothstein SJ, Dyer JM, Mullen RT (2009) Arabidopsis thaliana GPAT8 and GPAT9 are localized to the ER and possess distinct ER retrieval signals: functional divergence of the dilysine ER retrieval motif in plant cells. Plant Physiol Biochem 47:867–879
Gidda SK, Shockey JM, Falcone M, Kim PK, Rothstein SJ, Andrews DW, Dyer JM, Mullen RT (2011) Hydrophobic-domain-dependent protein–protein interactions mediate the localization of GPAT enzymes to ER subdomains. Traffic 12:452–472
Gunstone FD (2008) Disappearance. Lipid Technol 20:48
Gunstone FD, Hamilton RJ (2001) Oleochemical manufacture and applications. Sheffield Academic Press, UK
Guo Y, Mietkiewska L, Francis T, Katavic V, Brost JM, Giblin M, Barton DL, Taylor DC (2009) Increase in nervonic acid content in transformed yeast and transgenic plants by introduction of a Lunaria annua L. 3-ketoacyl-CoA synthase (KCS) gene. Plant Mol Biol 69:565–575
Hayes DG, Kleiman R (1996) A detailed triglyceride analysis of Lesquerella fendleri oil: column chromatographic fractionation followed by supercritical fluid chromatography. J Am Oil Chem Soc 73:267–269
Hettiarachchi D, Liu Y, Fox J, Sunderland B (2010) Western Australian sandalwood seed oil: new opportunities. Lipid Technology 22:27–29
Holic R, Yazawa H, Kumagai H, Uemura H (2012) Engineered high content of ricinoleic acid in fission yeast Schizosaccharomyces pombe. Appl Microbiol Biotechnol 95:179–187
Hu Z, Ren Z, Lu C (2012) The phosphatidylcholine diacylglycerol cholinephosphotransferase is required for efficient hydroxy fatty acid accumulation in transgenic Arabidopsis. Plant Physiol 158:1944–1954
Huang C-Y, Chung C-I, Lin Y-C, Hsing Y-IC, Huang AHC (2009) Oil bodies and oleosins in Physcomitrella possess characteristics representative of early trends in evolution. Plant Physiol 150:1192–1203
Ichihara K, Takahashi T, Fujii S (1988) Diacylglycerol acyltransferase in maturing safflower seeds: its influences on the fatty acid composition of triacylglycerol and on the rate of triacylglycerol synthesis. Biochim Biophys Acta 958:125–129
Isbell TA, Mund MS, Evangelista RL, Dierig DA (2008) Method for analysis of fatty acid distribution and oil content on a single Lesquerella fendleri seed. Ind Crop Prod 28:231–236
Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126:861–874
Jenderek MM, Dierig DA, Isbell TA (2009) Fatty-acid profile of Lesquerella germplasm in the National Plant Germplasm System collection. Ind Crops Prod 29:154–164
Kim HU, Li Y, Huang AH (2005) Ubiquitous and endoplasmic reticulum-located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis. Plant Cell 17:1073–1089
Kim HU, Lee K-R, Go YS, Jung JH, Suh M-C, Kim JB (2011) Endoplasmic reticulum-located PDAT1-2 from castor bean enhances hydroxy fatty acid accumulation in transgenic plants. Plant Cell Physiol 52:983–993
Kleiman R (1990) Chemistry of new industrial oilseed crops. In: Janick J, Simon JE (eds) Advances in new crops. Timber Press, Portland, pp 196–203
Kroon JT, Wei W, Simon WJ, Slabas AR (2006) Identification and functional expression of a type 2 acyl-CoA:diacylglcerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry 67:2541–2549
Kumar R, Wallis JG, Skidmore C, Browse J (2006) A mutation in Arabidopsis cytochrome b5 reductase identified by high-throughput screening differentially affects hydroxylation and desaturation. Plant J 48:920–932
Lager I, Yilmaz JL, Zhou XR, Jasieniecka K, Kazachkov M, Wang P, Zou J, Weselake R, Smith MA, Bayon S, Dyer JM, Shockey JM, Heinz E, Green A, Banas A, Stymne S (2013) Plant acyl-CoA:lysophosphatidylcholine acyltransferases (LPCATs) have different specificities in their forward and reverse reactions. J Biol Chem 288:36902–36914
Lands WE (1965) Lipid metabolism. Ann Rev Biochem 34:313–346
Lardizabal KD, Mai JT, Wagner NW, Wyrick A, Voelker T, Hawkins DJ (2001) DGAT2 is a new diacylglycerol acyltransferase gene family: purification, cloning, and expression in insect cell of two polypeptides from Mortierella ramanniana with diacylglycerol acyltransferase activity. J Biol Chem 276:38862–38869
Lee M, Lenman M, Banas A, Bafor M, Singh S, Schweizer M, Nilsson R, Liljenberg C, Dahlqvist A, Gummeson P, Sjödahl S, Green A, Stymne S (1998) Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation. Science 280:915–918
Li Y, Beisson F, Koo AJK, Molina I, Pollard M, Ohlrogge J (2007) Identification of acyltransferases required for cutin biosynthesis and production of cutin with suberin-like monomers. Proc Natl Acad Sci USA 104:18339–18344
Li R, Yu K, Wu Y, Tateno M, Hatanaka T, Hildebrand DF (2012) Vernonia DGATs can complement the disrupted oil and protein metabolism in epoxygenase-expressing soybean seeds. Metab Eng 14:29–38
Li-Beisson Y, Shorrosh B, Beisson F, Andersson MX, Arondel V, Bates PD, Baud S, Bird D, Debono A, Durrett TP, Franke RB, Graham IA, Katayama K, Kelly AA, Larson T, Markham JE, Miquel M, Molina I, Nishida I, Rowland O, Samuels L, Schmid KM, Wada H, Welti R, Xu C, Zallot R, Ohlrogge J (2013) Acyl-lipid metabolism. Arabidopsis Book 2013(11):e0161. doi:10.1199/tab.0161
Lin JT, Turner C, Liao LP, McKeon TA (2003) Identification and quantification of the molecular species of acylglycerols in castor oil by HPLC using ELSD. J Liquid Chromatography & Related Technologies 26:773–780
Lu C, Kang J (2008) Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium-mediated transformation. Plant Cell Rep 27:273–278
Lu C, Fulda M, Wallis J, Browse J (2006) A high-throughput screen for genes from castor that boost hydroxy fatty acid accumulation in seed oils of transgenic Arabidopsis. Plant J 45:847–856
Lu C, Xin Z, Ren Z, Miquel M, Browse J (2009) An enzyme regulating triacylglycerol composition is encoded by the ROD1 gene of Arabidopsis. Proc Natl Acad Sci USA 106:18837–18842
Lü S, Song T, Kosma DK, Parsons EP, Rowland O, Jenks MA (2009) Arabidopsis CER8 encodes LONG-CHAIN ACYL-COA SYNTHETASE 1 (LACS1) that has overlapping functions with LACS2 in plant wax and cutin synthesis. Plant J 59:553–564
Malathi B, Ramesh S, Venkateswara Rao K, Dashavantha Reddy V (2006) Agrobacterium-mediated genetic transformation and production of semilooper resistant transgenic castor (Ricinus communis L.). Euphytica 147:441–449
Mantle PG, Nisbet LJ (1976) Differentiation of Claviceps purpurea in axenic culture. J Gen Microbiol 93:321–334
Meesapyodsuk D, Qiu X (2008) An oleate hydroxylase from the fungus Claviceps purpurea: cloning, functional analysis, and expression in Arabidopsis. Plant Physiol 147:1325–1333
Metzger JO (2009) Fats and oils as renewable feedstock for chemistry. Eur J Lipid Sci Technol 111:865–876
Millar AA, Smith MA, Kunst L (2000) All FAs are not equal: discrimination in plant membrane lipids. Trends Plant Sci 5:95–101
Moire L, Rezzonico E, Goepfert S, Poirier Y (2004) Impact of unusual fatty acid synthesis on futile cycling through beta-oxidation and on gene expression in transgenic plants. Plant Physio 134:432–442
Moon H, Smith MA, Kunst L (2001) A condensing enzyme from the seeds of Lesquerella fendleri that specifically elongates hydroxyl fatty acids. Plant Physiol 127:1635–1643
Moreau RA, Stumpf PK (1981) Recent studies of the enzymic synthesis of ricinoleic acid by developing castor beans. Plant Physiol 67:672–676
Nishida I, Tasaka Y, Shiraishi H, Murata N (1993) The gene and the RNA for the precursor to the plastid-located glycerol-3-phosphate acyltransferase of Arabidopsis thaliana. Plant Mol Biol 21:267–277
Ohlrogge JB, Pollard MR, Stumpf PK (1978) Studies on biosynthesis of waxes by developing jojoba seed tissues. Lipids 13:203–210
Okuley J, Lightner J, Feldmann K, Yadav N, Lark E, Browse J (1994) Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 6:147–158
Princen LH, Rothfus JA (1984) Development of new crops for industrial raw materials. J Am Oil Chem Soc 61:281–289
Ramadan MF, Morsel JT (2002) Oil composition of coriander (Coriandrum sativum L.) fruit-seeds. Eur Food Res Technol 215:204–209
Reed DW, Taylor DC, Covello PS (1997) Metabolism of hydroxy fatty acids in developing seeds in the genera Lesquerella (Brassicaceae) and Linum (Linaceae). Plant Physiol 114:63–68
Rossak M, Smith M, Kunst L (2001) Expression of the FAE1 gene and FAE1 promoter activity in developing seeds of Arabidopsis thaliana. Plant Mol Biol 46:717–725
Sailaja M, Tarakeswari M, Sujatha M (2008) Stable genetic transformation of castor (Ricinus communis L.) via particle gun-mediated gene transfer using embryo axes from mature seeds. Plant Cell Rep 27:1509–1519
Schnurr JA, Shockey JM, de Boer G-J, Browse JA (2002) Fatty acid export from the chloroplast. Molecular characterization of a major plastidial acyl-coenzyme A synthetase from Arabidopsis. 129:1700–1709
Schnurr J, Shockey J, Browse J (2004) The Acyl-CoA synthetase encoded by LACS2 is essential for normal cuticle development in Arabidopsis. Plant Cell 16:629–642
Shanklin J, Cahoon EB (1998) Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol 49:611–641
Shintani DK, Ohlrogge JB (1995) Feedback inhibition of fatty-acid synthesis in tobacco suspension cells. Plant J 7:577–587
Shockey JM, Fulda MS, Browse JA (2002) Arabidopsis contains nine long-chain acyl-coenzyme A synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol 129:1710–1722
Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18:2294–2313
Singh S, Thomaeus S, Lee M, Stymne S, Green A (2001) Transgenic expression of a delta 12-epoxygenase gene in Arabidopsis seeds inhibits accumulation of linoleic acid. Planta 212:872–879
Slack CR, Campbell LC, Browse JA, Roughan PG (1983) Some evidence for the reversibility of the cholinephosphotransferase catalysed reaction in developing linseed cotyledons in vivo. Biochim Biophys Acta 754:10–20
Slack CR, Roughan PG, Browse JA, Gardiner SE (1985) Some properties of cholinephosphotransferase from developing safflower cotyledons. Biochim Biophys Acta 833:438–448
Smith CR Jr (1971) Occurrence of unusual fatty acids in plants. Prog Chem Fats Other Lipids 11:137–177
Smith CR Jr, Wilson TL, Melvin EH, Wolff IA (1960) Dimorphecolic Acid—a unique hydroxydienoid fatty acid. J Am Chem Soc 82:1417–1421
Smith MA, Moon H, Chowrira G, Kunst L (2003) Heterologous expression of a fatty acid hydroxylase gene in developing seeds of Arabidopsis thaliana. Planta 217:507–516
Snapp AR, Kang J, Qi X, Lu C (2014) A fatty acid condensing enzyme from Physaria fendleri increases hydroxy fatty acid accumulation in transgenic oilseeds of Camelina sativa. Planta 240:599–610
Somerville CR, Browse J, Jaworski J, Ohlrogge J (2000) Lipids. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and Molecular Biology of Plants, Chap 10. (Rockville, MD: American Society of Plant Physioloists), pp 456–526
Sorda G, Banse M, Kemfert C (2010) An overview of biofuel policies across the world. Energy Policy 38:6977–6988
Ståhl U, Banas A, Stymne S (1995) Plant microsomal phospholipid acyl hydrolases have selectivities for uncommon fatty-acids. Plant Physiol 107:953–962
Stålberg K, Ståhl U, Stymne S, Ohlrogge J (2009) Characterization of two Arabidopsis thaliana acyltransferases with preference for lysophosphatidylethanolamine. BMC Plant Biol 9:60
Stymne S, Stobart AK (1984) Evidence for the reversibility of the acyl-CoA: lysophosphatidylcholine acyltransferase in microsomal preparations from developing safflower (Carthamus tinctorius L.) cotyledons and rat liver. Biochem J 223:305–314
Sujatha M, Sailaja M (2005) Stable genetic transformation of castor (Ricinus communis L.) via Agrobacterium tumefaciens-mediated gene transfer using embryo axes from mature seeds. Plant Cell Rep 23:803–810
Sujatha M, Lakshminarayana M, Tarakeswari M, Singh PK, Tuli R (2009) Expression of the cry1EC gene in castor (Ricinus communis L.) confers field resistance to tobacco caterpillar (Spodoptera litura Fabr) and castor semilooper (Achoea janata L.). Plant Cell Rep 28:935–946
Troncoso-Ponce MA, Kilaru A, Cao X, Durrett TP, Fan J, Jensen JK, Thrower NA, Pauly M, Wilkerson C, Ohlrogge JB (2011) Comparative deep transcriptional profiling of four developing oilseeds. Plant J 68:1014–1027
United States Department of Agriculture (2014) Oilseeds: world markets and trade. http://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf
van de Loo FJ, Broun P, Turner S, Somerville C (1995) An oleate 12-hydroxylase from Ricinus communis L. is a fatty acyl desaturase homolog. Proc Natl Acad Sci USA 92:6743–6747
van Erp H, Bates PD, Burgal J, Shockey J, Browse J (2011) Castor phospholipid: diacylglycerol acyltransferase facilitates efficient metabolism of hydroxy fatty acids in transgenic Arabidopsis. Plant Physiol 15:683–693
Wang L, Shen W, Kazachkov M, Chen G, Chen Q, Carlsson AS, Stymne S, Weselake RJ, Zou J (2012) Metabolic interactions between the Lands cycle and the Kennedy pathway of glycerolipid synthesis in Arabidopsis developing seeds. Plant Cell 24:4652–4669
Weiss SB, Kennedy EP (1956) The enzymatic synthesis of triglycerides. J Am Chem Soc 78:3550
Weiss SB, Kennedy EP, Kiyasu JY (1960) The enzymatic synthesis of triglycerides. J Biol Chem 235:40–44
Xiao S, Chye M-L (2009) An Arabidopsis family of six acyl-CoA-binding proteins has three cytosolic members. Plant Physio Biochem 47:479–484
Xu J, Carlsson AS, Francis T, Zhang M, Hoffman T, Giblin ME, Taylor DC (2012) Triacylglycerol synthesis by PDAT1 in the absence of DGAT1 activity is dependent on re-acylation of LPC by LPCAT2. BMC Plant Biol 12:4
Yang W, Pollard M, Li-Beisson Y, Beisson F, Feig M, Ohlrogge J (2010) A distinct type of glycerol-3-phosphate acyltransferase with sn-2 preference and phosphatase activity producing 2-monoacylglycerol. Proc Natl Acad Sci USA 107:12040–12045
Yurchenko OP, Nykiforuk CL, Moloney MM, Ståhl U, Banaś A, Stymne S, Weselake RJ (2009) A 10-kDa acyl-CoA-binding protein (ACBP) from Brassica napus enhances acyl exchange between acyl-CoA and phosphatidylcholine. Plant Biotechnol J 7:602–610
Zhao L, Katavic V, Li F, Haughn GW, Kunst L (2010) Insertional mutant analysis reveals that long-chain acyl-CoA synthetase 1 (LACS1), but not LACS8, functionally overlaps with LACS9 in Arabidopsis seed oil biosynthesis. Plant J 64:1048–1058
Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou J (2003) Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acytransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15:1872–1887
Zheng P, Allen WB, Roesler K, Williams ME, Zhang S, Li J, Glassman K, Ranch J, Nubel D, Solawetz W, Bhattramakki D, Llaca V, Deschamps S, Zhong G-Y, Tarczynski MC, Shen B (2008) A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nat Genet 40:367–372
Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC (1999) The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. Plant J 19:645–653
Acknowledgments
This study was conducted with the support of the Agricultural Research Project Program (project no. PJ010075) of the National Academy of Agricultural Science, the “Next-Generation BioGreen 21 Program” (SSAC, project no. PJ009484012014) of the Rural Development Administration, Republic of Korea, the US Department of Agriculture-Agricultural Research Service-Current Research Information System Project 2030-21410-020-00D, and the USDA Trust Fund Cooperative Agreement with RDA (Agreement number: 58 0212 9 036F). The authors wish to thank Dr. Colleen McMahan for critical reading of the manuscript. USDA is an equal opportunity provider and employer. Mention of a specific product name by the United States Department of Agriculture does not constitute an endorsement and does not imply a recommendation over other suitable products.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Neal Stewart.
Rights and permissions
About this article
Cite this article
Lee, KR., Chen, G.Q. & Kim, H.U. Current progress towards the metabolic engineering of plant seed oil for hydroxy fatty acids production. Plant Cell Rep 34, 603–615 (2015). https://doi.org/10.1007/s00299-015-1736-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-015-1736-6