Abstract
The impact of golimumab (GLM) on remission or low disease activity (LDA) was evaluated in patients with moderate-to-severe rheumatoid arthritis (RA), progressive psoriatic arthritis (PsA), or severe axial spondyloarthritis (axSpA), who failed previous treatment for their rheumatic disease with one initial tumor necrosis factor α inhibitor (TNFi). This is a multicenter, prospective, real-world observational 18-month study, conducted in Greece. The primary endpoint, assessed at 6 months, included the proportion of patients attaining LDA and/or remission (Disease Activity Score for 28 joints based on C-reactive protein [DAS28-CRP] ≤ 3.2), minimal disease activity (MDA; MDA criteria), and moderate disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] score 4–7), respectively. Other endpoints evaluated the persistence to GLM treatment and its impact on patients’ work productivity (Work Productivity and Activity Impairment [WPAI] instrument) and quality of life (QoL; EuroQoL5 dimensions 3 levels [EQ-5D-3L] questionnaire). Descriptive statistics, the Wilcoxon signed-rank test, and Kaplan–Meier method were used for analyses. At 6 months, LDA was achieved by 46.4% of patients with RA, MDA by 57.1% of patients with PsA, and BASDAI 4–7 by 24.1% of patients with axSpA. For all study patients, persistence rates on GLM were high (85.1–93.7%) over 18 months; all WPAI domain scores and the EQ-5D-3L index score improved significantly (p < 0.001) from baseline to 18 months. GLM treatment was effective in patients with RA, PsA, or axSpA who had failed previous treatment with one TNFi and led to significant WPAI and QoL improvements. Persistence rates were high. Trial registration number and date of registration: As per the local regulations the study has been registered at the national registry for non-interventional studies https://www.dilon.sfee.gr/studiesp_d.php?meleti_id=MK8259-6995.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic inflammatory rheumatic diseases, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (axSpA), are frequently observed in clinical practice [1]. The main clinical manifestations of RA include pain in the joints and potential damage of bone and cartilage, as well as disability [2], PsA involves a diverse symptomatology and local inflammation pathways, including uveitis, dactylitis, osteitis, as well as skin and nail disease [3]. AxSpA is associated with chronic back pain and stiffness, primarily of the pelvis and the lower back, although any part of the spine can be involved [4]. These diseases are of predominant interest to rheumatologists due to their significant impact on the patient’s quality of life (QoL) and their substantial burden for patients and society [5].
Significant advances have been achieved in the clinical outcomes of these diseases, based mainly on evolved treatment strategies and the availability of multiple effective therapies. The current treatment strategy paradigm is the treat-to-target concept in RA and PsA, aiming at disease remission or low disease activity (LDA) [6, 7], and the maximization of QoL in axSpA [8]. Furthermore, several disease-modifying antirheumatic drugs (DMARDs) with diverse modes of action are available (conventional synthetic [cs], biologic [b], and targeted synthetic DMARDs), allowing for a tailored approach for a specific patient. The bDMARDs comprise, among other agents, the tumor necrosis factor α inhibitors (TNFis) etanercept, infliximab, adalimumab, certolizumab pegol, and golimumab.
The use of TNFis in the treatment of autoimmune disorders has improved clinical outcomes. However, treatment failure with TNFis is a common occurrence in RA [9], PsA [10], and axSpA [11]. In RA, reasons for discontinuation mainly include lack of efficacy, followed by physician preference, safety, patient preference, and no access to treatment [12], while in PsA and axSpA, TNFi discontinuation/switching includes primarily inefficacy and lack of tolerability [13, 14]. As a result, persistence with TNFi treatment is negatively impacted. In RA, the median time to TNFi discontinuation was ~ 26 months [12], and the discontinuation of TNFi treatment was 59.5% in patients with an inadequate response to a previous DMARD and 74.1% in patients who had failed a first bDMARD [15]. In PsA and axSpA the approximate 1-year discontinuation rates in were 30% [16] and 15% [17], respectively. In general, physicians manage TNFi failure in all three indications by switching either to an alternative TNFi or to another class of targeted agent with a different mode of action [6,7,8].
Golimumab is a human monoclonal antibody that prevents the binding of tumor necrosis factor α to its receptors [18]. In the European Union, golimumab has been approved, among other indications, for the treatment of moderate-to-severe active RA (in combination with methotrexate), active and progressive PsA (alone or in combination with methotrexate), and axSpA [18]. Golimumab’s safety and efficacy in RA, PsA, and axSpA have been demonstrated in several pivotal randomized clinical trials and their long-term extensions [19]. Golimumab has also been evaluated in these indications in several retrospective [20,21,22] and prospective [23,24,25] real-world studies, albeit that only two of these studies focused exclusively on patients who had failed treatment with one previous TNFi [20, 25]; thus, further real-world, prospective data, in patients with RA, PsA and axSpA who had failed one previous TNFi treatment would fill this gap in knowledge.
The present prospective study, GO-BEYOND, assessed primarily the effectiveness of golimumab in patients with RA, PsA, or axSpA who had failed one previous TNFi. Other study objectives included the patients’ persistence to golimumab treatment, work productivity and activity impairment, and healthcare resource utilization (HCRU).
Methods
Study design and patient population
GO-BEYOND was a prospective, observational, 18-month study conducted in 21 rheumatologic sites (private practices and hospitals) in Greece, constituting a wide selection of centers throughout Greece. Participant enrolment lasted from 30 March 2018 to 28 June 2019, and the last patient visit occurred in 28 December 2022. Visits were scheduled at enrolment (baseline) and post-baseline at approximately 3, 6, 12, and 18 months per routine clinical care.
Treatment initiation with golimumab and any treatment changes during the observation period fell entirely into the responsibility of the treating physicians and were based on the approved therapeutic protocols. Golimumab was prescribed per label [18]. Briefly, across RA, PsA, and axSpA, the recommended golimumab dose is 50 mg monthly (given concomitantly with methotrexate in RA) or 100 mg monthly for patients with body weight > 100 kg who do not achieve an adequate clinical response after three to four 50 mg doses.
Eligible patients were 18 years of age or older, diagnosed with active moderate-to-severe RA, active and progressive PsA, or severe and active axSpA, as based on the physicians’ assessment, who had failed previous treatment for their rheumatic disease with one initial TNFi, with or without methotrexate. Previous TNFi treatment failure was defined as loss of efficacy after ≥ 6 months of treatment (secondary failure) or intolerability or inconvenience after ≥ 3 months of treatment. The criteria for exclusion included: (i) previous treatment with non-TNFi bDMARDs or ≥ 1 TNFi; (ii) previous treatment with other biological therapeutics (including anakinra and abatacept); (iii) RA patients on bDMARD monotherapy who could not be switched to golimumab plus methotrexate or those with intolerance to methotrexate; (iv) any contraindication to the use of golimumab as per label or a clinically serious adverse reaction, opportunistic infection, or allergic reaction to the initial TNFi; and (v) history of lymphoproliferative disease, malignancy, or history of malignancy within the previous five years.
Study endpoint measures
The primary endpoint was assessed at 6 months after golimumab initiation for all three disease groups and regarded the proportions of patients achieving: (i) LDA and/or remission (Disease Activity Score for 28 joints based on the high-sensitivity C-reactive protein [DAS28-CRP] < 3.2) in RA [26]; (ii) minimal disease activity (MDA; defined by the achievement of five of seven MDA criteria) in PsA [27] and (iii) moderate disease activity (Bath Ankylosing Spondylitis Disease Activity Index [BASDAI] between 4 and 7 in AxSpA [28].
The major secondary endpoints were set per disease group. For the RA group, the endpoints included the proportion of patients achieving LDA and/or remission (DAS28-CRP < 3.2) at 3, 12, and 18 months, and the proportion of patients achieving remission (DAS28-CRP < 2.6) and good and moderate EULAR response [29] at each post-baseline visit. For the PsA group, the endpoints included the proportion of patients achieving MDA at 3, 12, and 18 months, and the proportion of patients achieving remission (DAS28-CRP < 2.6) and good and moderate EULAR response at each post-baseline visit. For the axSpA group, the endpoints included the proportions of patients achieving a BASDAI score of < 4, 4–7, and ≤ 7 at 3, 12, and 18 months; the proportions of patients achieving inactive disease defined as an Ankylosing Spondylitis Disease Activity Score [ASDAS] < 1.3) [30] and BASDAI 50 (defined as a 50% of improvement of the initial BASDAI) [31] at each post-baseline visit; and the description of the BASDAI, ASDAS, and the Assessment of SpondyloArthritis international Society (ASAS) Health index (HI) [32] scores at baseline and at each post-baseline visit.
Other secondary endpoints, assessed at each post-baseline visit, included persistence with golimumab treatment, the patients’ work productivity and activity impairment (using the Work Productivity and Activity Impairment [WPAI] score) [33] the improvement in QoL (using the EuroQoL 5 dimensions 3 levels, EQ-5D-3L (https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/), and the HCRU (using a patient diary).
Statistical analysis
A precision-based sample size calculation was performed for each cohort (RA, PsA, axSpA). Assuming a response rate of 30% at 6 months, a sample size of 102 RA, 57 PsA, and 57 axSpA patients would offer a maximum margin of error of ~ 10% in estimating the response rate per disease group (alpha = 10%). Additionally, assuming a drop-out rate of 10%, 113 RA, 63 PsA, and 63 axSpA patients were planned to be enrolled, i.e., a total of 239 patients. All pre-specified analyses were performed in the overall eligible population, i.e., every eligible patient who gave informed consent and initiated golimumab treatment for RA, PsA, or axSpA, with available data for each endpoint (i.e., using the as-observed data). Analyses were mainly descriptive. Categorical data, including categories of continuous data, are presented in frequency tables. Continuous data are presented using the median value and 25 (Q1) and 75 (Q3) percent quartiles. Continuous variables were described by visit. Changes in continuous variables from baseline to each subsequent visit were assessed in subgroups of patients with paired assessments (i.e., patients with assessments available at baseline and at the specific post-baseline timepoint). No formal hypothesis testing was performed; however, changes from baseline were also statistically assessed using the Wilcoxon signed-rank test to provide a more coherent evaluation of the results.
Patients’ compliance was defined as the proportion of full doses taken over the total doses planned during the follow-up period. Persistence was defined as the duration of time from initiation to discontinuation of therapy. Persistence rates (i.e., patients remaining on golimumab treatment) at the post-baseline visits were estimated using the Kaplan–Meier method. All analyses were performed using SAS® version 9.4.
Results
Patient disposition and baseline characteristics
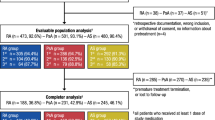
A total of 243 patients were enrolled, of whom one patient was excluded from the baseline analysis as he/she met the exclusion criterion of having received more than one previous TNFi. Of the remaining 242 patients, 117 (48.3%) had RA, 63 (26.0%) had PsA, and 62 (25.6%) had axSpA (Table 1). Compared to the PsA and axSpA groups, the RA group was older (ages, median: 52.0, 47.5, 61.0 years, respectively) and had a higher proportion of female patients (55.6%, 56.5%, 88.0%, respectively). The patients with extra-articular manifestations per disease group are shown in Supplementary Table 1. The disease duration was similar between the groups. All patients presented with active disease; the median (Q1–Q3) DAS28-CRP for the RA and PsA groups was 4.8 (4.5–5.3) and 4.7 (4.3–5.1), respectively, and the median (Q1–Q3) BASDAI score for the axSpA group was 6.2 (4.7–6.9). Before study entry, most patients with RA had received etanercept (47, 40.2%), and most patients with PsA had received adalimumab (23, 36.5%); almost equal numbers of patients with axSpA had received each of certolizumab pegol, etanercept, adalimumab, and infliximab. The median treatment period with previous TNFis ranged from 1.1 (RA) to 1.3 (axSpA) years. Almost all patients with RA and PsA had previously received methotrexate (96.6% and 88.9%, respectively), while the proportion of patients with axSpA who had received methotrexate was lower (32.3%).
Evolution of disease activity measurements during the observation period
Of the 242 patients included in the analysis, 233 (96.3%), 216 (89.3%), 203 (83.9%), and 190 (78.5%) patients attended the 3-, 6-, 12- and 18-month visits, respectively. Fifty-two (21.5%) patients withdrew prematurely during the observation period, most frequently due to patient lost to follow-up (26/52 patients, 50.0%) and adverse events (17/52 patients, 32.7%).
Patients with RA
The median (Q1–Q3) DAS28-CRP values showed a continuous improvement (decrease) from baseline through 18 months (4.8 [4.5–5.3]) to 2.6 [1.7–3.1], respectively), with similar improvements being observed for the corresponding CRP and ESR measures (Supplementary Table 2). The difference in the DAS28-CRP, CRP, and ESR values between the baseline and each visit was significant (p < 0.001 for all comparisons; Supplementary Table 2). For the primary objective, the proportion of patients achieving LDA and/or remission (DAS28-CRP < 3.2) at 6 months was 46.4% (45/97) (Table 2); the respective proportions at three, 12, and 18 months were 32.4% (35/108), 54.9% (50/91), and 77.6% (59/76). The proportions of patients achieving remission (DAS28-CRP < 2.6) at three, six, 12, and 18 months were 14.8% (16/108), 35.1% (34/97), 42.9% (39/91), and 48.7% (37/76), respectively, and the combined proportions of patients with good and moderate EULAR response were 65.1% (69/106), 81.7% (76/93), 93.1% (81/87), and 95.8% (69/72), respectively (Table 2).
Patients with PsA
The median (Q1–Q3) DAS28-CRP values showed a continuous improvement (decrease) from baseline through 18 months (4.7 [4.3–5.1]) to 2.3 [1.6–2.9], respectively), as did the median (Q1–Q3) BSA (8.0 [3.0–10.0] to 0.0 [0.0–1.0]) (Supplementary Table 2); improvements in these measures were observed even after 3 months of treatment. Similar improvements from baseline to 18 months were observed for CRP and ESR (Supplementary Table 2). The difference in the DAS28-CRP, BSA, ESR, and CRP values between the baseline and each visit was significant (p < 0.001 for all comparisons; Supplementary Table 2). For the primary objective, the proportion of patients achieving MDA at 6 months was 57.1% (32/56; Table 2); the respective proportions at 3, 12, and 18 months were 29.3% (17/58), 68.0% (34/50), and 86.0% (43/50). The proportion of patients achieving remission (DAS28-CRP < 2.6) at 3, 6, 12, and 18 months was 22.6% (12/53), 64.6% (31/48), 66.7% (28/42), and 56.7% (17/30), respectively, and the combined proportions of patients with good and moderate EULAR response were 80.4% (41/51), 93.5% (43/46), 100.0% (41/41), and 100.0% (29/29), respectively (Table 2).
Patients with axSpA
The median (Q1–Q3) BASDAI values improved from baseline through 18 months (6.2 [4.7–6.9]) to 1.0 [0.6–2.5], respectively), as did the median (Q1–Q3) values for the ASDAS (3.5 [2.8–4.0] to 1.4 [1.0–1.7], respectively) and ASAS HI (12.8 [11.0–15.9] to 5.0 [3.2–8.5], respectively) scores (Supplementary Table 2); improvements in all three disease activity measures were observed already after 3 months of treatment. The difference in all the above measure values between the baseline and each post-baseline visit was significant (p < 0.001 for all comparisons; Supplementary Table 2). For the primary endpoint, the proportion of patients with BASDAI score 4–7 at 6 months was 24.1% (13/54; Table 2). The proportions of patients with BASDAI < 4 continuously increased from 3 to 18 months (50.0% [28/56] to 90.2% [46/51], respectively; Table 2). The proportions of patients achieving BASDAI 50 at 3, 6, 12, and 18 months were 39.3% (22/56), 61.1% (33/54), 76.5% (39/51), and 83.0% (39/47), respectively (Table 2).
Persistence with golimumab treatment
For the entire study population, a median (Q1–Q3) of 19.0 (18.0–19.0) injections per patient were administered during the observation period; six (2.5%) patients missed ≥ 1 golimumab dose. The median (Q1–Q3) compliance rate was 100.0% (100.0–100.0). For all patients, the persistence rates (95% CI) at 3, 6, 12, and 18 months were 93.7% (89.9–96.2), 89.0% (84.3–92.4), 85.1% (79.9–89.1), and 85.1% (79.9–89.1), respectively (Fig. 1). Treatment persistence rates were similar among the three disease groups (data not shown).
Assessment of work productivity and activity impairment and quality of life
For the entire study population, all median (Q1–Q3) WPAI domain scores continuously decreased (improved) from baseline through 18 months; the change in all domain scores from baseline to each visit was significant (p < 0.001 for all comparisons; Fig. 2A–D). Similarly, the median (Q1–Q3) EQ-5D-3L UK index score continuously increased (improved) from baseline through 18 months, and the changes from baseline to each visit were significant (p < 0.001 for all comparisons; Fig. 3). The above-mentioned changes in the WPAI domain scores and the EQ-5D-3L UK index score between baseline and each post-baseline visit were also observed at the disease group level (p < 0.001 for all comparisons; data not shown).
Evolution of the WPAI domain scores for all patients during 18 months of treatment with golimumab, evaluating absenteeism (A), presenteeism (B), work productivity loss (C) and activity impairment (D). Data are patients (n) or median (Q1–Q3) change from baseline. For the post-baseline visits, data for patients with paired assessments (i.e., data available both at baseline and at the respective visit) are shown. The changes in values from baseline to each post-baseline visit were assessed with the Wilcoxon signed-rank test. Q1 first quartile, Q3 third quartile, WPAI work productivity and activity impairment
Evolution of the EQ-5D-3L UK index score for all patients during 18 months of treatment with golimumab. Data are patients (n) or median (Q1–Q3) change from baseline. For the post-baseline visits data for patients with paired assessments (i.e., data available both at baseline and at the respective visit) are shown. The changes in values from baseline to each post-baseline visit were assessed with the Wilcoxon signed-rank test. EQ-5D-3L EuroQol 5 dimensions 3 levels, Q1 first quartile, Q3 third quartiles
Disease-related healthcare resource utilization
During the 6 months before baseline, all patients (100.0%) had laboratory tests (Table 3); the total number of laboratory tests performed was 1071. During the same period, specialist consultations, hospitalizations, biopsies and/or imaging tests, and physiotherapies occurred in 34.3%, 9.5%, 24.8%, and 4.5% of all patients, respectively.
At each post-baseline visit, HCRU was mainly driven by laboratory tests and specialist consultations, with approximately 90.0% of patients reporting laboratory tests and approximately 20.0% reporting visits to specialists (Table 3). The HCRU remained stable and at the expected (as per clinical practice) level during the 18 months of observation. Notably, no hospitalizations were recorded during this time.
Discussion
Patients with RA, PsA, or axSpA and an inadequate response or intolerance to previous treatment with TNFi (or other bDMARDs) are routinely encountered in everyday clinical practice [12, 13, 20]; the management of these patients is challenging in terms of achieving and maintaining clinical response.
The present, real-world, prospective study focused exclusively on patients with RA, PsA, or axSpA who had previously failed treatment with one TNFi and primarily assessed the efficacy of second-line golimumab. Our study showed that by 6 months, the time of the primary endpoint assessment, 46.4% of patients with RA attained LDA and/or remission (DAS28-CRP < 3.2), 57.1% of patients with PsA attained MDA, and 24.1% of patients with axSpA achieved a BASDAI score of 4–7. Furthermore, by 18 months, which was the end of our observational period, 77.6% of patients with RA achieved LDA and/or remission, 86.7% of patients with PsA achieved MDA, and 91.5% of patients with axSpA achieved BASDAI < 4. Taken together, these outcomes indicate that golimumab is a reasonable second-line TNFi option for the treat-to-target strategy for these patients.
Although not directly comparable, our findings are generally aligned with those from previous real-world studies assessing the efficacy of golimumab in patients with autoimmune rheumatoid disorders. According to current knowledge, only two real-world prospective studies, the German GO-NICE [1] and the Italian GO-BEYOND [25] studies, assessed the proportion of patients with RA who achieved LDA and/or remission with golimumab at 6 months. The proportion of patients achieving LDA and/or remission was similar between our study and GO-NICE (46.4% and 49.2%, respectively), although the latter included both biological-experienced and biological-naïve patients. In contrast, the Italian GO-BEYOND study, which included only patients with an inadequate response to the first TNFi as our study, reported a higher proportion (68.0%) of patients achieving LDA and/or remission. The available data cannot explain this difference in LDA and/or remission rates between the current and the Italian GO-BEYOND studies, especially given that the RA populations in both studies had moderate disease (median DAS28-CRP 4.8 and 4.1, respectively); furthermore, the disease duration in the current study was numerically lower than that of the patients included in the Italian GO-BEYOND study (3.6 and 11 years, respectively), and it has been established that shorter RA duration is associated with better clinical outcomes with bDMARDs [34].
Regarding the patients with PsA, the rate of patients achieving MDA at 6 months was similar with the rate reported for golimumab-treated patients in a real-world, retrospective study conducted in Canada (57.1% and 53.9%, respectively) [35]; it is noted, however, that the latter study included both bDMARD-naïve patients and patients previously treated with one bDMARD. Finally, regarding the patients with axSpA, the current study showed that the median BASDAI score was reduced from 6.2 at baseline to ≤ 2.2 at 6 months and was sustained thereafter. A post hoc analysis of the previously-mentioned GO-NICE study [23] found that golimumab as a second-line biologic agent in patients with AS significantly reduced the mean BASDAI from 4.9 at baseline to 3.3, at 6 months.
In this study, high persistence rates with golimumab treatment (range 85.1%–93.7%) were observed over 18 months of observation, despite patients having already been treated with a previous TNFi. High 2-year persistence rates with treatment golimumab treatment were also observed in a recent real-world retrospective study conducted in Italy in patients with autoimmune rheumatoid disorders who had previously failed treatment with one TNFi (RA: 61.4%; PsA: 72.5%; and spondyloarthritis: 80.0%) [21]. Similarly, high 1-year probability of persistence rates (80%) were reported in a recent real-world retrospective study conducted in Spain in patients with PsA and axSpA who discontinued treatment with an initial TNFi [20]. The high persistence rates to treatment with golimumab in patients who have failed one previous TNFi is an important finding of this and previous real-world studies that could inform rheumatologists when choosing between agents for such patients.
For all patients, components of the WPAI score (absenteeism, presenteeism, work productivity and activity impairment) and the EQ-5D-3L UK index scores were significantly reduced from baseline to 3, 6, 12, and 18 months (p < 0.001, all comparisons). Previously, the GO-ART [36] and the GO-NICE [37] studies reported that golimumab treatment in patients with RA, PsA, and AS improved all WPAI domain scores within 3 months and resulted in substantial improvements in the EQ-5D-3L domain scores within 6 months.
Lastly, HCRU measures overall decreased during the observation period compared to the 6 months before study entry. This decrease was noteworthy for hospitalizations, with no reported hospitalizations during the observation period.
Limitations should be considered when interpreting the study findings. Patient selection bias cannot be ruled out, as the choice of treatment with golimumab was based on the investigator's judgment. As the study was non-comparative, outcomes cannot be directly compared to other TNFi or DMARDs. The follow-up period of 18 months may limit the extrapolation of the results to the longer-term, given that RA, PsA, and axSpA are chronic conditions. The possibility of patient recall bias needs to be considered for patient-reported outcomes (e.g., QoL, WPAI) and HCRU. However, every effort was made to mitigate patient recall bias with the use of patient-reported outcomes with a short recall period and validated in the Greek language; also, a patient diary was used for prospectively recording HCRU data. The statistical accuracy in estimating study outcomes at each time point may have decreased due to patients lost to follow-up. Additionally, the study was not designed to test any formal hypotheses, and p-values were only presented to allow a more comprehensive evaluation of the findings; no adjustment for multiple comparisons was made.
Conclusions
This real-world, prospective study in patients with RA, PsA, and axSpA and a previous TNFi failure, showed that golimumab treatment over 18 months was efficacious in these challenging patients. This finding combined with the high persistence to golimumab treatment and the substantial improvements in work productivity, activity impairment, and QoL support the use of golimumab as a second line treatment option in these diseases.
Data availability
Data are available from the corresponding author upon reasonable request. There are circumstances that may prevent MSD Greece from sharing the requested data.
Code availability
All data were analyzed using SAS v9.4 (SAS Institute, Cary, NC).
References
Krüger K, Burmester GR, Wassenberg S, Bohl-Bühler M, Thomas MH (2018) Effectiveness and safety of golimumab in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis under real-life clinical conditions: non-interventional GO-NICE study in Germany. BMJ Open 8(6):e021082. https://doi.org/10.1136/bmjopen-2017-021082
Smolen JS, Aletaha D, McInnes IB (2016) Rheumatoid arthritis. Lancet 388(10055):2023–2038. https://doi.org/10.1016/S0140-6736(16)30173-8
Veale DJ, Fearon U (2018) The pathogenesis of psoriatic arthritis. Lancet 391(10136):2273–2284. https://doi.org/10.1016/S0140-6736(18)30830-4
Sieper J, Poddubnyy D (2017) Axial spondyloarthritis. Lancet 390(10089):73–84. https://doi.org/10.1016/S0140-6736(16)31591-4
Michelsen B, Fiane R, Diamantopoulos AP et al (2015) A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS One 10(4): e0123582. https://doi.org/10.1371/journal.pone.0123582
Smolen JS, Landewé RBM, Bijlsma JWJ et al (2020) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 79(6):685–699. https://doi.org/10.1136/annrheumdis-2019-216655
Gossec L, Baraliakos X, Kerschbaumer A et al (2020) EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 79(6):700–712. https://doi.org/10.1136/annrheumdis-2020-217159
van der Heijde D, Ramiro S, Landewé R et al (2017) 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 76(6):978–991. https://doi.org/10.1136/annrheumdis-2016-210770
Rubbert-Roth A, Szabó MZ, Kedves M, Nagy G, Atzeni F, Sarzi-Puttini P (2019) Failure of anti-TNF treatment in patients with rheumatoid arthritis: The pros and cons of the early use of alternative biological agents. Autoimmun Rev 18(12):102398. https://doi.org/10.1016/j.autrev.2019.102398
Alten R, Conaghan PG, Strand V et al (2019) Unmet needs in psoriatic arthritis patients receiving immunomodulatory therapy: results from a large multinational real-world study. Clin Rheumatol 38(6):1615–1626. https://doi.org/10.1007/s10067-019-04446-z
Juanola X, Ramos MJM, Belzunegui JM, Fernández-Carballido C, Gratacós J (2022) Treatment failure in axial spondyloarthritis: insights for a standardized definition. Adv Ther 39(4):1490–1501. https://doi.org/10.1007/s12325-022-02064-x
Strand V, Miller P, Williams SA, Saunders K, Grant S, Kremer J (2017) Discontinuation of biologic therapy in rheumatoid arthritis: analysis from the corrona RA registry. Rheumatol Ther 4(2):489–502. https://doi.org/10.1007/s40744-017-0078-y
Stober C, Ye W, Guruparan T, Htut E, Clunie G, Jadon D (2018) Prevalence and predictors of tumour necrosis factor inhibitor persistence in psoriatic arthritis. Rheumatology (Oxford) 57(1):158–163. https://doi.org/10.1093/rheumatology/kex387
Manica SR, Sepriano A, Pimentel-Santos F et al (2020) Effectiveness of switching between TNF inhibitors in patients with axial spondyloarthritis: is the reason to switch relevant? Arthritis Res Ther 22(1):195. https://doi.org/10.1186/s13075-020-02288-8
Choquette D, Bessette L, Alemao E et al (2019) Persistence rates of abatacept and TNF inhibitors used as first or second biologic DMARDs in the treatment of rheumatoid arthritis: 9 years of experience from the Rhumadata® clinical database and registry. Arthritis Res Ther 21(1):138. https://doi.org/10.1186/s13075-019-1917-8
Gossec L, Siebert S, Bergmans P et al (2021) Persistence and effectiveness of the IL-12/23 pathway inhibitor ustekinumab or tumour necrosis factor inhibitor treatment in patients with psoriatic arthritis: 1-year results from the real-world PsABio Study. Ann Rheum Dis 81(6):823–830. https://doi.org/10.1136/annrheumdis-2021-221640
Bekele DI, Cheng E, Reimold A et al (2022) Tumor necrosis factor inhibitor (TNFi) persistence and reasons for discontinuation in a predominantly male cohort with axial spondyloarthritis. Rheumatol Int 42(11):1925–1937. https://doi.org/10.1007/s00296-021-05024-w
European Medicines Agency. Simponi Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/simponi-epar-product-information_en.pdf. Accessed 06 June 2023
Flipo RM, Tubach F, Goupille P et al (2021) Real-life persistence of golimumab in patients with chronic inflammatory rheumatic diseases: results of the 2-year observational GO-PRACTICE study. Clin Exp Rheumatol 39(3):537–545. https://doi.org/10.55563/clinexprheumatol/zizo0l
Alegre-Sancho JJ, Juanola X, Rodríguez-Heredia JM et al (2021) Effectiveness and persistence of golimumab as a second biological drug in patients with spondyloarthritis: a retrospective study. Medicine (Baltimore) 100(13):e25223. https://doi.org/10.1097/MD.0000000000025223
Iannone F, Favalli EG, Caporali R et al (2021) Golimumab effectiveness in biologic inadequate responding patients with rheumatoid arthritis, psoriatic arthritis and spondyloarthritis in real-life from the Italian registry GISEA. Joint Bone Spine 88(1):105062. https://doi.org/10.1016/j.jbspin.2020.07.011
Akar S, Kalyoncu U, Dalkilic E et al (2021) GO-BEYOND: a real-world study of persistence of golimumab in patients with axial spondyloarthritis and rheumatoid arthritis in Turkey. Immunotherapy 13(10):841–850. https://doi.org/10.2217/imt-2020-0296
Krüger K, Burmester GR, Wassenberg S, Thomas MH (2020) Golimumab as the first-, second-, or at least third-line biologic agent in patients with rheumatoid arthritis, psoriatic arthritis, or ankylosing spondylitis: post hoc analysis of a noninterventional study in Germany. Rheumatol Ther 7(2):371–382. https://doi.org/10.1007/s40744-020-00204-9
Michelsen B, Sexton J, Wierød A, Bakland G, Rødevand E, Krøll F, Kvien TK (2020) Four-year follow-up of inflammatory arthropathy patients treated with golimumab: Data from the observational multicentre NOR-DMARD study. Semin Arthritis Rheum 50(1):12–16. https://doi.org/10.1016/j.semarthrit.2019.07.003
D'Angelo S, Tirri E, Giardino AM et al (2022) Effectiveness of Golimumab as Second Anti-TNFα Drug in Patients with Rheumatoid Arthritis, Psoriatic Arthritis and Axial Spondyloarthritis in Italy: GO-BEYOND, a Prospective Real-World Observational Study. J Clin Med 11(14):4178. https://doi.org/10.3390/jcm11144178
Anderson J, Caplan L, Yazdany J et al (2012) Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken) 64(5):640–647. https://doi.org/10.1002/acr.21649
Mease PJ, Coates LC (2018) Considerations for the definition of remission criteria in psoriatic arthritis. Semin Arthritis Rheum 47(6):786–796. https://doi.org/10.1016/j.semarthrit.2017.10.021
Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A (1994) A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 21(12):2286–2291
Fransen J, van Riel PL (2005) The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 23(5 Suppl 39):S93–S99
Machado P, Landewé R, Lie E et al (2011) Assessment of SpondyloArthritis international Society. Ankylosing Spondylitis Disease Activity Score (ASDAS): defining cut-off values for disease activity states and improvement scores. Ann Rheum Dis 70(1):47–53. https://doi.org/10.1136/ard.2010.138594
Rudwaleit M, Listing J, Brandt J et al (2004) Prediction of a major clinical response (BASDAI 50) to tumour necrosis factor α blockers in ankylosing spondylitis. Ann Rheum Dis 63:665–670. https://doi.org/10.1136/ard.2003.016386
Kiltz U, van der Heiijde D, Boonen A et al (2015) Development of a health index in patients with ankylosing spondylitis (ASAS HI): final result of a global initiative based on the ICF guided by ASAS. Ann Rheum Dis 74(5):830–835. https://doi.org/10.1136/annrheumdis-2013-203967
Reilly MC, Zbrozek AS, Dukes EM (1993) The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics 4(5):353–365. https://doi.org/10.2165/00019053-199304050-00006
Smolen JS, Aletaha D (2015) Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 11(5):276–289. https://doi.org/10.1038/nrrheum.2015.8
Rahman P, Zummer M, Bessette L, et al (2017) Real-world validation of the minimal disease activity index in psoriatic arthritis: an analysis from a prospective, observational, biological treatment registry. BMJ Open 7(8):e016619. https://doi.org/10.1136/bmjopen-2017-016619
Krueger K, Remstedt S, Thiele A, Hohenberger S (2020) Golimumab improves patient-reported outcomes in daily practice of inflammatory rheumatic diseases in Germany. J Comp Eff Res 9(12):891–902. https://doi.org/10.2217/cer-2020-0092
Krüger K, Burmester GR, Wassenberg S, Bohl-Bühler M, Thomas MH (2019) Patient-reported outcomes with golimumab in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: non-interventional study GO-NICE in Germany. Rheumatol Int 39(1):131–140. https://doi.org/10.1007/s00296-018-4180-4
Acknowledgements
The authors wish to thank Dimitrios Zisopoulos, Michail P. Migkos, Ilias Bournazos, Eleni Kteniadaki for their assistance and feedback.
Funding
This study was sponsored by Merck Sharp & Dohme (MSD) Greece. The sponsor did not have any involvement at all stage of the research and submission, except providing funding.
Author information
Authors and Affiliations
Contributions
Substantial contribution to the conception or design of the work; Drafting the work or revising it critically for important intellectual content: EP, DB; acquisition and interpretation of data and writing: PA, DP, AG, GK, AT, SG, PS, MT, AB, AK, PV, GS, DV; analysis of data: ZH. All authors were involved in drafting the article or revising it critically for important intellectual content, approved final version of manuscript to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All co-authors take full responsibility for the integrity and accuracy of all aspects of all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
Dr Prodromos Sidiropoulos has received support for the present manuscript from MSD, in addition to research grants from Pfizer, Genesis, UCB, GSK, MSD, Abbvie, Novartis, Roche, Eli Lilly and Amgen. He has also received consultation payments from Pfizer, Abbvie and Eli Lilly, and honoraria from Pfizer, UCB, Abbvie, Novartis and Eli Lilly. He has received support for attending a Data Safety Monitoring Board or Advisory Board from Pfizer, Abbvie, Novartis and Eli Lilly. Dr Dimitrios Vassilopoulos has received support for the present manuscript from MSD. Dr Periklis Vounotrypidis has received support for the present manuscript from MSD, in addition to research grants from Abbvie, Genesis pharma and Novartis. Dr Andreas Bounas has received support for the present manuscript from MSD, in addition to grants or contracts from Abbvie, Amgen, Genesis, MSD, Novartis and Pfizer. He has also received honoraria from Abbvie, Aeonorasis, Amgen, Bausch Health, Faran, Genesis, GSK, Janssen, MSD, Novartis, Pfizer and UCB, as well as for participation on a Data Safety Monitoring Board or Advisory Board from Abbvie, Aeonorasis, Amgen, Bausch Health, Faran, Genesis, GSK, Janssen, MSD, Novartis, Pfizer and UCB. Dr Anna Kandyli has received consultation payments from Abbvie, Mylan and Genesis, as well as honoraria from Abbvie and Novartis. She has received support for attending meetings and/or travel from UCB, and for participating on a Data Safety Monitoring Board or Advisory Board from Genesis and Amgen. Dr Gkikas Katsifis has received honoraria from Abbvie, Aenorasis, Amgen, Janssen, Jenessis, Lilly, MSD, Novartis, Sobi, Roche, Pfizer and UCB. Also, the author has received support for attending meetings from AbbVie, Sandoz, Roche, UCB, and Lilly, and for his participation on a Data Safety Monitoring Board or Advisory Board from ELPEN. Dr Grigorios Sakellariou has received support for the present manuscript from MSD, as well as research grants for education activities from Pfizer, Genesis, UCB, GSK, and MSD, and support for attending meetings and/or travel from Abbvie and MSD. Dr Maria Tektonidou has received support for the present manuscript from MSD, as well as research grants from Genesis, UCB, GSK, MSD, and Amgen, and consultation payments from Genesis, GSK, Novartis and EI Lilly. Zhiping Huang is an employee of Merck & Co., Inc., Rahway, NJ, USA, and Evangelia Petrikkou is an employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, who owns stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA. Drs Panagiotis Athanassiou, Dimitrios Psaltis, Athanasios Georgiadis, Athina Theodoridou, Souzana Gazi, and Dimitrios Bounas have declared no conflicts of interest.
Ethical approval
The study was conducted in accordance with the EU Directive 2001/20/EC section for non-interventional studies and the applicable laws and regulations of Greece. All included patients provided written informed consent before study entry. The present study was performed in accordance with the Helsinki declaration of 1964, and its later amendments. The study was approved by the competent Institutional Review Boards (IRBs) of all participating hospital sites. Participation of private practice investigators was approved by the IRB of a participating hospital located in the same geographic region as the Private Practice. Specifically, the IRBs that reviewed and approved the study, and corresponding ethical review reference numbers include IRB of “Laiko” General Hospital (reference number: 6046/24-Apr-2018), IRB of “KAT” Regional General Hospital (reference number: 188/14-Jun-2018), IRB of Private Practice under “Laiko” General Hospital (reference number: 59/15-Feb-2018), IRB of University General Hospital of Heraklion, Crete (reference number: 17991/22-Nov-2018), IRB of “Olympion”Rehabilitation Center of Patras (reference number: 14-Mar-2018), IRB of Private Practice under University General Hospital of Ioannina (reference number: 5th/08-Apr-2018), IRB of Private Practice under European Interbalkan Medical Center (reference number 17-Jan-2018), IRB of Private Practice under European Interbalkan Medical Center (reference number 22-Jan-2019), IRB of “Attikon” University General Hospital (reference number 5th/29-May-2018), IRB of “Ag. Pavlos” of Thessaloniki (reference number: 123/08-Mar-2018), IRB of “Ippokrateio”General Hospital of Athens (reference number: 4552/19-Mar-2018), IRB of Naval Hospital of Athens (reference number: 3/18/02-Apr-2018).
Consent to participate
All persons gave their informed consent prior to their inclusion in the study.
Consent to publish
All participants provided consent for publication of the material collected in the context of this study in a non-identifiable manner.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dimitrios T. Boumpas: Lead Author.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Athanassiou, P., Psaltis, D., Georgiadis, A. et al. Real-world effectiveness of golimumab in adult patients with rheumatoid arthritis, psoriatic arthritis, and axial spondyloarthritis and an inadequate response to initial TNFi therapy in Greece: the GO-BEYOND prospective, observational study. Rheumatol Int 43, 1871–1883 (2023). https://doi.org/10.1007/s00296-023-05376-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-023-05376-5