Abstract
The objective of this study is to investigate the validity and reliability of the Turkish version of the Arthritis Impact Measurement Scale 2-Short Form (AIMS2-SF). Subjects fulfilling the ACR 2010 classification criteria for RA were enrolled into the study. Scale reliability was investigated using test–retest reliability (intra-class correlation coefficient—ICC) and internal consistency approaches (Cronbach’s α). Spearman’s rank correlation coefficients evaluated relationships between quantitative parameters and validity. Construct validity was assessed by correlating AIMS2-SF with clinical parameters and functional parameters including, Nottingham Health Profile (NHP), Health Assessment Questionnaire (HAQ), Beck Depression Inventory (BDI) and Duruöz Hand Index (DHI). One hundred and sixteen patients (105 females and 11 males) were recruited. The mean age ± standard deviation (SD) was 52.45 ± 11.48 years. Cronbach’s α was 0.88 and the ICC was 0.91. There were significant correlations (rho and p values) with parameters directly related to health-related quality of life (HRQoL); NHP subscales (energy levels: 0.54, pain: 0.62, emotional reaction: 0.50, sleep 0.44, social interaction: 0.51, physical activity: 0.61; p < 0.0005), HAQ (0.60, p < 0.0005), BDI (0.63, p < 0.001) and DHI (0.63, p < 0.0005). Poor or non-significant correlations were found for parameters not directly related to QoL, such as age (0.07, p = 0.45) and disease duration (0.12, p = 0.21); however, disease activity (0.43, p < 0.0005) and NRS pain (0.46, p < 0.0005) were correlated with AIMS2-SF as moderate. The Turkish AIMS2-SF version is a reliable and valid tool that may be used to evaluate QoL for RA. The scale can be easily used in daily practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory and lifelong destructive disease, with a considerable impact on the activities of daily living and quality of life (QoL) [1]. Clinical manifestations of RA vary from mild symptoms to severe disease, with joint deformities and extra-articular manifestations [2]. Even though innovative RA treatments have led to improved clinical results, RA is still associated with functional disability and poor QoL, when compared with the general population. Currently, RA treatment strategies are based on treat-to-target approaches that identify the best treatment methods, with a tight monitoring of disease activity [3]. Treating to target may vary depending on the treatment goals [4] Clinical remission is the primary treatment target, which ultimately contributes to the maximization of long-term health-related quality of life (HRQoL) [5] which is recommended as a primary treatment goal in RA patients. A HRQoL focuses on the functioning and well-being of physical, mental, and social domains related to health [6]. HRQoL measurements are increasingly required to evaluate self-perceived health issues, to assess the effectiveness of various treatment methods in clinical research and routine practice. Disease-specific measurements are more responsive in detecting small changes, when compared to generic QoL measurements, since they focus on symptoms and impairments in daily-life activities, and social or occupational functioning related to a specific disease [7]. Therefore, practical, trusted and validated disease-specific HRQoL RA measurements are essential in making treatment decisions and assessing their efficacy.
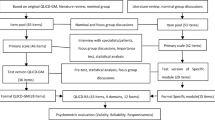
The Arthritis Impact Measurement Scale (AIMS) and its revised version (AIMS2) are commonly used as patient-reported instruments in assessing RA patient HRQoL [8, 9]. These instruments were developed in English, then translated and validated for several populations. However, completing an AIMS2 scale is a long process; it is difficult and time-consuming during routine practice, and reduces patient participation in researches [10, 11]. Therefore, Guillemin et al. developed and validated a short version of AIMS2 (AIMS2-SF) to make the instruments more practical for patients and researchers [12]. While the AIMS2 was validated in a Turkish population some years ago [13], the AIMS2-SF has not been similarly validated.
Therefore, we investigated the validity and reliability of the Turkish version of AIMS2-SF in RA patients.
Materials and methods
Participants
Patients diagnosed with RA, according to the American College of Rheumatology 2010 classification criteria [14], and aged 18–70 years, were included. This study has been approved by the ethical committee of Marmara University School of Medicine on April 2015 (approval number: 09.2015.102). All recruited patients were fully informed about the study and signed a consent form. The study was conducted in accordance with the principles of the Declaration of Helsinki. Those patients with insufficient cognitive understanding and cooperation, who were pregnant, had fibromyalgia, uncontrolled psychiatric conditions, and a history of surgical musculoskeletal interventions were excluded. Following socio-demographic and clinical data collection including age, gender, education level, disease duration, erythrocyte sedimentation rate, Disease Activity Score-28 (DAS-28), Numeric Rating cale (NRS) pain score, patients were asked to complete the following: AIMS2-SF, Health Assessment Questionnaire (HAQ), the Nottingham Health Profile (NHP), Beck Depression Inventory (BDI) and Duruöz Hand Index (DHI).
The Arthritis Impact Measurement Scale 2-Short Form (AIMS2-SF)
The AIMS2-SF is a 26-item short form of AIMS2, and covers five HRQoL components: physical (12 items), symptom (three items), affect (five items), social interaction (four items) and role (two items) [12]. Each item is evaluated on a 5-point Likert scale (0–4). Once tallied, the total scores of each subgroup are normalized from 0 (perfect health status) to 10 (worst health status). The 26 items of the AIMS2-SF were derived from 57 items of the Turkish AIMS2 which has been found to be valid and reliable with the procedures consisted of forward–backward translation method, cultural adaptation and the analysis of validity and reliability in Turkish population [13].
The Health Assessment Questionnaire (HAQ)
The HAQ is a self-reported outcome instrument, developed for RA patients to evaluate their functional status. It is a 20-item questionnaire consisting of eight sections: dressing, rising, eating, walking, hygiene, reach, grip, and usual activities. Scoring within each section is from 0 to 3 (0 = without any difficulty, 1 = with some difficulty, 2 = with much difficulty and 3 = unable to do). Total scores are calculated by summing the worst scores within each section and dividing this sum by eight. The score range is 0–3. The questionnaires reliability and validity have been studied in a Turkish population [15].
The Nottingham Health Profile (NHP)
The NHP is a generic HRQoL instrument with six health domains: physical mobility (eight items), pain (eight items), sleep (eight items), social isolation (five items), energy level (three items) and emotional reactions (nine items) [16]. It is a practical, comprehensive and commonly used instrument in clinical trials and routine practice. Answers to items are yes or no, and all items have specific weighted values. The total score for each domain is 0–100, with higher score signifying a worse HRQoL. This instrument has been validated in a Turkish population [17].
The Beck Depression Inventory (BDI)
The BDI measures the risk of depression and the severity of depressive symptoms in patients. It consists of 21 questions, and each item is scored 0–3. Total scores range from 0 to 63. A 0–9 score indicates minimal depression, 10–16 mild depression, 17–29 moderate depression, and 30–63 severe depression. A BDI validity and reliability study was performed by Hisli et al. in a Turkish population [18].
The Duruöz Hand Index (DHI)
The DHI assesses functional disability and functional handicap by assessing limitations in both hands, during daily living activities in RA patients [19]. The index consists of 18 daily-activity questions, and three factor groups involving the hands which evaluate hand functional disability. The DHI was validated for other hand arthropathies [20, 21]. Each question is scored 0–5, i.e., without difficulty to impossible, respectively. Total DHI scores range from 0 to 90, and patients complete it in 3–4 min. A high score is indicative of higher disability levels.
Statistical analysis
SPSS® version 22 (IBM Corp., Armonk, NY, USA) was used for statistical analyses. The data were analyzed using descriptive statistical methods (mean, standard deviation and frequencies).
AIM2-SF reliability was evaluated by internal consistency (Cronbach’s α coefficient) where α > 0.70 was considered acceptable. Floor and ceiling effects were calculated when evaluating the content validity and considered present when more than 15% of the patients achieved the highest or lowest possible score, respectively. The AIMS2-SF was managed two times to all patients in two weeks of intervals to assess the test-retest reliability.
To evaluate the construct properties of AIMS2-SF, convergent and discriminant validity were conducted. Convergent validity measures whether items that are expected to be related to AIMS2-SF are in fact related, while discriminant validity tests whether believed unrelated constructs are in fact unrelated. These measures were estimated by calculating the Spearman’s rank-order correlation coefficient. A p value of < 0.05 was considered statistically significant.
Results
In total, 116 RA patients (105 females, 11 males) were recruited. The average age and illness duration was 52.45 years (SD ± 11.48) and 94.07 months(SD ± 81.53), respectively. Patient demographic and clinical characteristics are outlined (Table 1). The floor and ceiling effects of the Turkish AIMS2-SF version were both 0. The mean time to fill in the form was five minutes. At the time of enrollment, 52 (44.8%) participants were working.
Reliability
The internal consistency (Cronbach’s α) of the AIMS2-SF was 0.88, demonstrating scale reliability. The test–retest reliability, assessed using the intra-class correlation coefficient (ICC), was 0.91.
Validity
Patients noted that questions of AIMS2-SF were related to some QoL dimensions. In interviews, patients easily answered all questions. There were no missing data. According to these data, AIMS2-SF was found to have face validity. The Turkish AIMS2-SF version exhibited good correlations with functional parameters that showed its convergent validity (Table 2). There were significant correlations between AIMS2-SF and all NHP subgroups. Among these subgroups, the strongest correlations were with the NHP-physical mobility (rho = 0.64, p < 0.0005) and pain (rho:0.62; p < 0.0005) components (Tables 3 and 4).
Moreover, a moderate to strong relationship existed between AIMS2-SF and HAQ (0.60, p < 0.0005), BDI (0.63, p < 0.0005) and DHI (0.63, p < 0.0005). Non-significant correlations were found for parameters not directly related to QoL, such as age (0.07, p = 0.45) and disease duration (0.12, p = 0.21); however, disease activity (0.43, p < 0.0005) and NRS pain (0.45, p < 0.0005) were correlated with AIMS2-SF as moderate. AIMS2-SF adequately differentiated patients with high disease and NRS pain scores.
Discussion
This study revealed that the AIMS2-SF is a valid and reliable questionnaire in a Turkish population. Items were clearly understood by patients, there were no missing data, and the scale was completed in approximately 5 min. Cronbach’s α reliability for internal consistency was 0.88, which reflected scale reliability in our Turkish context. Moreover, the test–retest reliability was excellent, with an ICC value of 0.91. These scores are compatible with the data from the original AIMS2-SF instrument, and other validated forms [11, 12, 22]. We also assessed correlations between the AIMS2-SF and other scales, including NHP, HAQ, DHI and BDI, to show convergent validity of the short form.
As originally developed, the AIMS2-SF had five domains representing physical, symptoms, affects, social interactions and role. However, previous studies divided the physical domains as upper body and lower body limitations. Lower limb involvement was frequently observed in RA patients and may have independently resulted in a worsening QoL [23]. Therefore, it was important to be distinctive and evaluate physical functions in lower and upper limb functions, separately. All AIMS2-SF domains were significantly correlated with HAQ. Since both HAQ and AIMS2-SF are disease-specific QoL measurements, this result was important in reporting that AIMS2-SF had good construct validity. The level of physical disability, as assessed by HAQ scores, proved to be the most significant predictor of QoL in RA patients. This finding agreed with a previous study that reported a strong association between physical disability and HRQoL in RA patients [24].
Almost all AIMS2-SF domains were significantly correlated with BDI, DHI and NHP scales. Depressed individuals have less interest in leisure activities, experience increased fatigue, and have problems with social interaction; thus, they are at risk of increased disability and decreased QoL [25]. Bazzichi et al. suggested that even mild depressive symptomatology can contribute, independent of functional disability, to an impaired QoL [24]. In this study, the BDI scale and the ER component of the NHP were significantly associated with the AIMS2-SF total score and all subgroup scores, with the affect subgroup being strongest. In a previous study, the Michigan Hand Outcomes Questionnaire (MHQ) was used to assess hand function in RA, with significant moderate correlations observed within subgroups of the Short Form-36 (SF-36). The most noticeable correlation existed between MHQ and SF-36 subgroups for physical function (rho = 0.52, p < 0.001) and pain (rho = 0.48, p < 0.001) [26]. Consistent with this study, hand function as measured by DHI had the strongest correlation with the ULF (rho: 0.60) and symptom (rho: 0.48) components of AIMS2-SF, respectively. While there were moderate to strong correlations between the scales that evaluated the similar functional domains as expected, the correlation between the social interaction domain of AIMS2-SF and the social isolation subgroup of NHP was weaker than expected (rho: 0.31). We believe this weak correlation was due to different types of questions in these scales. While questions in the AIMS2-SF scale covered patient social interactions, NHP questions were related to the effects of social isolation on mental health. Therefore, when compared to the social interaction subgroup, the affect subgroup of AIMS2-SF has a stronger correlation (rho: 0.58) with the social isolation subgroup of NHP. While there were no correlations between AIMS2-SF scores and age, disease duration, and erythrocyte sedimentation rates, patients with high DAS-28 and NRS pain scores had significantly worse AIMS2-SF scores. Although no correlations were found between AIMS2-SF and erythrocyte sedimentation rates, the moderate correlation between AIMS2-SF and DAS-28 was believed to be related to patient-reported global assessment used to calculate DAS-28 scores.
In conclusion, Turkish AIMS2-SF is a valid and reliable outcome measure to evaluate RA patients in routine care and clinical trials. AIMS2-SF is a feasible and comprehensive disease-specific outcome measure. On the other hand, the fact that it is more practical than the AIMS2 scale makes it more important to demonstrate the validity and reliability of this questionnaire. The greatest strength of this study was the use of several well-known outcome measures to validate AIMS2-SF. Conversely, this study has some limitations which should be pointed out. First, a priori analysis was not performed to determine the sample size. There are no widely accepted calculation formulas or absolute rules for the sample size required to validate a questionnaire; however, the use of respondent–item ratio is generally recommended by guidelines. In this study, the ratio of included sample size to AIMS2-SF items is approximately 5:1, which is acceptable for a validation study. Second limitation of this study was the lack of responsiveness data for AIMS2-SF. Because it is a disease-specific measure, it is expected to be more sensitive in detecting minor changes in patients with arthritis compared to generic measurements, but further studies are still needed to explore the responsiveness and sensitivity to the changes of this scale.
Data Availability
The second limitation of this study is not the existence of a responsiveness study.
References
van Vilsteren M, Boot CR, Knol DL, van Schaardenburg D, Voskuyl AE, Steenbeek R, Anema JR (2015) Productivity at work and quality of life in patients with rheumatoid arthritis. BMC Musculoskel Disord 16(1):107
Katchamart W, Narongroeknawin P, Chanapai W, Thaweeratthakul P, Srisomnuek A (2020) Prevalence of and factors associated with depression and anxiety in patients with rheumatoid arthritis: A multicenter prospective cross-sectional study. Int J Rheum Dis 23:302–308
Burmester GR, Pope JE (2017) Novel treatment strategies in rheumatoid arthritis. Lancet 389(10086):2338–2348
van Vollenhoven R (2019) Treat-to-target in rheumatoid arthritis—are we there yet? Nat Rev Rheumatol 15(3):180–186
Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, Kvien TK, Navarro-Compán MV, Oliver S, Schoels M (2016) Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 75(1):3–15
Karimi M, Brazier J (2016) Health, health-related quality of life, and quality of life: what is the difference? Pharmacoeconomics 34(7):645–649
Wiebe S, Guyatt G, Weaver B, Matijevic S, Sidwell C (2003) Comparative responsiveness of generic and specific quality-of-life instruments. J Clin Epidemiol 56(1):52–60
Meenan RF, Gertman PM, Mason JH (1980) Measuring health status in arthritis. Arth Rheum 23(2):146–152
Meenan RF, Mason JH, Anderson JJ, Guccione AA, Kazis LE (1992) AIMS2. The content and properties of a revised and expanded arthritis impact measurement scales health status questionnaire. Arth Rheum 35(1):1–10
ten Klooster PM, Veehof MM, Taal E, van Riel PL, van de Laar MA (2008) Confirmatory factor analysis of the Arthritis Impact Measurement Scales 2 short form in patients with rheumatoid arthritis. Arth Care Res 59(5):692–698
Rosemann T, Korner T, Wensing M, Schneider A, Szecsenyi J (2005) Evaluation and cultural adaptation of a German version of the AIMS2-SF questionnaire (German AIMS2-SF). Rheumatology 44(9):1190–1195
Guillemin F, Coste J, Pouchot J, Ghézail M, Bregeon C, Sany J (1997) The AIMS2-SF. A short form of the arthritis impact measurement scales. Arthr Rheum 40(7):1267–1274
Atamaz F, Hepguler S, Oncu J (2005) Translation and validation of the Turkish version of the arthritis impact measurement scales 2 in patients with knee osteoarthritis. J Rheumatol 32(7):1331–1336
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO III, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD (2010) 2010 rheumatoid arthritis classification criteria: an American college of rheumatology/european league against rheumatism collaborative initiative. Arthritis Rheum 62(9):2569–2581
Küçükdeveci AA, Sahin H, Ataman S, Griffiths B, Tennant A (2004) Issues in cross-cultural validity: example from the adaptation, reliability, and validity testing of a Turkish version of the stanford health assessment questionnaire. Arthritis Care Res (Hoboken) 51(1):14–19
Hunt SM, McKenna S, McEwen J, Williams J, Papp E (1981) The nottingham health profile: subjective health status and medical consultations. Soc Sci Med A 15(3):221–229
Kücükdeveci A, McKenna S, Kutlay S, Gürsel Y, Whalley D, Arasil T (2000) The development and psychometric assessment of the Turkish version of the nottingham health profile. Int J Rehabil Res 23(1):31–38
Hisli N (1989) The validity and reliability of the beck depression inventory among university students. Turki J Psychol 7(23):3–13
Duruöz MT, Poiraudeau S, Fermanian J, Menkes C-J (1996) Functional disability scale that assesses functional handicap. J Rheumatol 23(7):1167–1172
Duruöz MT (2019) Assessment of hand function. In: Duruöz MT (ed) Hand function, 2nd edn. Springer-Nature, Cham, pp 3–53
Turan Y, Duruöz MT, Aksakalli E, Gürgan A (2009) Validation of duruöz hand index for diabetic hand dysfunction. J Investig Med 57(8):887–891
Askary-Ashtiani AR, Mousavi SJ, Parnianpour M, Montazeri A (2009) Translation and validation of the persian version of the arthritis impact measurement scales 2-short form (AIMS2-SF) in patients with rheumatoid arthritis. Clin Rheumatol 28(5):521–527
Toprak CŞ, Duruöz MT, Gündüz OH (2018) Static and dynamic balance disorders in patients with rheumatoid arthritis and relationships with lower extremity function and deformities: a prospective controlled study. Arch Rheumatol 33(3):328
Bazzichi L, Maser J, Piccinni A, Rucci P, Del Debbio A, Vivarelli L, Catena M, Bouanani S, Merlini G, Bombardieri S (2005) Quality of life in rheumatoid arthritis: impact of disability and lifetime depressive spectrum symptomatology. Clin Exp Rheumatol 23(6):783
Katz P, Yelin E (1993) Prevalence and correlates of depressive symptoms among persons with rheumatoid arthritis. J Rheumatol 20(5):790–796
Durmus D, Uzuner B, Durmaz Y, Bilgici A, Kuru O (2013) Michigan Hand outcomes questionnaire in rheumatoid arthritis patients: relationship with disease activity, quality of life, and handgrip strength. J Back Musculoskel Rehabil 26(4):467–473
Funding
There is no funding to report for this submission.
Author information
Authors and Affiliations
Contributions
CST, MTD and CUU participated in the conception and design of the study. Data acquisition was conducted by NO, ED, CUU and CST. Data analyses was performed by CST. All authors contributed to interpretation of the results. MTD, CST and CUU contributed to the drafting of the manuscript. All authors contributed to the critical revision of the manuscript for important intellectual content, approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no competing financial interests exist.
Ethical approval
This study was approved by the Marmara University School of Medicine ethics committee (approval number: 09.2015.102, date: April 2015) and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
All recruited patients were fully informed about the study and signed an informed consent form. All authors have read and approved submission of the manuscript and the manuscript has not been published and is not being considered for publication elsewhere in whole or part in any language except as an abstract.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanal-Toprak, C., Unal-Ulutatar, Ç., Duruöz, E. et al. The validity and reliability of the Turkish version of the Arthritis Impact Measurement Scale 2-Short Form (AIMS2-SF) for rheumatoid arthritis. Rheumatol Int 43, 751–756 (2023). https://doi.org/10.1007/s00296-022-05257-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05257-3