Abstract
Autoimmune rheumatic diseases have their own specific clinical presentation, and can affect multiple systems. Neurological involvement of autoimmune rheumatic diseases may involve both the central and peripheral nervous systems. Inflammation of neural tissue, autoantibody-mediated reactions, and small vessel vasculitis may be effective in the pathogenesis of neuropathy in autoimmune rheumatological diseases. Autoimmune rheumatic disease with pure motor neuron involvement is very rare in the literature. The case is here presented of a 58-year-old female patient who presented with the complaints of increasing pain and weakness in the extremities and was diagnosed with lower motor neuron disease and overlap syndrome. The patient was treated with cyclophosphamide, pulse steroid, hydroxychloroquine and intravenous immunoglobulin. After 3 months of treatment, a significant improvement was observed in the patient's clinical complaints and laboratory parameters. In conclusion, some patients with undiagnosed autoimmune rheumatic diseases may have neurological complaints. Clinicians should investigate patients with such neurological complaints for autoimmune rheumatic diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autoimmune connective tissue diseases are characterized by the presence of autoantibodies in the blood and chronic inflammation of the tissues. Many tissues and organs have autoimmunity-related microvasculitis. They can affect various organs of the body especially the skin, joints, hematopoietic system, kidneys, and nervous system. Although rheumatic diseases are usually seen alone in a patient, sometimes more than one rheumatic disease can be seen simultaneously in the same patient (overlap syndrome) [1]. Systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and Sjögren's syndrome (SS) are some of the well-known autoimmune rheumatic diseases. Patients with these diseases may have central and peripheral neurological problems. Neurological involvement incidence and prevalence among patients with RA, primary SS and SLE vary greatly [2,3,4,5,6]. Although the etiology of rheumatic diseases and neurological diseases is multifactorial, autoimmunity plays a role in the etiology of both disease groups. For this reason, some rheumatic diseases can cause problems with the central and peripheral nervous system. Studies to elucidate the common etiopathogenesis have primarily focused on autoantibodies, cytokines, chemokines and factors that cause blood–brain barrier dysfunction, how these factors function in the manifestation of neurological involvement in rheumatic diseases is not fully understood [7]. It is accepted that inflammation in the blood–brain barrier facilitates the transfer of autoantibodies in the serum to the cerebrospinal fluid and is one of the important causes of central nervous system involvement in rheumatic diseases. Small vessel vasculitis plays an important role in affecting the central and peripheral nervous systems [8,9,10]. In addition, cytokines and chemokines play a role in neurologic involvement in autoimmune diseases by activating Th1 and B cells [11]. Antibodies developed against antigens, such as myelin oligodendrocyte glycoprotein (MOG), neurofascin 186 (NF186), glutamate decarboxylase 65 (GAD65), have been shown to be associated with neurological involvement in SLE [12].
In this article, a case with overlap syndome and neurological involvement is discussed. The case we present is the first case reported in the literature as a motor neuron disease accompanying overlap syndrome.
Search strategy
We performed a literature search on PubMed database using Medical Subject Headings (MeSH) Terms (“amyotrophic lateral sclerosis” and "rheumatoid arthritis"), (“amyotrophic lateral sclerosis” and “Sjogren's Syndrome”), (“amyotrophic lateral sclerosis” and "systemic lupus erythematosus"), (“motor neuron disease” and "rheumatoid arthritis"), (“motor neuron disease” and " Sjogren's Syndrome "), (“motor neuron disease” and "systemic lupus erythematosus"). We found a total of 122 English written articles on PubMed (Fig. 1).
Articles related to different subjects from our case report, such as motor neuropathy due to drug side effects in rheumatic diseases and other neurodegenerative syndromes in rheumatic diseases, were excluded from the study. Ten articles related to patients with rheumatoid arthritis or Sjogren’s syndrome or systemic lupus erythematosus as well as motor neuron disease or amyotrophic lateral sclerosis (ALS) were included in our study. Information about 13 cases reported in a total of 10 articles is presented in Table 1.
Case report
A 58-year-old female patient presented with the complaints of increasing pain and weakness in the extremities, which had been ongoing for 1 year. From the patient history, it was learned that there had been a diagnosis of seropositive rheumatoid arthritis 7 years previously according to the ACR/EULAR 2010 rheumatoid arthritis classification criteria [22]. For 7 years, the patient had been using methotrexate 15 mg/week regularly and methylprednisolone 8 mg/day irregularly. She had no complaints other than occasional arthralgia The patient's medical history revealed weakness, fatigue, photosensitivity, dry mouth and eyes for the last six months.
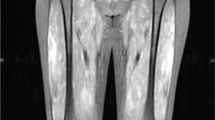
The patient had experienced difficulty while walking for last three months. In the physical examination, there were no signs of arthritis. A photosensitive rash was detected on the face and neck. Both hand and wrist movements were within normal limits but minimally painful. Left shoulder abduction was painful and limited to 90 degrees. Muscle atrophy and weakness were detected in both shoulder girdles, bilateral thenar, hypothenar, and interosseous muscles in the hands (Figs. 2, 3). In the neurological assessment, bilateral deep tendon reflexes could not be obtained in the upper extremities, and were bilaterally hypoactive in the lower extremities. No pathological reflex was detected. Gower’s sign indicating proximal muscle weakness was detected. Sensory examinations of the extremities and examination of the cranial nerves were normal.
Laboratory findings were as follows: white blood cell (WBC)1480/mL (4.5 to 11.0 × 109/L); lymphocytes 630/mL (42.9%); neutrophils 570/mL (38.4%); hemoglobin (Hgb): 12.5 g/dL; mean corpuscular volume (MCV): 89.2; mean corpuscular hemoglobin (MCH): 28.7 picograms/per cell; PLT: 304.000/mL; Urea: 28 mg/d; creatinine: 0.44 mg/dL; eGFR: 112; AST: 16 U/L; ALT: 41 U/L; creatine kinase (CK): 44 U/L; CRP: 9 mg/L (0–5 mg/L); erythrocyte sedimentation rate (ESR): 43 mm/h, rheumatoid factor: 69.7 IU/ml (0–15); Anti- Cyclic Citrullinated Peptide: > 200 U/ml, antiphospholipid antibodies: negative, and C3c: 1.0 g/L (0.9–1.8), C4: 0.2 g/L ( 0.1–0.4). Urinalysis showed no pathological findings. The random urinary protein-to-creatinine ratio was 97 mg/g (< 200).
The immunological test results showed signs of autoimmune disease, including antinuclear antibodies (ANA) 1:1000 (+ 3) positive antinuclear antibodies with homogenous granular pattern, Anti-ds DNA-ELISA: 256 (< 100), Anti-SS-A: + 2, Anti-Ro-52 + , and Anti-SS-B: negative.
The serology tests results for viruses were negative. COVID-19 reverse transcription polymerase chain reaction (RT-PCR) with nasopharyngeal swab was negative.
Radiological examination revealed changes due to rheumatoid arthritis (Fig. 4). Abdominal ultrasonography, thorax computed tomography and transesophageal echocardiography revealed no pathological findings.
In the cranial MR examination, gliotic changes consistent with the patient’s age were observed. Bilateral deltoid muscle atrophy was detected in the shoulder MRI examination. No pathological findings were detected on cervical, lumbar, and brachial plexus MRI.
Electroneuromyography (ENMG) revealed normal sensory and motor nerve conduction studies. Spontaneous activity potentials were observed in bilateral triceps, tibialis anterior, and rectus femoris muscles. Chronic neurogenic motor unit potential (MUP) changes, giant MUPs and widespread MUP loss were detected in muscles. These EMG findings were consistent with diffuse anterior horn motor neuron involvement.
The Schirmer test results for the right and left eye were 5 mm. Salivary gland biopsy showed periductal localized lymphoid aggregates (> 50 lymphocytes in one focus) in two areas, which was evaluated as Chisholm–Mason grade 3.
The patient was diagnosed with RA according to the ACR/EULAR 2010 rheumatoid arthritis classification criteria [22] (> 6 points: 4–10 small joints; 3 points: high positive RF or high positive ACPA; 3 points, duration of symptoms longer than 6 weeks; 1 point).
SLE was diagnosed by obtaining a total of 6 points from the 17 criteria defined by The Systemic Lupus International Collaborating Clinics (SLICC) group, 3 clinical (arthritis, leukopenia, photosensitive lupus rash) and 3 laboratory (ANA, Anti-DNA bodies, low complement) points. According to these criteria, at least 4 points should be obtained for the diagnosis of SLE [23]. The patient was also diagnosed with Sjögren’s syndrome (Anti-SS-A:3, salivary gland biopsy:3) according to the 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria [24]. The patient with three simultaneous rheumatic diseases was accepted as overlap syndrome.
The musculoskeletal system findings of the patient with a diagnosis of rheumatoid arthritis, SLE, and Sjögren’s syndrome were found to be compatible with anterior horn motor neuron disease. The patient was treated with cyclophosphamide (500 mg intravenously every 15 days for a total of 6 times), pulse steroid (1000 mg of methylprednisolone for 3 days), hydroxychloroquine (200 mg/day), and intravenous immunoglobulin (0.4 g/kg/day for 5 days, repeated 3 times). After 3 months of treatment, a significant improvement was observed in the patient’s clinical complaints (Table 2). Laboratory parameters including sedimentation, CRP and Anti-ds DNA-ELISA returned to normal limits (27 mm/h, 1 mg/L,90, respectively).
Written informed consent for publication of this report was obtained from the patient.
Discussion
Central and peripheral neurologic systems may be affected in rheumatic diseases. Aseptic meningitis, cerebrovascular disease, seizure disorder, myelopathy, cognitive dysfunction, acute confusional state are among the central nervous system-related problems seen in autoimmune rheumatic diseases [11]. Guillain–Barre syndrome, autonomic neuropathy, mononeuropathy, myasthenia gravis, plexopathy, polyneuropathy, axonal sensory neuropathy, acute motor and sensory axonal neuropathy are peripheral neuropathy types seen in autoimmune rheumatic diseases [11, 20, 25]. While central nervous system involvement is noteworthy in SLE, peripheral involvement is more prominent in Sjögren's syndrome. In rheumatoid arthritis, the central nervous system may be affected by rheumatoid meningitis or vasculitis, and myelopathy due to cervical spine instability may be seen [26]. Neurological involvement in Sjögren’s syndrome is generally in the form of peripheral and axonal sensorimotor neuropathy. Demyelinating polyradiculoneuropathy, cranial neuropathy, and autonomic neuropathy may also be seen [27].
Although the mechanism of neurological involvement in autoimmune diseases is not fully known, it is accepted that direct targeting of neural tissues through autoantibodies, cytokines and lymphocytes may be effective in addition to autoimmune vasculopathy and coagulopathy [25, 28]. Studies have shown T cell and monocyte infiltration in the central nervous system, immunoglobulin deposits in motor neurons, and increased cytokines and chemokines in the peripheral circulation [29,30,31,32]. Wang et al. compared serum samples of 245 ALS patients with a healthy control group. They found that serum C4 levels in ALS patients were lower than in the healthy control group [33]. Depending on the understanding of the role of the immune system in motor neuron diseases and the developments in drug technology, some studies investigating the effectiveness of immune modulator biological agents in the treatment of motor neuron disease have started [34, 35].
ALS is the most common and best known of the motor neuron diseases and its incidence is 3–5 per 100,000. It is a progressive paralytic disease and patients often die within a few years of diagnosis [36]. Poliomyelitis, genetic–familial diseases, such as spinal muscular atrophy and hereditary spastic paraparesis, are other types of motor neuron disease [37]. It is typical for patients with motor neuron disease to have progressive muscle weakness without sensory deficits. ALS is a neurodegenerative disease with both upper and lower motor neuron manifestations. Increased deep tendon reflexes and the presence of pathological reflexes are seen in patients. Complaints such as difficulty in speaking and swallowing due to cranial nerve involvement are also expected. In the current case, there was no clinical finding of upper motor neuron involvement, and no pathology was found in the upper motor neuron regions in the cranial and cervical MRI examinations. Few cases with ALS-SLE coexistence and ALS-RA coexixstence have been reported in the literature. (Table 1). In Sjögren's syndrome, cases with upper motor neuron involvement without lower motor neuron findings have been reported [20, 25]. Yang et al. recently reported rapidly progressive motor neuron disease in a patient with Sjögren's syndrome. The patient did not respond to treatment and died [21]. In a study reported by Seeliger et al., 184 patients with polyneuropathy associated with limb weakness were evaluated for Sjögren's syndrome (ACR-EULAR classification criteria), and Sjögren's syndrome was determined in 44 patients [38]. This association is important in terms of showing the role played by autoimmunity in the etiology of neuropathy. Patients with Neuro-Sjögren often present with axonal and or demyelinating damage [38]. The interesting aspect of the current case was that there was only anterior horn motor neuron involvement without sensorimotor or demyelinating involvement. ALS is the most well-known anterior horn motor neuron disease. In addition, the absence of upper motor neuron type findings and good response to immunosuppressive therapy are not expected features of ALS. In the case presented here, there were no upper motor neuron findings and spinal cord MRI revealed no findings of myelitis. The patient received pulse corticosteroids, intravenous immunoglobulins, and immunosuppressant treatment. IVIG treatment shows its effect by suppressing the effect of opsonin, neutralizing cytokines and decreasing antibody production [39]. The clinical findings of the current patient improved with immunosuppressive therapy.
Conclusion
In patients with autoimmune rheumatism, extremity weakness and muscle atrophy develop due to non-use, but the presence of fasciculation and disproportionate muscle atrophy in particular should suggest motor neuron disease. Although neurological findings developed after the diagnosis of autoimmune rheumatic disease in the current patient, it is known that some patients with autoimmune rheumatic diseases first present at neurology clinics with neurological symptoms. It is important to investigate patients with such neurological complaints in terms of autoimmune rheumatic diseases. In suspicious cases, neurological examination and nerve conduction studies should be performed.
References
Pepmueller PH (2016) Undifferentiated connective tissue disease, mixed connective tissue disease, and overlap syndromes in rheumatology. Mo Med 113:136–140
Segal B, Carpenter A, Walk D (2008) Involvement of nervous system pathways in primary Sjögren’s syndrome. Rheum Dis Clin North Am 34:885–906
Ahn GY, Kim D, Won S, Song ST, Jeong HJ, Sohn IW et al (2018) Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus 27:1338–1347
Hanly JG, Urowitz MB, Su L, Bae SC, Gordon C, Wallace DJ et al (2010) Systemic lupus international collaborating clinics (SLICC). Prospective analysis of neuropsychiatric events in an international disease inception cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 69:529–535
CarvajalAlegria G, Guellec D, Mariette X, Gottenberg JE, Dernis E, Dubost JJ et al (2016) Epidemiology of neurological manifestations in Sjögren’s syndrome: data from the French ASSESS cohort. RMD Open 2(1):e000179
Padovan M, Caniatti LM, Trotta F, Govoni M (2011) Concomitant rheumatoid arthritis and amyotrophic lateral sclerosis: report of two new cases and review of literature. Rheumatol Int 31:715–719
Carrión-Barberà I, Salman-Monte TC, Vílchez-Oya F, Monfort J (2021) Neuropsychiatric involvement in systemic lupus erythematosus: a review. Autoimmun Rev 20:102780
Lvovich S, Goldsmith DP (2017) Neurological complications of rheumatic disease. Semin Pediatr Neurol 24:54–59
Birnbaum J (2019) The nervous system in rheumatic disease. In: Hochberg MC, Gravallese EM, Sılman AJ, Smolen JS, Weinblatt ME, Weisman MH (eds) Rheumatology, 7th edn. Elsevier, Philadelphia, pp 317–324
Magaki S, Chang E, Hammond RR, Yang I, Mackenzie IR, Chou BT et al (2016) Two cases of rheumatoid meningitis. Neuropathology 36:93–102
Deijns SJ, Broen JCA, Kruyt ND, Schubart CD, Andreoli L, Tincani A et al (2020) The immunologic etiology of psychiatric manifestations in systemic lupus erythematosus: a narrative review on the role of the blood brain barrier, antibodies, cytokines and chemokines. Autoimmun Rev 19:102592
Pröbstel AK, Thanei M, Erni B, Lecourt AC, Branco L, André R et al (2019) Swiss systemic lupus erythematosus cohort study group. Association of antibodies against myelin and neuronal antigens with neuroinflammation in systemic lupus erythematosus. Rheumatology (Oxford) 58:908–913
Schady W, Metcalfe RA, Holt PJ (1989) Rheumatoid arthritis and motor neurone disease–an association? Br J Rheumatol 28:70–73
Forns X, Bosch X, Graus F, Navarro M, Font J (1992) Development of amyotrophic lateral sclerosis in the course of systemic lupus erythematosus. Clin Rheumatol 11:578–579
Forns X, Bosch X, Graus F, Navarro M, Font J (1993) Amyotrophic lateral sclerosis in a patient with systemic lupus erythematosus. Lupus 2:133–134
M’Bappé P, Moguilevski A, Arnal C, Cocheton JJ, Roullet E (2000) Concomitant rheumatoid arthritis and amyotrophic lateral sclerosis. A puzzle illustrated by a new case. Joint Bone Spine 67:242–244
Maldonado ME, Williams RC Jr, Adair JC, Hart BL, Gregg L, Sibbitt WL Jr (2002) Neuropsychiatric systemic lupus erythematosus presenting as amyotrophic lateral sclerosis. J Rheumatol 3:633–635
Rao TV, Tharakan JK, Jacob PC (2004) Systemic lupus erythematosus presenting as amyotrophic lateral sclerosis. Clin Neuropathol 3:99–101
Dziadzio M, Reddy V, Rahman S, Mummery C, Keat A (2006) Is TNFalpha really a good therapeutic target in motoneuronal degeneration? A case of amyotrophic lateral sclerosis in a patient with RA receiving infliximab. Rheumatology (Oxford) 45:1445–1446
Hagiwara K, Murai H, Ochi H, Osoegawa M, Shigeto H, Ohyagi Y et al (2008) Upper motor neuron syndrome associated with subclinical Sjögren’s syndrome. Intern Med 47:1047–1051
Yang H, Jing X, Yan J, Ma D (2020) Sjögren’s syndrome with rapidly progressive motor neuron disease: a case report. J Int Med Res 48:300060520974465
Kay J, Upchurch KS (2012) ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford) 51(6):vi5-9
Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR et al (2012) Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686
Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM et al (2017) International Sjögren’s syndrome criteria working group. 2016 American college of Rheumatology/European League against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 69:35–45
Chen YM, Su KY (2021) Acute motor and sensory axonal neuropathy in association with primary Sjögren’s syndrome: a case report. BMC Neurol 21:161
Bhattacharyya S, Helfgott SM (2014) Neurologic complications of systemic lupus erythematosus, sjögren syndrome, and rheumatoid arthritis. Semin Neurol 34:425–436
Jaskólska M, Chylińska M, Masiak A, Siemiński M, Ziętkiewicz M, Czuszyńska Z et al (2020) Uncommon or underestimated problem? Brain Behav 10(8):e01665
Mukaino A, Nakane S, Higuchi O, Nakamura H, Miyagi T, Shiroma K et al (2016) Insights from the ganglionic acetylcholine receptor autoantibodies in patients with Sjögren’s syndrome. Mod Rheumatol 26:708–715
Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G et al (2012) Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 122:3063–3087
Engelhardt JI, Soós J, Obál I, Vigh L, Siklós L (2005) Subcellular localization of IgG from the sera of ALS patients in the nervous system. Acta Neurol Scand 112:126–133
Cereda C, Baiocchi C, Bongioanni P, Cova E, Guareschi S, Metelli MR et al (2008) TNF and sTNFR1/2 plasma levels in ALS patients. J Neuroimmunol 194:123–131
Sun Q, Huo Y, Bai J, Wang H, Wang H, Yang F et al (2021) Inflammatory cytokine levels in patients with sporadic amyotrophic lateral sclerosis. Neurodegener Dis 21:87–92
Wang M, Liu Z, Du J, Yuan Y, Jiao B, Zhang X et al (2021) Evaluation of peripheral immune activation in amyotrophic lateral sclerosis. Front Neurol 12:628710
Lin TJ, Cheng GC, Wu LY, Lai WY, Ling TY, Kuo YC et al (2022) Potential of cellular therapy for ALS: current strategies and future prospects. Front Cell Dev Biol 16(10):851613
Kim SH, Oh KW, Jin HK, Bae JS (2018) Immune inflammatory modulation as a potential therapeutic strategy of stem cell therapy for ALS and neurodegenerative diseases. BMB Rep 51:545–546
Brown RH, Al-Chalabi A (2017) Amyotrophic lateral sclerosis. N Engl J Med 377:162–172
Foster LA, Salajegheh MK (2019) Motor neuron disease: pathophysiology, diagnosis, and management. Am J Med 132:32–37
Seeliger T, Prenzler NK, Gingele S, Seeliger B, Körner S, Thiele T et al (2019) Peripheral neuropathy with limb weakness in Sjögren’s syndrome. Front Immunol 11(10):1600
Dalakas MC (1998) Mechanism of action of intravenous immunoglobulin and therapeutic considerations in the treatment of autoimmune neurologic diseases. Neurology 51(6 Suppl 5):S2-8
Author information
Authors and Affiliations
Contributions
EA, KG, and HB wrote the manuscript. FGY, TG, ŞE were involved in the clinical management; EY and HB supervised the entire process.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest related to this case report.
Informed consent
Written informed consent for publication of this report was obtained from the patient.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atalar, E., Yurdakul, F.G., Gök, K. et al. Motor neuron disease in a patient with overlap syndrome (rheumatoid arthritis; systemic lupus erythematosus, Sjogren’s syndrome). Rheumatol Int 43, 367–372 (2023). https://doi.org/10.1007/s00296-022-05207-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05207-z