Abstract
Relapse in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) is associated with significant morbidity and mortality. Utility of ANCA for prediction of relapses is still controversial. PubMed/MEDLINE, Scopus, and WebOfScience were searched, screened and confirmed for inclusion [PROSPERO No: CRD42020220308]. Studies measuring serial ANCA by ELISA or indirect immunofluorescence (IF), reporting relapses with sufficient data to calculate sensitivity and specificity were included. Diagnostic odds ratio (OR), sensitivity, specificity and likelihood ratios (LR) were synthesized using a bivariate mixed-effect regression model. Sub-group analysis included a comparison between ELISA and IIF, anti-myeloperoxidase (MPO) and -proteinase 3(PR3), and type of rise in ANCA. For meta-analysis of survival outcomes, hazard ratios were synthesized using a random-effect model. QUADAS-2 was used for assessing quality of studies, I2 statistic for heterogeneity Begg’s test for publication bias. 2946 abstracts and 43 full-texts were reviewed to identify 26 eligible studies that included 2623 patients with AAV and 848 relapses. Overall heterogeneity was high [I2 = 99%] and the overall risk of bias was low to moderate. ANCA positivity by either ELISA or immunofluorescence for predicting relapse of AAV had a sensitivity of 0.70(95% CI 0.58–0.81), specificity of 0.66(0.55–0.76), positive LR of 2.1(1.6–42.7) and negative LR of 0.44(0.30–0.60). ELISA performed marginally better [OR: 5(3–7)] than IIF [OR: 4(2–9)] with similar sensitivity, specificity, PLR and NLR. The area under the curve for PR3 was 0.74(0.7–0.77), while that for MPO was not computed as the number of eligible studies was only three. In the survival analysis, the hazard ratio for relapse was 3.11(1.7–5.65). The meta-analysis shows modest accuracy of ANCA in predicting relapses of ANCA vasculitis and supports the use of serial ANCA monitoring as a biomarker for relapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) is associated with high morbidity and mortality. Prediction of relapse is important to minimize damage accrual, and indeed, a major treatment target in AAV is to prevent relapse.
Over the last five decades, the face of AAV has transformed from a near-fatal ailment to a chronic relapsing disease with the use of oral cyclophosphamide [1]. With subsequent trials employing effective and safe treatment regimens, outcomes have significantly improved over time with relapse rates as low as 5–10% with the use of rituximab-based courses for maintenance [2, 3]. Despite these advances, relapses are still associated with damage accrual, increased cumulative drug use, and comorbidities like cardiovascular disease. On the other hand, increasing immunosuppression in anticipation of a relapse which may not occur exposes patients to undue drug adverse effects and costs. Thus, it is imperative to have reliable biomarkers predicting relapses.
Our understanding of AAV has uncovered a definite role of ANCAs in the diagnosis and pathogenesis [4], with initial reports describing their role in predicting disease flares [5]. With the obvious importance of the need for appropriate biomarkers, multiple studies have evaluated and shown conflicting results regarding the value of ANCA measurements in predicting disease flares. The last meta-analysis in this area was carried out almost a decade ago [6,7,8]. The major limitations observed in these systematic reviews were heterogeneity across the studies which precluded definitive analysis on prediction of relapses. We expect the evidence has changed with better accuracy of tests in addition to increased availability of data from both trials and registries in the last decade. Moreover, the recent use of B-cell depletion therapies in AAV adds to the relevance of this question since they might have a direct bearing on antibody levels. Thus, there is an unmet need for an update on whether rising titres or persistently high titres of ANCA are useful in predicting future relapses in AAV in light of these new advances.
One recent meta-analysis in AAV has looked at relapse after cyclophosphamide therapy while another has looked at overall predictors of relapse [9, 10]. The first study included 24 studies and analyzed the cumulative relapse rates over time. A meta-analysis of 28 studies showed an association with lower level of baseline serum creatine, proteinase 3 (PR3)-ANCA positivity at diagnosis, an ANCA rise, extrarenal organ involvement, intravenous (vs oral) cyclophosphamide induction, a shorter course of immunosuppressant maintenance, and maintenance with mycophenolate mofetil (vs azathioprine). Risk ratios of relapse associated with persistently positive ANCA were not estimated [9]. Whereas, the second study included 16 studies and analyzed baseline ANCA positivity and not rising titres as a predictor of relapse [10]. No recent meta-analysis has previously determined diagnostic accuracy (sensitivity, specificity, positive and negative likelihood ratios) of ANCA titres or ANCA positivity in predicting relapse. Thus, we carried out a systematic review and meta-analysis of published literature to update the current evidence on the utility of ANCA titres for predicting relapses of AAV.
Methods
Research question
The meta-analysis was planned for estimating the utility of ANCA (positivity, rising titres, method of ANCA testing) for predicting relapses in AAV.
The protocol was prospectively registered with PROSPERO (CRD42020220308). Results are reported as per preferred reporting of systematic reviews and meta-analyses (PRISMA) guidelines for diagnostic test accuracy studies [11].
Literature search
Systematic searches were done on PubMed/MEDLINE, Scopus, and the WebOfScience databases, including conference proceedings and abstracts. A search was performed using various combinations of antibody, antineutrophil cytoplasmic, vasculitis, remission, relapse, disease activity, recurrence, follow-up, cohort and prediction with a filter for the English language. Search results till 26/11/2020 were included. Antibodies, Antineutrophil Cytoplasmic [MESH] AND vasculitis AND ( recurrence OR relapse)Antibodies, Antineutrophil Cytoplasmic [MESH] AND vasculitis AND disease activity for PubMed; (‘‘antineutrophil cytoplasmic antibodies’’ OR ‘‘ANCA’’) AND ‘‘vasculitis’’ AND ( relapse OR recurrence) AND (‘‘cohort’’ OR ( follow AND up)) AND ( predict OR prediction) for SCOPUS; and ("antineutrophil cytoplasmic antibodies" OR "ANCA") AND "vasculitis" AND (relapse OR recurrence) AND ("cohort" OR follow-up) for WebofScience. (Supplementary File 1).
Study selection
SA performed the literature search and excluded the duplicate studies from the total. In phase I, PM and AB independently screened titles and abstracts, and then the full-text review of all the eligible articles. SA and SP finalized the selection of studies wherever there was any discrepancy between PM and AB.
Inclusion criteria
Studies that used a definitive diagnosis of ANCA vasculitis; reported serial ANCA measurements performed during the maintenance phase of AAV; included at least 10 patients in the study; had data that could be extracted to calculate both sensitivity and specificity and were published in English.
Exclusion criteria
Studies were excluded if the ANCA rise was only concurrent with relapse of disease (no serial monitoring during remission); if only the number of relapses but not the number of patients with and without relapse during follow-up could be extracted; if ANCA levels were used to define remission or relapse or were published as case reports or case series or was unpublished data present only in pre-print repositories.
Data extraction
AB and PM independently extracted data into a pre-specified spreadsheet. This included country of origin, sample size, median or mean age, median or mean follow-up, stratification by subtype of AAV and ANCA, method of ANCA testing, the definition of positivity and rise in titer, number of relapses, correlation of ANCA status at the time of relapse and classification into ANCA positivity/rise if mentioned.
Quality assessment
SP and MP assessed the quality of included studies using QUADAS-2. [12]
Publication bias
Publication bias was assessed using Begg’s test by MP.
Statistical analysis
Summary estimates of sensitivity and specificity were obtained using a bivariate mixed-effect regression model which preserves the two-dimensional structure of data and incorporates the correlation between sensitivity and specificity [13]. The summary measures included for diagnostic test accuracy were sensitivity, specificity, diagnostic odds ratio (OR) as positive as well as negative likelihood ratios (LR). Using a Bayesian approach, we calculated the post-test probability based on pre-test probability and likelihood ratio. Wherever relevant, summary receiver operating characteristic (ROC) curves were synthesized. Sub-group analyses were performed for IIF vs ELISA, Anti-PR3 versus Anti-MPO, persistently high ANCA titres versus a rise in ANCA titer, persistently positive ANCA versus negative to positive conversion. Also, for meta-analysis of survival outcomes, hazard ratios were synthesized using a random-effect model. I2 statistic was used to assess heterogeneity. Publication bias was assessed using Begg’s test. All the analyses were performed using Stata Version 15.1(Statacorp, Texas, USA).
Results
Literature search and selected studies
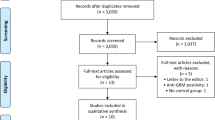
We screened 2946 titles and abstracts, of which 43 articles were selected for full-text review. Seventeen were not included in the final analysis because of missing/incomplete data in 10 [14,15,16,17,18,19,20,21,22,23], no serial monitoring in one [24], different outcomes in two [25, 26], one being a correction [27] and no access to data in three [28,29,30]. Twenty-six studies were selected for data extraction and analysis (Fig. 1, Supplementary Table 1). The details of the studies included are given in Table 1. Eleven were retrospective studies and 15 were prospective of which two were randomized controlled trials (RCT) [22, 31]. Two were based on a long-term follow-up of an RCT [2, 32].
These 26 studies cumulatively included 2623 AAV patients and 848 relapses. Eleven studies used clinical definitions of relapse, 13 used Birmingham Vasculitis Activity Score (BVAS) and two used Disease Extent Index (DEI). The results of QUADAS-2 analysis of the quality of the studies are presented in Fig. 2. The risk of bias was moderate in the domain of patient selection, whereas it was low in the remaining three domains. No publication bias was found for the comparison of studies.
Positive ANCA
Of the 26 studies included for the analysis, 11 were retrospective. All studies but one (ALBIA—Addressable Laser Bead Immunoassay, [33]) used either ELISA or both IIF and ELISA. The frequency of ANCA testing was not mentioned in nine studies [32, 34,35,36,37,38,39,40,41]. Eleven studies did not mention clearly whether ANCA turned positive from a negative report or whether there was a rise from baseline [32, 34, 36,37,38, 40,41,42,43,44,45]. The ROC curve of the studies showed an AUC of 0.74(95% CI 0.70–0.78). Sensitivity was 0.71(95% CI 0.59–0.80) and specificity 0.67(0.56–0.76), positive LR 2.1(95% CI 1.6–2.7), negative LR 0.44(95% CI 0.32–0.60) and an OR of 5 (95% CI 3–8). The forest plot is shown in Fig. 3a. The Fagan plot (Fig. 3b) shows a lower likelihood of disease relapse if the rise in ANCA is not seen. The heterogeneity between studies was significantly high with an I2 of 99.
IIF versus ELISA
Nine studies utilized IIF techniques for the detection of ANCA [22, 31, 32, 35, 36, 43, 45,46,47]. Five studies [22, 43, 45,46,47] were included for this analysis, three of them being retrospective. The meta-analysis of the five studies provided an AUC of 0.73(95% CI 0.69–0.76), sensitivity of 0.72(0.56–0.84), specificity of 0.62(95% CI 0.45–0.77), positive LR of 1.9(95% CI 1.3–2.8), negative LR of 0.45(95% CI 0.28–0.7) and an OR of 4(95% CI 2–9).
ELISA as a diagnostic method was used in nearly all of the studies (25 of 26), 11 being retrospective. Meta-regression showed AUC of 0.73(95% CI 0.69–0.77), sensitivity 0.71(95% CI 0.60–0.80), specificity 0.65(95% CI 0.54–0.74), positive LR 2(95% CI 1.6–2.6), negative LR 0.45(95% CI 0.32–0.61) and OR of 5(95% CI 3–7). The comparison of IIF ANCA and ELISA PR3/MPO is shown in Supplementary Fig. 1a, b and Supplementary Fig. 2a, b, respectively. The study heterogeneity was significantly high with I2 of 80 for IIF and 99 for ELISA.
PR3 and MPO
Five studies included PR3 [22, 39, 45, 47, 48] and three included MPO alone (32,49,50), whereas the remaining analyzed both. The AUC for PR3 was 0.69 (95% CI 0.65–0.973), sensitivity 0.83(95% CI 0.3–0.98), specificity 0.57(95% CI 0.37–0.75), positive LR 1.9(95% CI 1.5–2.6), negative LR 0.29(95% CI 0.06–1.52) and OR 7(95% CI 1–37). The study heterogeneity was significantly high with an I2 of 86. The meta-analysis was not done for MPO as the number of studies was only three. The Forest plot and ROC curve for PR3 are shown in Supplementary Fig. 3a and 3b, respectively.
Rising ANCA
Ten studies specified the total number of patients who had a rise in ANCA and relapses associated with it [19, 31, 33, 39, 46,47,48, 50,51,52]. AUC for rise in ANCA and association with relapse was 0.71(95% CI 0.66–0.74), sensitivity 0.70 (95% CI 0.51–0.84), specificity 0.65 (95% CI 0.55–0.73), positive LR 2(95% CI 1.4–2.8), negative LR 0.47 (95% CI 0.26–0.82) and OR of 4 (95% CI 2–10). The study heterogeneity was significantly high with an I2 of 91. The Forest plot and Fagan plot for rising ANCA are shown in Fig. 4a, b, respectively.
Negative to positive conversion of ANCA
Eight studies specified the association of conversion to a positive ANCA after turning negative with a relapse [19, 22, 31, 35, 48,49,50, 53]. AUC for conversion to a positive ANCA and association with a relapse was 0.74 (95% CI 0.7–0.78), sensitivity 0.65 (95% CI 0.44–0.81), specificity 0.72 (95% CI 0.62–0.80), positive LR 2.3 (95% CI 1.8–2.8), negative LR 0.49(0.31–0.79) and OR of 5 (95% CI 3–8). The study heterogeneity was significantly high with an I2 of 92. The Forest plot and Fagan plot for PR3 are shown in Fig. 5a, b, respectively.
Survival analysis
Nine studies were included in the survival analysis, out of which five studies were prospective [19, 32, 40, 48, 52] and four retrospective (22,33,39,50). The overall HR was 3.11 (95% CI 1.7–5.65). The forest plot is shown in Fig. 6a, b, respectively.
Discussion
Our meta-analysis revealed a moderate sensitivity (0.71) and specificity (0.67) for a positive ANCA as a predictor of relapse. Furthermore, ANCA testing by IIF and ELISA yielded a similar sensitivity and specificity for the same. Sub-group analysis with PR3 ANCA revealed a good sensitivity (0.83) and poor specificity (0.57); rising ANCA and negative to positive conversion of ANCA, both revealed a moderate sensitivity (0.7, 0.65) and specificity (0.65, 0.72) for the prediction of relapses. This was supported by the likelihood ratios. The overall HR for relapse with a positive ANCA was 3.11 on survival analysis. Thus, serial monitoring of ANCA may be of modest help in predicting relapse. However, as the AUC is between 0.7 and 0.8, its utility in isolation is questionable.
The consistent role of ANCA in the pathogenesis of AAV has attracted much attention for exploring its role as a biomarker for the prediction of the clinical course of AAV. However, its utility for the same is supported by incongruous results from various studies as a result of varied methodology of ANCA testing, variable follow-up intervals for measuring ANCA, heterogenous clinical subgroups and different organ involvement amongst patients, definitions of rise and persistence of ANCA and definitions of response and relapse.
In this analysis, we aimed to address some aspects of this conundrum—predictability of relapse with persistent or rising ANCA titres, use of IIF compared with ELISA for the same, and bearing of the subtype of ANCA on predictability of relapse. We found that a combined analysis of rise and persistent ANCA has modest sensitivity and specificity for the prediction of relapse with a positive LR + of 2.1 and negative LR- of 0.4, so with a pre-test probability of 20%, post-test risk of relapse is 40% of there is a positive ANCA and 10% if ANCA is negative (Fig. 3b). Although there is a lack of sufficient evidence supporting the practice of antibody-based guidance in deciding therapy for patients, it is often observed in routine clinical practice if the clinician perceives the pre-test possibility of relapse to be high. Rise or persistence may be associated with intensification of immunosuppression used, thus preventing a relapse subsequently. This may explain the modest sensitivity and specificity that we have reported in our analysis [50].
Although ELISA is a more sensitive method than IIF for diagnosis of ANCA and the majority of the studies used ELISA as a method for serial monitoring, on subgroup analysis, both ELISA and IIF had similar sensitivity and specificity in predicting relapses. (Supplementary Figs. 1, 2).
Individual studies have highlighted better predictability of relapse with ANCA monitoring under certain clinical settings. The RTX in AAV trial (RAVE) compared RTX with oral cyclophosphamide followed by azathioprine for induction and found RTX to be non-inferior [3]. A subsequent analysis of data from the RAVE trial demonstrated that rising PR3 ANCA predicted a relapse with a HR of 4.7(95% CI 2.16–10.37), and the risk was higher for those with renal disease or alveolar hemorrhage [52]. A single-center cohort study from Netherlands identified 110 patients with AAV treated with Rituximab. They found a significantly higher risk of relapse with persistently positive PR-3ANCA or a reappearance of PR-3ANCA and relapses in MPO- ANCA positive patients were restricted to patients with persistent MPO positivity [54]. Another cohort study from Netherlands found an association between ANCA and relapses only in renal disease regardless of the ANCA subtype [19]. However, some recent trials like Maintenance of Remission using RTX in AAV (MAINRITSAN II) which evaluated a tailored versus fixed RTX regimen in AAV based on ANCA and B-cell repopulation did not find any association between ANCA rise/positivity and relapses.
In our study, when PR3 was analyzed separately, its levels had better sensitivity than combined MPO and PR3 (Supplementary Fig. 3) similar to a previous meta-analysis [7]. However, we did not stratify studies based on organ involvement and on previous treatment received. On studying the association of relapse with a rise in titer versus the conversion from a negative to a positive report, similar sensitivity and specificity were found.
Our results are consistent with a previous analysis done on this subject with similar LRs. A rise in ANCA titres yielded a positive LR of 2.84 (95% CI 1.65–4.9) and negative LR of 0.49 (95% CI 0.27–0.87) for relapses whereas persistent positivity had a positive LR of 1.97(95%CI 1.43–2.7) and negative LR of 0.73(95% CI 0.5–1.1) for relapses [7]. We additionally looked at various subgroups based on testing methodology, ANCA subtype, and a negative to positive conversion supplementing the results of overall positivity and rise in ANCA. There were two recent metanalyses that have looked at various risk factors for relapse. One of which found the HR for positive PR3 ANCA computed from 3 studies to be 1.69 [10, 34, 55, 56]. Another study found the HR for PR3 positivity to be 2 and rising ANCA to be 8. These results are again in line with our study with a slight difference as we have focused only on ANCA as a biomarker for relapse.
There are several strengths regarding our approach to this analysis. We have included studies with serial monitoring of ANCA titres with both rising and conversion into positive results being included for predictability of relapses. Furthermore, we have also studied the effect of method of ANCA testing, the subtype of ANCA, rise in ANCA, negative to positive conversion on the predictability of relapses. It provides a basis for the inclusion of serial monitoring of ANCA titres in clinical trials and cohorts to generate a better quality of data. Furthermore, dosing regimens based on ANCA titres have been explored with a non-significant trend of increased relapse in the tailored arm (17% vs 10%) but with a significantly reduced need for drug infusions [19]. Thus, in a disease that has a severe dearth of biomarkers available for use in the clinic, monitoring ANCA may serve as a readily available biomarker, especially in low- and middle-income countries where the cost of therapy is borne out-of-pocket by the patients or their kin. The high negative likelihood ratio of the test may help to mitigate the extra expense of treatment along with the increased risk of infection associated with overzealous treatment.
Our assessment of the quality of individual studies showed a moderate to low risk of bias. Also, including all studies with at least 10 patients ensured that our meta-analysis had higher power with narrow confidence intervals. The varied study designs and the nature of AAV itself (with myriad manifestations) contributed to high heterogeneity. Moreover, such high heterogeneity between studies is common in meta-analyses involving observational studies [7, 9, 10].
The limitation in the interpretation of the above results is that we did not stratify patients based on the type of therapy received, organ involvement and severity of relapse as that could have a bearing on the utility of ANCA as biomarkers. Also, most studies have more PR3-AAV as compared to MPO-AAV. The definition of ANCA “increase” varied amongst studies, and thus, we are not able to assess the minimum rise in titres to qualify as an increase.
The main focus of our analysis was to estimate the diagnostic accuracy of ANCA as a biomarker in predicting relapses. We would like to reiterate that escalating therapy in AAV should not be solely based on ANCA results. The ideal may be a composite model of clinical phenotype, organ involvement, previous therapy received, ANCA titres and type, inflammatory markers to predict the future risk of relapse for an individual patient.
Thus, in conclusion, the summary estimates of the accuracy of ANCA as a biomarker for relapses are likely to be near the true value despite heterogeneity between included studies. We found that a positive ANCA along with stratification based on testing methodology (IIF, ELISA), PR3 positivity, negative to positive conversion and rise of ANCA are consistent to support a moderate sensitivity and specificity of ANCA in predicting relapse in AAV. Balancing the risks of morbidity and mortality associated with relapses against the expense and adverse effects of immunosuppression demand the use of clinical acumen in addition to ANCA results. Our analysis paves the way for further exploration of this plausible biomarker in a more systematic way to assess the optimum frequency of monitoring and best cut-offs to define rise of ANCA titres.
References
Fauci AS, Wolff SM, Johnson JS (1971) Effect of cyclophosphamide upon the immune response in Wegener’s granulomatosis. N Engl J Med 285:1493–1496
de Groot K, Harper L, Jayne DRW, Flores Suarez LF, Gregorini G, Gross WL et al (2009) Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 150:670–680
Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS et al (2010) Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 363:221–232
Xiao H, Heeringa P, Hu P, Liu Z, Zhao M, Aratani Y et al (2002) Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J Clin Invest 110:955–963
Davies DJ, Moran JE, Niall JF, Ryan GB (1982) Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J 285:606
Birck R, Schmitt WH, Kaelsch IA, van der Woude FJ (2006) Serial ANCA determinations for monitoring disease activity in patients with ANCA-associated vasculitis: systematic review. Am J Kidney Dis Off J Natl Kidney Found 47:15–23
Tomasson G, Grayson PC, Mahr AD, Lavalley M, Merkel PA (2012) Value of ANCA measurements during remission to predict a relapse of ANCA-associated vasculitis—a meta-analysis. Rheumatology 51:100–109
Mukhtyar C, Flossmann O, Hellmich B, Bacon P, Cid M, Cohen-Tervaert JW et al (2008) Outcomes from studies of antineutrophil cytoplasm antibody associated vasculitis: a systematic review by the European league against rheumatism systemic vasculitis task force. Ann Rheum Dis 67:1004–1010
He P, Hu J-P, Tian X-J, He L-J, Sun S-R, Huang C (2020) Prevalence and risk factors of relapse in patients with ANCA-associated vasculitis receiving cyclophosphamide induction: a systematic review and meta-analysis of large observational studies. Rheumatology 60(3):1067–1079
King C, Druce KL, Nightingale P, Kay E, Basu N, Salama AD et al (2021) Predicting relapse in anti-neutrophil cytoplasmic antibody-associated vasculitis: a systematic review and meta-analysis. Rheumatol Adv Pract. https://doi.org/10.1093/rap/rkab018
Salameh J-P, Bossuyt PM, McGrath TA, Thombs BD, Hyde CJ, Macaskill P et al (2020) Preferred reporting items for systematic review and meta-analysis of diagnostic test accuracy studies [PRISMA-DTA]: explanation, elaboration, and checklist. BMJ 370:m2632
Bristol U 2021 QUADAS-2 University of Bristol; https://www.bristol.ac.uk/population-health-sciences/projects/quadas/quadas-2/
Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982–990
Han WK, Choi HK, Roth RM, McCluskey RT, Niles JL (2003) Serial ANCA titers: useful tool for prevention of relapses in ANCA-associated vasculitis. Kidney Int 63:1079–1085
Gapud EJ, Manno R, Seo P, Hanouneh M, Geetha D (2018) Long-term clinical course of antineutrophil cytoplasmic antibody-associated vasculitis patients off maintenance therapy. Cureus 10:e2372
Davenport A, Lock RJ, Wallington T (1995) Clinical significance of the serial measurement of autoantibodies to neutrophil cytoplasm using a standard indirect immunofluorescence test. Am J Nephrol 15:201–207
van Pesch V, Jadoul M, Lefèbvre C, Lauwerys BR, Tomasi JP, Devogelaer JP et al (1999) Clinical significance of antiproteinase 3 antibody positivity in cANCA-positive patients. Clin Rheumatol 18:279–282
Sinico RA, Radice A, Corace C, Toma DI, L, Sabadini E, (2006) Value of a new automated fluorescence immunoassay [EliA] for PR3 and MPO-ANCA in monitoring disease activity in ANCA-associated systemic vasculitis. Ann N Y Acad Sci 1050:185–192
Kemna MJ, Damoiseaux J, Austen J, Winkens B, Peters J, van Paassen P et al (2015) ANCA as a predictor of relapse: useful in patients with renal involvement but not in patients with nonrenal disease. J Am Soc Nephrol JASN 26:537–542
Bader L, Koldingsnes W, Nossent J (2010) B-lymphocyte activating factor levels are increased in patients with Wegener’s granulomatosis and inversely correlated with ANCA titer. Clin Rheumatol 29:1031–1035
Blockmans D, Stevens E, Mariën G, Bobbaers H (1998) Clinical spectrum associated with positive ANCA titres in 94 consecutive patients: is there a relation with PR-3 negative c-ANCA and hypergammaglobulinaemia? Ann Rheum Dis 57:141–145
Sanders J-SF, de Joode AAE, DeSevaux RG, Broekroelofs J, Voskuyl AE, van Paassen P et al (2016) Extended versus standard azathioprine maintenance therapy in newly diagnosed proteinase-3 anti-neutrophil cytoplasmic antibody-associated vasculitis patients who remain cytoplasmic anti-neutrophil cytoplasmic antibody-positive after induction of remission: a randomized clinical trial. Nephrol Dial Transp 31:1453–1459
Land J, Abdulahad WH, Arends S, Sanders J-SF, Stegeman CA, Heeringa P et al (2017) Prospective monitoring of in vitro produced PR3-ANCA does not improve relapse prediction in granulomatosis with polyangiitis. PLoS ONE 12:e0182549
Fijolek J, Wiatr E, Petroniec V, Augustynowicz-Kopec E, Bednarek M, Gawryluk D et al (2019) Antineutrophil cytoplasmic antibodies and their relationship with disease activity and presence of staphylococcal superantigens in nasal swabs in patients having granulomatosis with polyangiitis: results of a study involving 115 patients from a single center. Clin Rheumatol 38:3297–3305
Bulanov NM, Makarov EA, Shchegoleva EM, Zykova AS, Vinogradova ES, Novikov PI et al (2018) Relationship between serologic profile [ANCA type] and clinical features of renal involvement in ANCA-associated vasculitides. Ter Arkh 90:15–21
Ara J, Mirapeix E, Rodriguez R, Saurina A, Darnell A (1999) Relationship between ANCA and disease activity in small vessel vasculitis patients with anti-MPO ANCA. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc Eur Ren Assoc 14:1667–1672
Rheumatism BPGL and ELA (2018) Correction: Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial [MAINRITSAN2]. Ann Rheum Dis 78:e101–e101
Pettersson E, Heigl Z (1992) Antineutrophil cytoplasmic antibody [cANCA and pANCA] titers in relation to disease activity in patients with necrotizing vasculitis a longitudinal study. Clin Nephrol 37:219–28
Verstockt B, Bossuyt X, Vanderschueren S, Blockmans D (2015) There is no benefit in routinely monitoring ANCA titres in patients with granulomatosis with polyangiitis. Clin Exp Rheumatol 33:72–76
Rasmussen N, Salmela A, Ekstrand A, de Groot K, Gregorini G, Cohen Tervaert JW et al (2013) Changes in proteinase 3 anti-neutrophil cytoplasm autoantibody levels in early systemic granulomatosis with polyangiitis [Wegener’s] may reflect treatment rather than disease activity. Clin Exp Rheumatol 31:S38-44
Charles P, Terrier B, Perrodeau É, Cohen P, Faguer S, Huart A et al (2018) Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, phase III trial [MAINRITSAN2]. Ann Rheum Dis 77:1143–1149
Terrier B, Pagnoux C, Perrodeau É, Karras A, Khouatra C, Aumaître O et al (2018) Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis 77:1150–1156
Thompson GE, Fussner LA, Hummel AM, Schroeder DR, Silva F, Snyder MR et al (2020) Clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3 in ANCA-associated vasculitis. Front Immunol 11:2053
Morgan MD, Szeto M, Walsh M, Jayne D, Westman K, Rasmussen N et al (2017) Negative anti-neutrophil cytoplasm antibody at switch to maintenance therapy is associated with a reduced risk of relapse. Arthritis Res Ther 19:129
Terrier B, Saadoun D, Sène D, Ghillani P, Amoura Z, Deray G et al (2009) Antimyeloperoxidase antibodies are a useful marker of disease activity in antineutrophil cytoplasmic antibody-associated vasculitides. Ann Rheum Dis 68:1564–1571
Nowack R, Grab I, Flores-Suarèz LF, Schnülle P, Yard B, van der Woude FJ (2001) ANCA titres, even of IgG subclasses, and soluble CD14 fail to predict relapses in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 16:1631–1637
De Oliviera J, Gaskin G, Dash A, Rees AJ, Pusey CD (1995) Relationship between disease activity and anti-neutrophil cytoplasmic antibody concentration in long-term management of systemic vasculitis. Am J Kidney Dis 25(3):380–389
Jayne DR, Gaskin G, Pusey CD, Lockwood CM (1995) ANCA and predicting relapse in systemic vasculitis. QJM Mon J Assoc Physicians 88:127–133
Joshi L, Lightman SL, Salama AD, Shirodkar AL, Pusey CD, Taylor SRJ (2011) Rituximab in refractory ophthalmic Wegener’s granulomatosis: PR3 titers may predict relapse, but repeat treatment can be effective. Ophthalmology 118:2498–2503
Hogan SL, Falk RJ, Chin H, Cai J, Jennette CE, Jennette JC et al (2005) Predictors of relapse and treatment resistance in antineutrophil cytoplasmic antibody-associated small-vessel vasculitis. Ann Intern Med 143:621–631
Lin W, Chen M, Zhao M-H (2008) Follow-up of avidity and titer of anti-myeloperoxidase antibodies in sera from patients with primary ANCA-associated vasculitis. Autoimmunity 42:198–202
McClure ME, Wason J, Gopaluni S, Tieu J, Smith RM (2019) Jayne DR et al [1999] Evaluation of PR3-ANCA status after rituximab for ANCA-associated vasculitis. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis 25(5):217–223
Kyndt X, Reumaux D, Bridoux F, Tribout B, Bataille P, Hachulla E et al (1999) Serial measurements of antineutrophil cytoplasmic autoantibodies in patients with systemic vasculitis. Am J Med 106(5):527–533
Alberici F, Smith RM, Jones RB, Roberts DM, Willcocks LC, Chaudhry A et al (2015) Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology 54:1153–1160
Thai L-H, Charles P, Resche-Rigon M, Desseaux K, Guillevin L (2014) Are anti-proteinase-3 ANCA a useful marker of granulomatosis with polyangiitis [Wegener’s] relapses? Results of a retrospective study on 126 patients. Autoimmun Rev 13:313–318
Boomsma MM, Stegeman CA, van der Leij MJ, Oost W, Hermans J, Kallenberg CG et al (2001) Prediction of relapses in Wegener’s granulomatosis by measurement of antineutrophil cytoplasmic antibody levels: a prospective study. Arthr Rheumatol 43:2025–2033
Damoiseaux J, Andrade LE, Fritzler MJ, Shoenfeld, (2015) Autoantibodies 2015: From diagnostic biomarkers toward prediction, prognosis and prevention. Autoimmun Rev 14:555–563
Finkielman JD, Merkel PA, Schroeder D, Hoffman GS, Spiera R, St Clair EW et al (2007) Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 147:611–619
Watanabe S, Nakashima I, Misu T, Miyazawa I, Shiga Y, Fujihara K et al (2007) Therapeutic efficacy of plasma exchange in NMO-IgG-positive patients with neuromyelitis optica. Mult Scler Houndmills Basingstoke Engl 13:128–132
Yamaguchi M, Ando M, Kato S, Katsuno T, Kato N, Kosugi T et al (2015) Increase of Antimyeloperoxidase antineutrophil cytoplasmic antibody [ANCA] in patients with renal anca-associated vasculitis: association with risk to relapse. J Rheumatol 42:1853–1860
Lurati-Ruiz F, Spertini F (2005) Predictive value of antineutrophil cytoplasmic antibodies in small-vessel vasculitis. J Rheumatol 32:2167–2172
Fussner LA, Hummel AM, Schroeder DR, Silva F, Cartin-Ceba R, Snyder MR et al (2016) Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthr Rheum 68:1700–1710
Girard T, Mahr A, Noël LH, Cordier JF, Lesavre P, André MH et al (2001) Are antineutrophil cytoplasmic antibodies a marker predictive of relapse in Wegener’s granulomatosis? a prospective study. Rheumatology 40:147–151
van Dam LS, Dirikgil E, Bredewold EW, Ray A, Bakker JA, van Kooten C et al (2020) PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab. Nephrol Dial Transpl 36:1408–1417
Walsh M, Flossmann O, Berden A, Westman K, Höglund P, Stegeman C et al (2012) Risk factors for relapse of antineutrophil cytoplasmic antibody-associated vasculitis. Arthr Rheum 64:542–548
de Joode AAE, Sanders JSF, Puéchal X, Guillevin LP, Hiemstra TF, Flossmann O et al (2017) Long term azathioprine maintenance therapy in ANCA-associated vasculitis: combined results of long-term follow-up data. Rheumatology 56:1894–1901
Gaskin G, Savage CO, Ryan JJ, Jones S, Rees AJ, Lockwood CM et al (1991) Anti-neutrophil cytoplasmic antibodies and disease activity during long-term follow-up of 70 patients with systemic vasculitis. Nephrol Dial Transp 6:689–94
Author information
Authors and Affiliations
Contributions
PM and SA were involved in the conceptualization; SA, AB and PM were involved in the data extraction; MP was involved in the data analysis. PM, AB, MP, SA, SP were involved in the writing and reviewing of the manuscript. All authors have approved of the final manuscript and take full responsibility for the same.
Corresponding author
Ethics declarations
Conflict of interest
SA has received honorarium as speaker from Pfizer, DrReddy’s, Cipla and Novartis (unrelated to the current work). The other authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mehta, P., Balakrishnan, A., Phatak, S. et al. Diagnostic accuracy of antineutrophil cytoplasmic antibodies (ANCA) in predicting relapses of ANCA-associated vasculitis: systematic review and meta-analysis. Rheumatol Int 43, 437–448 (2023). https://doi.org/10.1007/s00296-022-05192-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-022-05192-3