Abstract
Chronic inflammation starting early in life and continuing into adulthood may predispose children with Juvenile Idiopathic Arthritis (JIA) to cardiovascular (CV) complications. To compare non-invasive CV risk markers- left ventricular mass index (LVMi), brachial artery flow mediated dilatation (FMD) and carotid artery intima-media thickness (CIMT) between patients with JIA and healthy controls. Measurements of LVMi, CIMT and FMD and lipid profile were compared between 4 and 18 year old 81 patients with JIA and 78 age and sex matched healthy controls. Among 81, 20 had systemic onset, 19 enthesitis related arthritis, 9 polyarticular rheumatoid factor (RF) + ve, 19 polyarticular RF –ve, 11 oligo-articular, and 3 un-differentiated JIA. FMD was significantly lower (p < 0.001), CIMT and LVMi significantly higher in patients (p ≤ 0.001). CIMT showed positive correlation with blood pressure (p = 0.001), disease duration (p ≤ 0.001) and negative correlation with high density lipoprotein (HDL) (p ≤ 0.001). FMD correlated positively with HDL (p = 0.006) and negatively with disease duration (p ≤ 0.001). CIMT (p = 0.017) and FMD (p = 0.04) were significantly worse in active than inactive disease. Children with JIA have worse lipid profile, increased LVMi, CIMT, and reduced brachial artery FMD, suggestive of early cardiovascular dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile Idiopathic Arthritis (JIA), is the most common rheumatic disease in children associated with chronic joint inflammation and long term complications [1]. Most of the published data on JIA are from developed countries with an annual incidence of 16–150 per 100,000 children [2]. A few studies are published from South Asia that report many clinical differences compared to Caucasians [3, 4] and a prevalence of ~ 48 per 100,000 [5]. It is a rare disease that is often diagnosed late due to lack of awareness among paediatricians, leading to complications of chronic inflammation and reduced quality of life [6, 7]. The link between chronic inflammation and atherosclerosis is well established. Early changes start with epithelial dysfunction that progresses to atherosclerosis and its complications over time. Diseases associated with chronic inflammation including adult rheumatic diseases e.g. rheumatoid arthritis (RA) are reported to have a higher incidence of cardiovascular morbidity and mortality [8].
With the onset of disease in childhood and its extension into adulthood, long periods of exposure to chronic inflammation may place JIA patients at high risk to develop CV complications. The etiological factors for JIA are shared with adult rheumatic diseases [9]. In the case of adults with RA, European League against Rheumatism (EULAR) recommends CV risk assessment once a year for all patients [10]. Whether such screening will be useful in children and adolescents with JIA is not known. As the chronic inflammation in these patients starts at a much younger age and continues for prolonged periods of time [11] early detection of cardiovascular changes may provide a window of opportunity to initiate preventive measures. Regular screening may help to potentially reduce morbidity and mortality later in life.
Ultrasonographic markers for endothelial dysfunction are well accepted non-invasive surrogate markers for early changes that precede atherosclerosis [12, 13]. A few researchers have studied this aspect of JIA with differing results [14,15,16,17]. We studied the traditional and ultrasonographic markers for assessment of CVD risk in children with JIA and compared with healthy controls to understand whether early screening could be useful for them.
Patients and methods
Ninety patients with JIA, attending the Pediatrics and Clinical Immunology departments of a tertiary care teaching hospital were screened. Two were excluded due to revised diagnosis and parents of seven refused consent. Age- and sex-matched healthy controls were recruited from among children attending hospital for immunisation or minor injuries or accompanying a sick sibling. Finally, 81 patients aged 4–18 years, and 78 controls were enrolled. Those with a history of direct or indirect exposure to smoke or family history of early (< 50 years age) cardiovascular morbidity or mortality were excluded from both groups. Approval for the study was granted by the institute ethics committee. Written informed consent was obtained from parents of all participants and assent of participants where appropriate.
The demographic data and serum lipid profile, erythrocyte sedimentation rate (ESR, normal < 15 mm in the first hour), high sensitivity C-reactive protein (hsCRP, normal < 1.0 mg/dL) and anti-nuclear antibody (ANA) status of patients were recorded from available records. A dilution of 1:100 was taken as the threshold for ANA positivity. Rheumatoid factor (RF) was analysed by nephelometry. American college of Rheumatology (ACR) criteria were used to differentiate between the presence and absence of disease activity [18]. Brachial artery flow-mediated dilatation (FMD) and carotid artery intima-media thickness (CIMT) were assessed by the trained cardiologist, blinded to the patient/control status of participants. Parents of the participants were present throughout the study to make the children comfortable. The study was performed with an Acuson Sequoia 512 Ultrasound Instrument (Siemens, Germany) with 6–10 MHz linear array transducer.
For CIMT, carotid arteries were imaged in longitudinal section and digitally recorded during systole for offline measurement as described earlier [19]. Brachial artery diameter was recorded for 3 min in longitudinal section, measurements gated to ECG. Ejection fraction, fractional shortening and LV mass measurements were recorded on 2D echocardiography. Left ventricular mass index (LVMi) was calculated by dividing LV mass by body surface area.
Statistical analysis
SPSS version 19 was used to analyse data. Categorical data were compared by Chi-square or Fisher’s exact test. Normally distributed continuous variables are presented as mean with standard deviation and compared using independent Student t test. Non-normal data are presented as median with range and compared by Mann–Whitney U or Kruskal–Wallis test. A p value < 0.05 was considered significant.
Results
We studied 81 patients with JIA and 78 age- and sex-matched healthy controls for CV risk markers. Clinical characteristics and laboratory investigations of patients are depicted in Table 1. Normality in the distribution of numeric variables was checked visually by P-P plot and statistically by Shapiro–Wilk test using SPSS 19.0 software. Baseline characteristics (Table 1) were comparable between patients and controls except mean BMI (p = 0.005). Patients had significantly higher systolic BP than controls (p < 0.001), but similar diastolic BP, though none had hypertension according to nomograms for South Indian children [20]. HLA-B 27, rheumatoid factor (RF) and ANA status of patients is given in Table 1. Mean ESR of patients was elevated and hsCRP was raised in 85% patients though only 42% had evidence of disease activity (Table 1). Mean HDL level was significantly lower and total cholesterol and LDL levels higher in patients (p < 0.001).
On comparing ultrasonographic parameters, there was a significant difference in CIMT, absolute and proportionate change in FMD and LVMi between patients and controls (p ≤ 0.001) (Table 2). CIMT was significantly higher and change in FMD lower in patients with active compared to inactive disease though the difference was less striking than that between patients and healthy controls (Table 3). Common carotid artery lumen diameter (CCA LD), ejection fraction (EF), fractional shortening (FS) and LVMi were similar between patients and controls (Table 2), and between active and inactive disease (Table 3). Comparison among subclasses of JIA did not reveal any significant difference. Steroid administration was also not found to be associated with any of the CV risk markers.
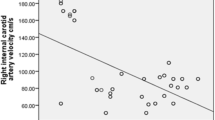
We found a significant positive correlation of CIMT with blood pressure, total and LDL cholesterol levels and negative correlation with HDL (Table 4). Absolute and proportionate change in FMD correlated positively with HDL and negatively with a duration of disease (p ≤ 0.001). Both FMD and CIMT correlated with duration of disease (p < 0.001) (Table 4).
Multiple linear regression analysis for LVMi, CIMT, absolute and FMD as dependent variables with traditional CV risk factors did not reveal any significant predictor for CIMT, FMD or LVMi.
Discussion
Subclinical atherosclerosis associated with chronic inflammatory processes, the harbinger of later cardiovascular complications has not been studied well in JIA, the most common childhood-onset chronic arthritis. We compared the traditional and non-invasive ultrasonographic surrogate markers for early atherosclerosis and its precursor endothelial dysfunction in 5–18 years old JIA patients with age- and gender-matched healthy controls. We found raised ESR, high hsCRP (even in patients with inactive disease) and worse lipid profile among patients with JIA. Ultrasonographic evaluation revealed significantly increased CIMT and LVMi and reduced brachial artery FMD among patients, indicating the presence of subclinical cardiovascular changes that may progress to atherosclerosis with chronic exposure to inflammation. The non-invasive risk markers also differed significantly between patients with active or inactive disease and showed a significant correlation with duration of disease.
Though ERA is the most common subtype of JIA in India [21], sicker patients with SoJIA made the most common class among our patients. The significantly lower mean BMI in our patients maybe the result of malnutrition associated with chronic inflammation, previously noted in JIA patients by other investigators [22, 23]. As low BMI is associated with increased CVD risk in adult patients with RA, the same maybe true for children [24]. Despite undernutrition, their reduced lean body mass and increased proportion of total body fat [25] may contribute to increased CVD risk. Lipid profile, the well-established risk factor for CVD was significantly worse among our patients than controls. Total cholesterol and LDL were significantly higher (p < 0.001), while HDL cholesterol was much below the normal range for age and significantly lower (p < 0.001) than controls.
The mean ESR was raised and high sensitivity CRP (hsCRP), a sensitive inflammatory marker, was elevated in 85% of our patients though 57% did not have active disease, suggestive of underlying chronic inflammation even in the clinically inactive phase of the disease. Such chronic inflammation predisposes to rise in the risk of developing CVD as reported in RA and other adult chronic inflammatory arthritic diseases [26, 27] supported by reports that vascular stiffness and cardiovascular dysfunction are more common in adult RA patients than healthy controls [28].
The blood pressure of our patients was higher than controls with a statistically significant difference in systolic BP (p ≤ 0.001) suggesting early compromise in cardiovascular compliance, though none had hypertension. Higher than normal BP, CIMT, LVMi, and lower FMD in JIA patients have been reported earlier [29]. Some studies have reported relatively lower CIMT in patients classified under milder subtype of JIA [30, 31] unlike the findings of ours and another study [32], supporting the premise that these changes may be less significant with less severe inflammation. Reduced arterial FMD was reported in obese children compared to non-obese, supporting endothelial dysfunction as an early marker of preclinical atherosclerosis [33]. Similarly, lower FMD among our patients compared to controls indicates some loss of arterial wall compliance, suggestive of early atherosclerotic changes.
We found a significant correlation of FMD and CIMT with duration of disease. This finding suggests that CV damage in patients with JIA progresses with ongoing exposure to inflammation. Thus, JIA patients with earlier onset and longer duration of chronic inflammation may be at higher risk of CV complications. Positive correlation of CIMT with BP, LDL cholesterol, its negative correlation with HDL cholesterol, and positive correlation between FMD and HDL cholesterol indicate that the deleterious changes in risk factors for CVD go hand in hand as a response to chronic inflammation in JIA.
Our patients with active disease had significantly higher CIMT and reduced change in FMD than those with inactive disease. The increased burden of acute inflammation in active stage of disease may be associated with enhanced vascular endothelial dysfunction. Patients with active disease had higher BMI than patients with inactive disease, though still less than normal children. Reduced physical activity and treatment with steroids during disease activity are likely to contribute to weight gain and increase in BMI.
We did not find an association of steroid administration with any of the risk markers that may be explained by offsetting of their atherosclerotic effect by a reduction in inflammation. With the dual effect of steroids as anti-inflammatory drugs and atherosclerosis promoting agents, it is difficult to clearly define the role of steroids in JIA.
Our study provides the evidence and brings into focus the need to incorporate screening for early CV changes as part of the regular follow-up of patients with JIA. As there is very little information on the important aspect of early-onset and ongoing development of atherosclerosis that can have devastating complications in future but are preventable by timely and appropriate interventions, this information sets the stage for multicentric studies with larger sample size in individual subclasses of JIA to identify the subsets of patients who may benefit the most through early intervention. However, we suggest that cardiovascular health-promoting interventions e.g. preventive dietary modifications, appropriate physical activity, avoiding smoking and alcohol intake etc. can be useful for all patients with JIA irrespective of the severity of the disease.
The limitation of our study is the small number of patients in individual subclasses of JIA. The degree of inflammation differs significantly among JIA subgroups, however, we could not evaluate the risk factors among individual classes though the risk of CVD is expected to vary with severity of disease.
Conclusion
Our study found significantly worse levels of traditional and ultrasonographic markers of endothelial and cardiovascular dysfunction at an early age in children and adolescents with JIA, suggesting early-onset subclinical atherosclerotic changes. We suggest that regular cardiovascular follow up is important for patients of JIA, especially those with a longer duration of active disease. This will provide an opportunity to initiate preventive interventions to reduce the risk of future cardiovascular complications.
Data availability
All data are available.
Code availability
Not applicable.
References
Ravelli A, Martini A (2007) Juvenile idiopathic arthritis. Lancet 369(9563):767–778
Prakken B, Albani S, Martini A (2011) Juvenile idiopathic arthritis. Lancet 377(9783):2138–2149
Jariwala M, Aggarwal M, Sawhney S (2012) Diagnostic value of the Assessment of Spondyloarthropathy International Society (ASAS) criteria for children with Enthesitis Related Arthritis (ERA): a single center study of 124 patients. Arthritis Rheum 64:S718
Roussou E, Chopra S, Ngandu DL (2013) Phenotypic and clinical differences between Caucasian and South Asian patients with psoriatic arthritis living in Northeast London. Clin Rheumatol 32(5):591–599
Abujam B, Mishra R, Aggarwal A (2014) Prevalence of musculoskeletal complaints and juvenile idiopathic arthritis in children from a developing country: a school-based study. Int J Rheum Dis 17(3):256–260
Pavo MR, de Inocencio J (2019) Pediatrician beliefs about Juvenile Idiopathic Arthritis may result in referral delays: a Spanish national survey. J Pediatrics 209(6):236–239.e2
Aggarwal R, Malaviya AN (2009) Diagnosis delay in patients with ankylosing spondylitis: factors and outcomes—an Indian perspective. Clin Rheumatol 28(3):327–331
del Rincón ID, Williams K, Stern MP, Freeman GL, Escalante A (2001) High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum 44(12):2737–2745
Hinks A, Barton A, John S, Bruce I, Hawkins C, Griffiths CEM, Donn R, Thomson W, Silman A, Worthington J (2005) Association between the PTPN22 gene and rheumatoid arthritis and juvenile idiopathic arthritis in a UK population: further support that PTPN22 is an autoimmunity gene. Arthritis Rheum 52(6):1694–1699
Peters MJL, Symmons DPM, McCarey D, Dijkmans BAC, Nicola P, Kvien TK et al (2010) EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69(2):325–331
Bohr A-H, Fuhlbrigge RC, Pedersen FK, de Ferranti SD, Müller K (2016) Premature subclinical atherosclerosis in children and young adults with juvenile idiopathic arthritis. A review considering preventive measures. Pediatr Rheumatol Online J 14(1):3
Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L et al (2009) Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension 54(5):919–950
Satija M, Yadav TP, Sachdev N, Chhabra A, Jahan A, Dewan V (2014) Endothelial function, arterial wall mechanics and intima media thickness in juvenile idiopathic arthritis. Clin Exp Rheumatol 32(3):432–439
Sozeri B, Atikan BY, Ozdemir K, Mir S (2016) Assessment of vascular function in systemic onset juvenile idiopathic arthritis. Clin Rheumatol 35(7):1699–1703
Aulie HA, Selvaag AM, Günther A, Lilleby V, Molberg Ø, Hartmann A, Holdaas H, Flatø B (2015) Arterial haemodynamics and coronary artery calcification in adult patients with juvenile idiopathic arthritis. Ann Rheum Dis 74(8):1515–1521
Evensen K, Aulie HA, Rønning OM, Flatø B, Russell D (2016) Carotid atherosclerosis in adult patients with persistently active juvenile idiopathic arthritis compared with healthy controls. J Rheumatol 43(4):810–815
Del Giudice E, Dilillo A, Tromba L, La Torre G, Blasi S, Conti F, Viola F, Cucchiara S, Duse M (2018) Aortic, carotid intima-media thickness and flow-mediated dilation as markers of early atherosclerosis in a cohort of pediatric patients with rheumatic diseases. Clin Rheumatol 37(6):1675–1682
Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N et al (2011) American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res 63(7):929–936
Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA et al (2002) Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 39(2):257–265
Sayeemuddin M, Sharma D, Pandita A, Sultana T, Shastri S (2015) Blood pressure profile in school children (6–16 years) of Southern India: a prospective observational study. Front Pediatr 3:24
Srivastava R, Agnihotry S, Aggarwal R, Bajpai P, Aggarwal A (2015) HLA-B27 subtypes in enthesitis-related arthritis category of juvenile idiopathic arthritis and ankylosing spondylitis in northern India. Clin Exp Rheumatol 33(6):931–935
Cleary AG, Lancaster GA, Annan F, Sills JA, Davidson JE (2004) Nutritional impairment in juvenile idiopathic arthritis. Rheumatol Oxf Engl 43(12):1569–1573
Haugen MA, Lien G, Flatø B, Kvammen JA, Vinje O, Sørskaar D, et al (2002) Minor impact of juvenile arthritis on nutritional status in young adult patients. Arthritis Rheum 15;47(6):623–629.
Kremers HM, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE (2004) Prognostic importance of low body mass index in relation to cardiovascular mortality in rheumatoid arthritis. Arthritis Rheum 50(11):3450–3457
Bechtold S, Roth J (2009) Natural history of growth and body composition in juvenile idiopathic arthritis. Horm Res 72(Suppl 1):13–19
Sattar N, McCarey DW, Capell H, McInnes IB (2003) Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 108(24):2957–2963
Kramer HR, Giles JT (2011) Cardiovascular disease risk in rheumatoid arthritis: progress, debate, and opportunity. Arthritis Care Res 63(4):484–499
Protogerou AD, Zampeli E, Fragiadaki K, Stamatelopoulos K, Papamichael C, Sfikakis PP (2011) A pilot study of endothelial dysfunction and aortic stiffness after interleukin-6 receptor inhibition in rheumatoid arthritis. Atherosclerosis 219(2):734–736
Głowińska-Olszewska B, Bossowski A, Dobreńko E, Hryniewicz A, Konstantynowicz J, Milewski R et al (2013) Subclinical cardiovascular system changes in obese patients with juvenile idiopathic arthritis. Mediators Inflamm 2013:436702
Jednacz E, Rutkowska-Sak L (2015) Assessment of the body composition and parameters of the cardiovascular risk in juvenile idiopathic arthritis. Biomed Res Int 2015:619023
Vlahos AP, Theocharis P, Bechlioulis A, Naka KK, Vakalis K, Papamichael ND et al (2011) Changes in vascular function and structure in juvenile idiopathic arthritis. Arthritis Care Res 63(12):1736–1744
Breda L, Di Marzio D, Giannini C, Gaspari S, Nozzi M, Scarinci A et al (2013) Relationship between inflammatory markers, oxidant-antioxidant status and intima-media thickness in prepubertal children with juvenile idiopathic arthritis. Clin Res Cardiol 102(1):63–71
Aggoun Y, Farpour-Lambert NJ, Marchand LM, Golay E, Maggio ABR, Beghetti M (2008) Impaired endothelial and smooth muscle functions and arterial stiffness appear before puberty in obese children and are associated with elevated ambulatory blood pressure. Eur Heart J 29(6):792–799
Funding
No funds were received from any agency for the study.
Author information
Authors and Affiliations
Contributions
KSH collected the data, reviewed the literature and drafted the first manuscript. RG conceptualized the study, reviewed the literature and critically reviewed the manuscript. SS performed the ultrasonographic cardiovascular assessment of participants and critically reviewed the manuscript. VSN participated in conceptualizing the study and critically reviewed the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
KS Hussain, R Gulati, Santhosh S and VS Negi declare that they have no conflict of interest.
Ethics approval
Approval for the study was granted by the Institute Ethics Committee. Institute Ethics Committee (Human studies), JIPMER, India. Protocol approval number JlP/lEC/2O14/8/423 date 05/12/2014.
Consent to participate
Written informed consent was received from parents of all participants and assent of participants where appropriate.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hussain, K.S., Gulati, R., Satheesh, S. et al. Early-onset subclinical cardiovascular damage assessed by non-invasive methods in children with Juvenile Idiopathic Arthritis: analytical cross-sectional study. Rheumatol Int 41, 423–429 (2021). https://doi.org/10.1007/s00296-020-04689-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-020-04689-z