Abstract
This study aimed to identify the parameters related to the area and adipose tissue content of thigh muscles that are associated with radiographic knee osteoarthritis grade. Fifty patients (mean age ± standard deviation, 73.0 ± 4.5 years) were divided into early osteoarthritis (n = 23) and established osteoarthritis (n = 27) groups based on Kellgren–Lawrence classification. The femorotibial angle was measured from anteroposterior radiographs of the lower limbs. Individual thigh muscle and adipose tissue areas were analyzed using axial T1-weighted magnetic resonance imaging. After intergroup comparison, logistic regression analysis was performed to determine independent parameters associated with established osteoarthritis. Moreover, correlation coefficients were assessed between the left–right differences of osteoarthritis grade and parameters. Established osteoarthritis exhibited a significantly greater femorotibial angle and increased adipose tissue content in the subcutaneous, intermuscle, and intramuscle of the adductor, vastus lateralis, vastus intermedius, as well as a lower vastus medialis area, in comparison to early osteoarthritis. A greater femorotibial angle, increased intermuscular adipose tissue, and a lower vastus medialis area to knee extensor ratio were significantly independently associated with established osteoarthritis (odds ratio 3.2, 1.8, and 2.0, respectively). The left–right differences of femorotibial angle and vastus medialis area were significantly correlated with osteoarthritis grade, whereas adipose tissue content had no significant correlations with osteoarthritis grade. Greater femorotibial angle and lower vastus medialis area were related with higher osteoarthritis grade. Greater intermuscular adipose tissue content was associated with established osteoarthritis; however, in the left–right differences, adipose tissue content was not related with osteoarthritis grade.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In general, knee osteoarthritis (KOA) is characterized by medial knee joint space narrowing, chronic knee pain, and knee varus deformities, often impairing activities of daily living (ADLs) in affected patients. Muscle strengthening exercises are recommended by the Osteoarthritis Research Society International (OARSI) Guidelines for the treatment of KOA [1]. In particular, knee extensor function is important for stabilizing the knee joint [2]. Winby et al. [3] reported that the muscles around the knee contribute to greater than 50% of knee stabilization during gait; in particular, the knee extensors contribute to more than 40% of knee stabilization. Previous studies have shown an association between knee extensor weakness and radiographic KOA and knee pain [4, 5].
From a clinical point of view, the evaluation of knee extensor strength is difficult in patients with KOA because the performance of knee extension tasks is associated with considerable knee pain. Blazevich et al. [6] proposed muscle volume measurement as an alternative gold standard for estimating knee extensor strength. Moreover, simpler methods of muscle volume estimation based on cross-sectional area (CSA) or muscle thickness have been reported and are widely used as indices of muscle strength. However, recent studies have shown that knee extensor CSA is not associated with KOA and/or knee pain [7,8,9,10,11]. Studies from other authors have shown that thigh subcutaneous adipose tissue (SAT), adipose tissue between muscles [intermuscular adipose tissue (inter-MAT)], and adipose tissue within muscles [intramuscular adipose tissue (intra-MAT)] but not knee extensor CSA are associated with KOA and/or knee pain [7, 9, 10, 12]. On the other hand, some authors have demonstrated that vastus medialis (VM) CSA loss is associated with increasing knee pain and KOA progression [13, 14]. Pan et al. [15] reported that greater vastus lateralis (VL)/VM CSA ratios were associated with better cartilage health in patients with KOA.
Therefore, the evaluation of individual thigh muscles and adipose tissue may be more important than the measurement of knee extensor CSA for detecting KOA-related thigh morphology. However, in a comparison between patients, the determination of whether individual thigh muscle CSA and adipose tissue content is more strongly associated with radiographic KOA grade and knee pain remains unclear. Moreover, whether these parameters associated with radiographic KOA grade and knee pain in a comparison between patients with KOA will be the same when analyzing the left and right sides of the same patient also remains unclear. This cross-sectional study sought to identify thigh muscle CSA and adipose tissue content associated with established radiographic KOA in a comparison between patients with KOA. Our secondary objective was to investigate thigh muscle CSA and adipose tissue content as it relates to knee pain in patients with KOA and to determine the left–right differences in parameters related to KOA grade and knee pain in the same patient.
Patients and methods
Patients
Fifty patients aged 60–80 years were included in this study who complained of knee pain in one and/or both knees, were able to walk without using any knee braces and walking aids, and were diagnosed with medial KOA using anteroposterior radiographs of both knees while standing on both legs with knees extended. A patient who had trauma and/or surgery on his/her lower limbs as past history was excluded. KOA grade was classified by the first author using the Kellgren–Lawrence classification (greater grades indicating worse disease). The classification was performed two times separately by the first author to evaluate intraobserver reproducibility. When the two classification results were inconsistent, the first classified grade was used. In the intergroup comparison between patients with KOA, the knee with the worse KOA grade was selected; the more painful knee was selected in cases with bilateral KOA with identical grades. Patients with radiographic KOA grade 1 were defined as early KOA (n = 23) and those with KOA grade ≥ 2 were defined as established KOA (n = 27). To analyze the right and left sides in the same patient, we studied left–right differences in 50 patients. Thirty-six patients had the same KOA grade in both knees, and 14 patients had the left–right difference with KOA grade ≥ 1. This study was conducted with the approval of the Institutional Review Board of Akita Hospital (approval date, 6. Jan. 2015; number, 56824). Written informed consent was obtained from all participants.
Assessment
Measurements of anatomical axis and self-reported knee score
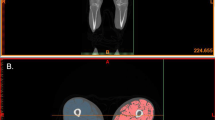
A weight-bearing anteroposterior radiograph of the entire lower extremities was obtained that included both legs; the radiograph was taken while the patient was in standing position with the knees extended, and there was a 2-m distance between knees and the X-ray bulb. The X-rays were acquired by a radiologist from Akita Hospital. The anatomical axis of the limb was measured from the radiograph as the femorotibial angle (FTA) between the femur anatomical axis and the tibia mechanical axis using Management Integrate Network Diagnosis Solution (MIND Solution; LEOCLAN Co., Ltd., Osaka, Japan) by the first author. The femur anatomical axis was measured from the distal half of the femur, and the tibia mechanical axis was defined as a line connecting the center of the knee and the ankle (Fig. 1) [16]. The measurement of FTA in the right side of randomly selected 20 patients was performed by the first author two times separately to evaluate intraobserver reproducibility. The Japanese Knee Osteoarthritis Measure (JKOM) score (100 full points) was used to evaluate knee pain (32 full points), ADLs (60 full points), and health perception (8 full points); higher JKOM scores were worse [17].
Weight-bearing anteroposterior radiograph of the entire lower extremities. Solid lines represent the femur anatomical axis in the distal one-half of the femur, and dotted lines represent the tibial mechanical axis between the center of the knee and the ankle. Lateral angles (femorotibial angle) are the anatomical axes of the limbs
Measurement of individual thigh muscle CSAs
A magnetic resonance imaging (MRI)-based measurement method for individual thigh muscle CSAs was used (1.5-T, Excel ART; Canon Corp., Tokyo, Japan). T1-weighted spin-echo with 10-mm-thick axial images (TR/TE, 625/15 ms; field of view, 250 mm; 512 × 512 matrix) were taken using a body coil. Yamauchi et al. [18] reported that the single CSA at the 10% distal region from the mid-thigh between the distal end of the lesser trochanter and the proximal end of the patella accurately reflected the individual muscle volume in elderly people, even if they had KOA. Therefore, in the present study, CSA at the 10% distal region was analyzed.
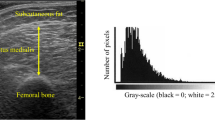
Individual thigh muscle CSAs were measured with manual segmentation using Medical Image Processing, Analysis, and Visualization software (MIPAV, v 7.1.1; National Institutes of Health, Bethesda, MD, USA) (Fig. 2). To calculate thigh muscle CSAs, those for rectus femoris (RF), VL, vastus intermedius (VI), VM, biceps femoris short head (BFS), biceps femoris long head (BFL), semitendinosus (ST), semimembranosus (SM), adductor (ADD), sartorius (SAR), and gracilis (GRA) were summed. To calculate knee extensor muscle, CSA, RF, VL, VI, and VM CSAs were summed. To calculate knee flexor muscle, CSA, BFS, BFL, ST, and SM CSAs were summed. The ratios of CSAs of the knee extensor, knee flexor, and ADD muscles to those of the thigh were calculated. The ratios of the individual knee extensor CSAs to the knee extensor and individual knee flexor CSAs to the knee flexor were calculated. The reproducibility of individual thigh muscle CSA measurement was excellent, as reported previously [18]. For the intraobserver reproducibility of the CSA measurement, the standard error of measurement (SEM) for the individual thigh muscle CSAs measurement was mean 3.7% and the SEM for the measurement of the individual thigh muscle CSA to muscle group ratios was mean 0.79%.
The dotted line represents the myofascial cross-sectional area (CSA) and solid lines are individual thigh muscle CSAs (left image). The white area within the myofascia is inter-MAT (muscle adipose tissue) (right upper quadrant), and the white area within individual muscle is intra-MAT (right lower quadrant)
Measurements of SAT, thigh inter-MAT, and individual thigh intra-MAT CSAs
Thigh circumference and myofascial CSA at the 10% distal region were semi-automatically segmented using MIPAV software (Fig. 1). Yoshiko et al. [19] reported that significant differences in the intra-MAT composition between healthy young individuals and elderly individuals were observed at the distal region of the thigh. Therefore, CSA at the 10% distal region was analyzed in the present study. SAT CSA (cm2) was calculated as thigh circumference CSA—myofascial CSA. Inter-MAT and intra-MAT areas (cm²) were measured by a radiologist from Akita Hospital using MIPAV software as follows [19, 20]: (1) The image heterogeneity at the distal 10% region was corrected using a nonparametric nonuniform intensity normalization (N3) algorithm. (2) In the corrected image, four regions of interest of 5 mm × 5 mm were set on muscle tissue and adipose tissue to define the Otsu threshold value using histogram shape-based thresholding techniques. Three times the mean values were used as the Otsu threshold value; (3) the pixel with higher value than Otsu threshold value was recognized as adipose tissue and the adipose tissue area was calculated. The inter-MAT area (cm2) was calculated as the adipose tissue within the myofascial CSA, excluding individual muscles, vessels, nerve tissue and bone area (Fig. 1, right upper quadrant). The intra-MAT area (cm2) was calculated within individual muscles (Fig. 1, right lower quadrant). To test the reproducibility of MAT measurement, inter and intra-MAT of the right thighs of randomly selected patients were analyzed twice by the radiologist, with a 2-month interval between the two measurement times.
Statistics
An a priori power analysis of Student’s t test between two groups in the parameters of SAT and inter-MAT area, and VM CSA revealed that a total sample size of 50 corresponded to a power = 0.8, p value = 0.05 and effect size d = 0.8 in allocation ratio = 1. The power analysis was performed using G*Power 3.1. software (Heinrich-Heine-Universität, Düsseldorf, Germany).
Intergroup comparisons of the parameters of patient characteristics, JKOM scores, thigh muscle CSA, and adipose tissue area were performed between early KOA and established KOA using Student’s t test if the data were distributed normally. When the data were not distributed normally, Mann–Whitney U tests were performed. When Student’s t test was performed, the data were expressed as the means ± standard deviation (SD), whereas data were expressed as medians (first quartile to third quartile) for Mann–Whitney U tests. The data distribution was tested by Shapiro–Wilk test. To examine sex differences between two groups, Chi-squared test was performed. To examine multicollinearity, correlation coefficients were assessed among the parameters that showed significant differences in the intergroup comparison for all observed parameters except the JKOM score. The Pearson correlation coefficient (r) was assessed for normally distributed data, and the Spearman correlation coefficient (rs) was assessed for nonnormally distributed data. Correlation coefficients ≥ 0.7 were considered to indicate a strong correlation, correlation coefficients < 0.7 and ≥ 0.4 indicated a moderate correlation, and correlation coefficients < 0.4 indicated a weak correlation. Finally, a stepwise logistic regression analysis was performed to determine independent parameters associated with early KOA and established KOA.
To detect parameters related to knee pain in patients with KOA, a correlation between knee pain score and BMI, FTA, thigh muscle CSA, and thigh adipose tissue area was examined. The Spearman correlation coefficient (rs) was assessed because knee pain score data were not normally distributed.
To detect parameters related with the left–right difference of KOA grade and knee pain in the same patient, a correlation analysis between the left–right differences of the parameters (FTA, thigh muscle CSA, and adipose tissue area) and the KOA grade and pain scores was performed using the Spearman correlation coefficient (rs) because the data were not normally distributed. The left–right differences in KOA grade, knee pain scores, and FTA were calculated as right–left. The left–right differences in individual muscle CSAs and adipose tissue areas were calculated as right/left ratios.
The kappa coefficient was assessed to test the intraobserver reproducibility of the KOA grade classification (kappa coefficients ≥ 0.81 were defined as almost perfect, kappa coefficients ≤ 0.80 and ≥ 0.61 were defined as substantial, kappa coefficients ≤ 0.60 and ≥ 0.41 were defined as moderate, and kappa coefficients ≤ 0.40 and ≥ 0.21 were defined as fair). To test the intraobserver reproducibility of the measurement of FTA and inter and intra-MAT area, an intraclass coefficient was assessed. The cut-offs of intraclass coefficient were same as those for the kappa coefficient.
All statistical tests were performed using SPSS statistical software (version 22; SPSS, Inc., Chicago, IL, USA). p values of < 0.05 were considered statistically significant.
Results
Patient characteristics and JKOM scores
Patients with KOA grade ≥ 2 had greater BMI and FTA than those with KOA grade = 1. In terms of total JKOM, pain, ADLs, and health perception scores, patients with KOA grade ≥ 2 had significantly higher scores than those with KOA grade = 1 (Table 1).
Comparison of thigh muscle CSA and adipose tissue area between early and established KOA
The knee extensor CSA to thigh ratio was significantly lower in patients with KOA grade ≥ 2 than in those with KOA grade = 1. The SAT area to thigh ratio, the inter-MAT area to thigh myofascial ratio, and the thigh intra-MAT area ratio were greater in patients with KOA grade ≥ 2 than in those with KOA grade = 1. In particular, the knee extensor intra-MAT area ratio and the ADD intra-MAT area ratio were greater in patients with KOA grade ≥ 2 than in those with KOA grade = 1 (Table 2).
The VM CSA to knee extensor ratio was on average 2.2% lower in patients with KOA grade ≥ 2 than in those with KOA grade = 1 (p = 0.006), whereas the VL and VI intra-MAT area ratios were greater (median, 6.1%, p = 0.023; mean, 4.2%, p = 0.019, respectively) in patients with KOA grade ≥ 2 than in those with KOA grade = 1. No significant differences were observed in other muscles (Fig. 3).
Independent parameters associated with early and established KOA
Strong correlations were observed between the SAT area and the SAT to thigh ratio (rs = 0.91, p < 0.001); the inter-MAT area and the inter-MAT to thigh myofascial ratio (r = 0.90, p < 0.001); and the ADD intra-MAT area and the ADD intra-MAT ratio (rs = 0.84, p < 0.001). Then, these ratio parameters were included in a logistic regression analysis. For the intra-MAT ratios, a strong correlation was observed between the thigh and the knee extensor (rs = 0.70, p < 0.001); the thigh and the ADD (rs = 0.85, p < 0.001); the knee extensor and the VL (rs = 0.73, p < 0.001); and the knee extensor and the VI (rs = 0.83, p < 0.001). The smaller muscle was selected for a logistic regression analysis.
Ultimately, strong correlations were not observed among BMI, FTA, the knee extensor CSA ratio, the VM CSA ratio, the SAT ratio, the inter-MAT ratio or the intra-MATs ratio for ADD, VL, or VI, and these parameters were incorporated in a stepwise logistic regression analysis. In a stepwise logistic regression analysis, FTA [odds ratio (OR) 3.2, 95% confidence interval (CI) 1.3–7.7, p = 0.01]; the VM CSA to knee extensor ratio (OR 2.0, 95% CI 1.0–3.7, p = 0.036); and the inter-MAT area to thigh myofascial ratio (OR 1.8, 95% CI 1.0–3.1, p = 0.037) were significantly independently associated with established KOA. The VL intra-MAT ratio had a tendency to be associated with established KOA (OR 1.3, 95% CI 0.98–1.7, p = 0.064).
Correlation between the knee pain score and parameters in patients with KOA
Knee pain score was significantly positively correlated with FTA (rs = 0.30, p = 0.033), whereas knee pain score had no significant correlations with age and BMI (rs = 0.004, p = 0.98 and rs = 0.16, p = 0.29, respectively). No significant correlations were noted between knee pain score, thigh muscle CSA and adipose tissue area (Table 3). Moreover, no significant correlations were observed between the knee pain score, the individual muscle CSA to muscle group ratios and the intra-MAT ratio of the individual muscles.
Correlation between the left–right differences of KOA grade and knee pain score and parameters
The left–right difference of KOA grade in the same patient was positively correlated with the left–right difference of knee pain score (rs = 0.34, p = 0.018). The left–right differences of KOA grade and knee pain score were positively correlated with the left–right difference of FTA and negatively correlated with the left–right difference of knee extensor CSA, particularly with regard to the left–right difference of VM CSA (Table 4). However, the left–right differences of inter-MAT area were not significantly correlated with the left–right differences in KOA grade and knee pain score (p = 0.39 and p = 0.17, respectively). The left–right differences of any intra-MATs area were not significantly correlated with the left–right differences in KOA grade and knee pain score (p = 0.17–0.96 for the KOA grade and p = 0.27–0.97 for the knee pain score).
Reproducibility of KOA grade classification, FTA, and adipose tissue area measurements
The kappa coefficient in the intraobserver reproducibility of classifying KOA grade was 0.88, and the reproducibility of the KOA grade classification was almost perfect. For the intraobserver reproducibility of the measurement of FTA and inter and intra-MAT area, the intraclass correlation coefficient was 0.98 (95% CI 0.94–0.99), and the SEM was 1.4 degrees for the FTA measurement. The reproducibility of the FTA measurement was almost perfect. The intraclass correlation coefficient was 0.92 (95% CI 0.70–0.98), and the SEM was 11.2% for inter-MAT measurement. During intra-MAT analyses of individual muscles, the intraclass correlation coefficients ranged from 0.76 (95% CI 0.10–0.94) with an SEM of 19.6% to 0.94 (95% CI 0.79–0.99) with an SEM of 12.9%. The reproducibility of the adipose tissue area measurement was substantial and almost perfect.
Discussion
Recent studies have shown stronger associations between KOA, SAT and inter-MAT and intra-MAT of the knee extensor compared to associations with the knee extensor CSA [7, 10, 12]. The results of the present study also show that lower knee extensor CSA is not independently associated with established KOA; however, novel findings include the observation that FTA and the VM CSA to knee extensor ratio are independently associated with established KOA. In addition, no parameters except FTA were related with knee pain in patients with KOA. On the other hand, Sattler et al. [21] reported that knee pain induced VL and VM atrophy on the affected side compared with the side without knee pain in an inpatient cohort. Similarly, in the present study, the left–right difference in knee pain is negatively correlated with the left–right differences of the knee extensor, VL, and VM CSA, as well as the lower thigh muscle CSA. Whereas the left–right differences of the KOA grade are negatively correlated with the left–right differences of knee extensor CSA and VM CSA. Interestingly, in the left–right differences, adipose tissue content (SAT, inter-MAT, and intra-MATs) exhibits no correlations with KOA grade and knee pain. These results indicate that greater adipose tissue content may not depend on KOA grade and knee pain but rather on physical activities, systematic calorie consumption, and BMI.
In the present study, greater FTA and lower VM CSA are associated with higher radiographic KOA grade in patients with KOA and were also related with the KOA grade when analyzing the left–right differences in the same patient. Some authors have reported that varus knee (greater FTA) is a risk factor for KOA progression [22, 23]. Varus knee increases the knee adduction moment and load in the medial compartment of the knee [23,24,25,26]. Interestingly, in the present study, FTA is not correlated with the VM CSA ratio, and these parameters were independent parameters associated with established KOA in patients with KOA. Some authors reported that greater FTA induces external patellar rotation, with the VM pulling the patella medially and stabilizing the patellofemoral joint [27, 28]. Therefore, VM atrophy might be a risk factor for medial KOA, potentially leading to greater FTA; if VM atrophy is successfully prevented in patients with varus knee, the progression of medial KOA could be prevented. In actuality, prospective studies have shown that decreases in VM CSA are associated with the progression of KOA [13, 14]. From a clinical perspective, focusing on the VM CSA to knee extensor ratio measured using MRI may be important to investigate rehabilitation efficacy and risk for medial KOA. The intraobserver reproducibility for the measurement of the VM CSA to knee extensor ratio had an SEM of 0.85% in our pilot study, which was lower than the mean difference of the VM CSA ratios observed between early and established KOA. Individual extensor muscle CSAs could be estimated from muscle thickness; therefore, measurement using MRI may represent an alternative to the measurement using sonography as a simpler method for a clinically use.
Bastick et al. [29] reported that baseline KOA grade severity was related to the progression of knee pain, whereas baseline knee pain was also related to the progression of radiographic KOA grade. In the present study, knee pain was weakly correlated with FTA in patients with KOA and left-side differences. Greater knee extensor strength was associated with reduced symptoms in patients with KOA and radiographic KOA progression, and knee extensor strengthening was recommended by the OARSI guidelines [1, 5]; however, in patients with varus knee, greater knee extensor strength was associated with KOA progression, and a knee extensor strengthening program revealed no significant improvement in knee pain compared with patients without varus knee [30, 31]. Therefore, future clinical studies are needed to determine whether increasing the VM to knee extensor ratio by rehabilitation may prevent greater FTA and knee pain impairment; further studies would also allow for an increased understanding of the development and progression of medial KOA as well as to investigate the relationship between these parameters over time.
There was a limitation in this study. To measure adipose tissue area, the present study used pixel-by-pixel analysis, which is a practical method using T1-weighted MRI; however, it was unclear whether this analysis was consistent with other methods, such as chemical shift-based water/fat separation methods (Dixon techniques) and iterative decomposition of water and fat with echo asymmetry and least-squares estimation; thus, future studies are needed for clarification of the agreement of these methods.
Conclusion
This study found that a greater FTA and a lower VM CSA to knee extensor ratio were associated with higher KOA grade. A greater inter-MAT ratio was associated with established KOA; however, adipose tissue was not related with KOA and knee pain when analyzing the left–right differences in the same patient. A future study on the effect of improvement of VM atrophy on FTA and KOA may be noteworthy.
References
Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, Bierma-Zeinstra S, Brandt KD, Croft P, Doherty M, Dougados M, Hochberg M, Hunter DJ, Kwoh K, Lohmander LS, Tugwell P (2010) OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr Cartil 18:476–499. https://doi.org/10.1016/j.joca.2010.01.01
Hudelmaier M, Glaser C, Hausschild A, Burgkart R, Eckstein F (2006) Effects of joint unloading and reloading on human cartilage morphology and function, muscle cross-sectional areas, and bone density—a quantitative case report. J Musculoskelet Neuronal Interact 6:284–290
Winby CR, Lloyd DG, Besier TF, Kirk TB (2009) Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech 42:2294–2300. https://doi.org/10.1016/j.jbiomech.2009.06.019
Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L (2008) Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc 40:422–427
Segal NA, Glass NA (2011) Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Phys Sportsmed 39:44–50. https://doi.org/10.1249/MSS.0b013e31815ef285
Blazevich AJ, Coleman DR, Horne S, Cannavan D (2009) Anatomical predictors of maximum isometric and concentric knee extensor moment. Eur J Appl Physiol 105:869–878. https://doi.org/10.1007/s00421-008-0972-7
Kumar D, Karampinos DC, MacLeod TD, Lin W, Nardo L, Li X, Link TM, Majumdar S, Souza RB (2014) Quadriceps intramuscular fat fraction rather than muscle size is associated with knee osteoarthritis. Osteoarthr Cartil 22:226–234. https://doi.org/10.1016/j.joca.2013.12.005
Ruhdorfer AS, Dannhauer T, Wirth W, Cotofana S, Roemer F, Nevitt M, Eckstein F, OAI investigators (2014) Thigh muscle cross-sectional areas and strength in knees with early vs knees without radiographic knee osteoarthritis: a between-knee, within-person comparison. Osteoarthr Cartil 22:1634–1638. https://doi.org/10.1016/j.joca.2014.06.002
Ruhdorfer A, Wirth W, Dannhauer T, Eckstein F (2015) Longitudinal (4 year) change of thigh muscle and adipose tissue distribution in chronically painful vs painless knees—data from the Osteoarthritis Initiative. Osteoarthr Cartil 23:1348–1356. https://doi.org/10.1016/j.joca.2015.04.004
Dannhauer T, Sattler M, Wirth W, Hunter DJ, Kwoh CK, Eckstein F (2014) Longitudinal sensitivity to change of MRI-based muscle cross-sectional area versus isometric strength analysis in osteoarthritic knees with and without structural progression: pilot data from the Osteoarthritis Initiative. MAGMA 27:339–347. https://doi.org/10.1007/s10334-013-0418-z
Beattie KA, Macintyre NJ, Ramadan K, Inglis D, Maly MR (2012) Longitudinal changes in intermuscular fat volume and quadriceps muscle volume in the thighs of women with knee osteoarthritis. Arthritis Care Res (Hoboken) 64:22–29. https://doi.org/10.1002/acr.20628
Dannhauer T, Ruhdorfer A, Wirth W, Eckstein F (2015) Quantitative relationship of thigh adipose tissue with pain, radiographic status, and progression of knee osteoarthritis: longitudinal findings from the osteoarthritis initiative. Invest Radiol 50:268–274. https://doi.org/10.1097/RLI.0000000000000113
Raynauld JP, Pelletier JP, Roubille C, Dorais M, Abram F, Li W, Wang Y, Fairley J, Cicuttini FM, Martel-Pelletier J (2015) Magnetic resonance imaging-assessed vastus medialis muscle fat content and risk for knee osteoarthritis progression: relevance from a clinical trial. Arthritis Care Res (Hoboken) 67:1406–1415. https://doi.org/10.1002/acr.22590
Wang Y, Wluka AE, Berry P, Siew T, Teichtahl AJ, Urquhart DM, Lloyd DG, Jones G, Cicuttini FM (2012) Increase in vastus medialis cross-sectional area is associated with reduced pain, cartilage loss, and joint replacement risk in knee osteoarthritis. Arthritis Rheum 64:3917–3925. https://doi.org/10.1002/art.34681
Pan J, Stehling C, Muller-Hocker C, Schwaiger BJ, Lynch J, McCulloch CE, Nevitt MC, Link TM (2011) Vastus lateralis/vastus medialis cross-sectional area ratio impacts presence and degree of knee joint abnormalities and cartilage T2 determined with 3T MRI—an analysis from the incidence cohort of the Osteoarthritis Initiative. Osteoarthr Cartil 19:65–73. https://doi.org/10.1016/j.joca.2010.10.023
Canale ST, Daugherty K, Jones L, Burns B (2003) Campbell’s operative orthopaedics, 10th edn. Mosby, Philadelphia, p 265
Akai M, Doi T, Fujino K, Iwaya T, Kurosawa H, Nasu T (2005) An outcome measure for Japanese people with knee osteoarthritis. J Rheumatol 32:1524–1532
18 Yamauchi K, Yoshiko A, Suzuki S, Kato C, Akima H, Kato T, Ishida K (2017) Estimation of individual thigh muscle volumes from a single-slice muscle cross-sectional area and muscle thickness using magnetic resonance imaging in patients with knee osteoarthritis. J Orthop Surg (Hong Kong) 25:2309499017743101. https://doi.org/10.1177/2309499017743101
Yoshiko A, Hioki M, Kanehira N, Shimaoka K, Koike T, Sakakibara H, Oshida Y, Akima H (2017) Three-dimensional comparison of intramuscular fat content between young and old adults. BMC Med Imaging 17:12. https://doi.org/10.1186/s12880-017-0185-9
Sled JG, Zijdenbos AP (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Sattler M, Dannhauer T, Ring-Dimitriou S, Sänger AM, Wirth W, Hudelmaier M, Eckstein F (2014) Relative distribution of quadriceps head anatomical cross-sectional areas and volumes–sensitivity to pain and to training intervention. Ann Anat 196:464–470. https://doi.org/10.1016/j.aanat.2014.07.005
Brouwer GM, van Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, Pols HA, Bierma-Zeinstra SM (2007) Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum 56:1204–1211
Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S (2002) Dynamic load at baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis 61:617–622
Tetsworth K, Paley P (1994) Malalignment and degenerative arthropathy. Orthop Clin N Am 25:367–377
Schipplein OD, Andriacchi TP (1991) Interaction between active and passive knee stabilizers during level walking. J Orthop Res 9:113–119
Hurwitz DE, Ryals AB, Case JP, Block JA, Andriacchi TP (2002) The knee adduction moment during gait in subjects with knee osteoarthritis is more closely correlated with static alignment than radiographic disease severity, toe out angle and pain. J Orthop Res 20:101–107
Worlicek M, Moser B, Maderbacher G, Zentner R, Zeman F, Grifka J, Keshmiri A (2017) The influence of varus and valgus deviation on patellar kinematics in healthy knees: an exploratory cadaver study. Knee 24:711–717. https://doi.org/10.1016/j.knee.2017.04.009
Elias JJ, Kilambi S, Goerke DR, Cosgarea AJ (2009) Improving vastus medialis obliquus function reduces pressure applied to lateral patellofemoral cartilage. J Orthop Res 27:578–583. https://doi.org/10.1002/jor.20791
Bastick AN, Belo JN, Runhaar J, Bierma-Zeinstra SM (2015) What are the prognostic factors for radiographic progression of knee osteoarthritis? A meta-analysis. Clin Orthop Relat Res 473:2969–2989. https://doi.org/10.1007/s11999-015-4349-z
Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW (2003) Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med 138:613–619
Lim BW, Hinman RS, Wrigley TV, Sharma L, Bennell KL (2008) Does knee malalignment mediate the effects of quadriceps strengthening on knee adduction moment, pain, and function in medial knee osteoarthritis? A randomized controlled trial. Arthritis Rheum 59:943–951. https://doi.org/10.1002/art.23823
Acknowledgements
We greatly appreciate Shigetoshi Suzuki, the staff of the radiology division for his assistance while conducting this study and the editors of American Journal Experts for correcting English language.
Funding
None.
Author information
Authors and Affiliations
Contributions
CK study conception and design. TK study conception and design.
Corresponding author
Ethics declarations
Conflict of interest
Koun Y, Chisato K, and Takayuki K declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yamauchi, K., Kato, C. & Kato, T. Characteristics of individual thigh muscles including cross-sectional area and adipose tissue content measured by magnetic resonance imaging in knee osteoarthritis: a cross-sectional study. Rheumatol Int 39, 679–687 (2019). https://doi.org/10.1007/s00296-019-04247-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04247-2