Abstract
The aims of this study were (1) to describe dietary protein intake, and (2) to evaluate the association between dietary protein intake and upper leg muscle strength in subjects with knee osteoarthritis (OA). Baseline data from the OA was used, in a cross-sectional study. All subjects were diagnosed with symptomatic and radiographic knee OA. Daily dietary protein intake was measured with the Block Brief 2000 food frequency questionnaire (g/kg body weight). The sum of knee flexion and extension strength of the index knee (N/kg bodyweight) was assessed with the Good Strength chair test. Linear regression analysis was used to test the association between dietary protein intake and muscle strength, adjusting for relevant confounders. Data from 1316 subjects (mean age 61.4 ± SD 9.1 years, 57.0% female) were used. The mean daily protein intake was 0.72 ± SD 0.30 g/kg bodyweight, and 65.1% of the subjects had a protein intake lower than the recommended daily allowance of 0.8 g/kg bodyweight. The mean muscle strength was 5.4 ± SD 2.1 N/kg bodyweight. Lower protein intake was significantly associated with lower muscle strength (B = 0.583, 95% CI 0.230–0.936, p = 0.001). The majority of the subjects with knee OA had a dietary protein intake lower than the recommended daily allowance. Lower protein intake was associated with lower upper leg muscle strength. Longitudinal observational and interventional studies are needed to establish whether dietary protein intake has a causal effect on muscle strength in subjects with knee OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lower muscle strength in subjects with knee osteoarthritis (OA) is strongly related to pain and activity limitations, and has been reported to be a risk factor for symptomatic and structural progression of the disease [1,2,3,4,5]. A wide variety of factors were found to be associated with lower muscle strength in knee OA, such as low muscle quality, more severe joint degeneration, pain and physical inactivity [6]. Remarkably, limited research was found on the relation between nutrition-related factors and muscle strength in knee OA [6]. While evidence is emerging on the impact of protein intake on muscle mass and strength in the general population [7,8,9,10], protein intake in relation to muscle strength has not been studied in patients with knee OA, to our knowledge. The impact of protein intake on muscle mass and strength is of particular interest in knee OA, because of the clinical relevance of muscle strength in knee OA [1,2,3,4,5].
Dietary protein intake can have a positive effect on the balance of muscle protein synthesis (i.e. building of muscle tissue) and muscle protein breakdown (i.e. the breakdown of muscle tissue) (see Fig. 1) [7, 11,12,13]. The long-term net balance between muscle protein synthesis and breakdown dictates the amount of muscle mass available [13, 14]. Dietary protein intake increases muscle protein synthesis and inhibits muscle protein breakdown, thereby favoring net muscle protein balance, resulting in an increase in muscle mass over time [13, 14]. The higher the amount of muscle mass available for a contraction, the higher the amount of force a person can generate, assuming neural input and drive is optimal [15]. Therefore, adequate dietary protein intake is important to preserve or increase muscle mass [11, 16]. The recommended daily allowance (RDA) for dietary protein intake is 0.8 g/kg of bodyweight in adults stated by the European Union Geriatric Medicine Society (EUGMS) [16]. Lower intakes than the RDA has been reported in 7–41% of the older adults [17, 18].
Patients with knee OA may need even higher protein intakes than the RDA, since they are prone to develop anabolic resistance. Anabolic resistance is a dampened response in muscle protein metabolism to dietary protein intake or exercise, which is prevalent in older adults [16, 19]. It is suggested that anabolic resistance might develop as a result of the age-related decline in physical activity and the age-associated low-grade inflammation [13]. Higher dietary protein intake is suggested to compensate for this dampened response. In healthy older adults an RDA of 1.0–1.2 g/kg/day is suggested by the EUGMS, while in older adults with acute or chronic diseases an RDA of 1.2–1.5 g/kg/day may be necessary according to the EUGMS [16, 20]. Patients with knee OA are mostly older adults, report low levels of physical activity [21], have chronic low-grade inflammation [22] and have a chronic disease [23]. This suggests that they are at risk for anabolic resistance and may require higher daily protein intakes. These considerations suggest that the relationship between dietary protein intake, muscle protein synthesis and muscle strength in patients with knee OA are not necessarily the same as in older adults.
To date, the amount of dietary protein intake per day and the association between daily dietary protein intake and muscle strength in knee OA is unknown. We aimed to (1) describe daily dietary protein intake, and (2) to evaluate the association between daily dietary protein intake and upper leg muscle strength in subjects with knee OA.
Subjects and methods
Subjects
Data were obtained from the Osteoarthritis Initiative (OAI) database (http://www.oai.ucsf.edu/) [24]. This study was approved by the Committee on Human Research, Institutional Review Board for the University of California, San Francisco (Approval Number: 10-00532, date: 24 Feb 2017). The OAI is a prospective cohort study focusing on studying biomarkers for the progression or development of knee OA. The OAI inclusion and exclusion criteria are described in “Appendix”. The OAI cohort is divided into three subcohorts: subjects with knee OA at baseline (progression subcohort), subjects at risk of developing knee OA (incidence subcohort) and healthy controls (reference control subcohort). In this study, only baseline data of the progression subcohort were used (see Fig. 2; specific datasets used are 0.2.2 and 0.2.3) [24]. The progression cohort includes subjects with frequent knee symptoms and radiographic tibiofemoral knee OA at baseline. Frequent knee symptoms were defined as pain, aching or stiffness in or around the knee for most days for at least 1 month during the past 12 months. Radiographic tibiofemoral knee OA was defined as the presence of definite tibiofemoral osteophytes [equivalent to a score of ≥ 2 within the Kellgren and Lawrence (KL) grade] on the fixed flexion radiograph. All collected data of the subjects was assessed during the screening visit or via self-reported questionnaires.
Dietary protein intake
Dietary protein intake in the OAI was measured with the use of the Block Brief 2000 Food Frequency (Block 2000 FFQ) Questionnaire (Nutritionquest©) [25, 26]. The Block 2000 FFQ has 102 items and measures participants’ usual eating habits focusing on the past 12 months. Patients were asked ‘how often, on average, did you eat the food during the past 12 months?’. In addition, portion sizes, per serving, were questioned. Using the information collected with the use of the questionnaire, daily intake of energy and numerous nutrients, including protein, were calculated.
Only subjects with valid dietary protein and energy intake were included. Unlikely values for caloric intake were excluded based on commonly used methods to exclude records in food frequency questionnaires [24]. These include kilocalories (kcal) less than 500, or greater than 5000 per day and when more than 15% of the questions were missing.
Dietary protein intake was divided by measured bodyweight and expressed as protein in grams per kg bodyweight per day. Dietary protein intake measured with the use of the Block 2000 FFQ was found not to be statistically different from dietary protein intake measured by food diaries [26, 27].
Index knee
For the purpose of this study, an index knee (most affected knee) was determined for each participant. The index knee was determined using the Kellgren and Lawrence-grade (KL-grade). The knee with the highest KL-grade was determined to be the index knee. In case of an equal KL grade between the left and right knee, the knee with the highest score on the WOMAC pain was determined to be the index knee. In case of an equal score on both KL grade and WOMAC pain, the index knee was chosen randomly.
Upper leg muscle strength
Upper leg muscle strength of each leg was measured, isometrically, for knee flexion and extension with the Good Strength chair (Metitur Oy) [24, 28, 29]. The maximal force produced was measured during isometric contractions of the right and left quadriceps and hamstring muscles at a knee angle of 60° from full extension on the Good Strength chair (Metitur Oy) [24, 28, 29]. During the measurement, the subject was positioned in the Good Strength chair and the waist and the tested upper leg were fixated. Before the measurement started, subjects performed a short warming up exercise and practiced the execution of the measurements. At least three correct measurements were performed per leg. The peak force of those three measurements was used. During the measurements, subjects were vocally motivated. After the measurement subjects were encouraged to move, stretch or shake their legs to reduce muscular pain and stiffness after the test. Pain in the knee during the measurement was assessed for each repetition [24]. Upper leg muscle strength of the index knee was expressed as the sum of both flexion and extension strength corrected for bodyweight (N/kg bodyweight) [30].
Potential confounders
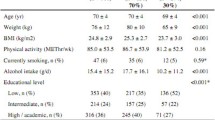
Based on previous studies investigating dietary protein intake and muscle strength in older adults [11, 16, 19, 31], the following potential confounders were considered relevant: age, gender, race, level of education, energy intake, alcohol consumption, knee pain, comorbidities and physical activity. These variables were expected to meet the following criteria of a confounder: the variable is considered a cause of the outcome, the variable is associated with the exposure and the variable is not an effect of the exposure or outcome [32]. Age, gender, race (Caucasian, Blacks or Afro-Americans, Others) and alcohol consumption were assessed by questionnaire. Alcohol consumption was classified into no alcohol consumption:‘none’, moderate alcohol consumptions ‘< 1/week to 7 drinks/week’ and high alcoholic consumption ‘≥ 8 consumptions per week’. Daily energy intake (kcal) was calculated based on the information from the Block brief 2000. The level of education was classified in three categories: high school graduate or less, some college or college graduate, some graduate school or graduate degree.
A self-reported questionnaire based on the Charlson index was used to collect information about comorbidities [33]. The Charlson index questionnaire scores comorbidities based on the mortality risk: a high score represents a high number of comorbidities [34]. The Charlson index score has been dichotomized into “no comorbidities” (score = 0) and “1 or more comorbidities” (score > 0). Physical activity was measured by the Physical Activity Scale for Elderly (PASE) and expressed in a score ranging from 0 to 793, with higher scores indicating greater physical activity [35].
Knee pain was examined as potential confounder. Knee pain was assessed for the index knee with the use of the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC, version LK 3.1) pain subscale, a validated questionnaire in the knee OA population [36]. The WOMAC-pain subscale was scored from 0 to 20; higher scores represent more pain [36].
Descriptive variables
Height (mm) and bodyweight (kg) were measured to calculate body mass index (BMI, kg/m2). The radiographic features of the index knee (joint space narrowing, osteophyte formation, sclerosis, cysts) within the tibiofemoral joints were scored on site according to the Osteoarthritis Research Society International (OARSI) atlas and expressed in KL grades. Scores of the KL grades were classified in five categories (0–4). Grade 0 represents no osteoarthritic features while grade 4 represents severe joint space narrowing, large osteophyte formation, sclerosis and cysts. Depression was assessed by the Center of Epidemiological Studies Depression Scale (CES-D), which was included in the questionnaire during the screening visit.
Statistical analysis
Descriptive values were calculated for all variables. Variables were carefully checked for possible outliers, based on Tukey’s method [37]. The assumptions for linear regression [e.g. no strong multicollinearity, homoscedasticity, linearity (checked by visual inspection of the normality plots and distribution of the residuals)] were checked [38]. Univariate linear regression analysis was performed, including protein intake per day per kilogram bodyweight (continuous variable) as an independent variable and muscle strength per kilogram bodyweight (continuous variable) as a dependent variable. In multivariate regression analysis, the model was adjusted to account for relevant confounders, including age, gender, race, alcohol consumption, level of education, comorbidities and physical activity. Race, alcohol consumption, level of education and comorbidities were added to the model as dummy variables. For WOMAC pain score it was unclear whether or not the criteria for confounding applied. This variable may also be in the causal pathway and adjustment might result in an overcorrection of the model. Therefore, a separate model with an adjustment for WOMAC pain score was reported separately. We performed an additional analysis in which dietary protein intake was dichotomized. The binary split was performed based on the recommended protein intake according to the guidelines. Dietary protein intake was scored 0 for intakes < 0.8 g/kg/day, and 1 for intakes ≥ 0.8 g/kg/day. To assess whether the level of physical activity would influence the association between protein intake and muscle strength an interaction term (daily protein intake*physical activity) was added to the model. A p-value of 0.1 was used to examine the presence of an interaction effect. SPSS version 18.0 (SPSS Inc., 2009) was used to perform the analyses.
Results
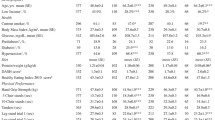
The selection of the subjects of this study is depicted in Fig. 2. Characteristics of the included subjects (n = 1316) are presented in Table 1. The mean daily protein intake was 0.72 ± SD 0.30 g/kg bodyweight, and 65.1% of the subjects had a protein intake lower than the recommended daily allowance of 0.8 g/kg bodyweight. The mean muscle strength of the index knee was 5.4 ± SD 2.1 N/kg bodyweight.
In Table 2, the univariate and multivariate associations between daily dietary protein intake and muscle strength are presented. In the univariate model, lower dietary protein intake was associated with lower muscle strength (B = 1.030, 95% CI 0.635–1.425, p < 0.001). After adjusting for age, gender, race, alcohol consumption, level of education, comorbidities and physical activity, the association between dietary protein intake and muscle strength remained significant (B = 0.583, 95% CI 0.230–0.936, p = 0.001; r2 = 0.268). Energy intake could not be added to the model as a potential confounder, because of multicollinearity (the association between daily dietary protein intake and daily energy intake was r = 0.822, p < 0.001). Finally, after additional adjustment for WOMAC pain score, the association between dietary protein intake and muscle strength remained significant (B = 0.524, 95% CI 0.177–0.871, p = 0.003). When dietary protein intake was dichotomized, dietary protein intake lower than 0.8 g/kg/day was associated with lower muscle strength compared to an intake equal or greater than 0.8 g/kg/day, both in the univariate (B = 0.558, 95% CI 0.309–0.807, p < 0.001) and multivariate model (B = 0.315, 95% CI 0.258–0.372, p < 0.001). No statistically significant interaction between dietary protein intake and physical activity on muscle strength was found.
Discussion
The average daily dietary protein intake of subjects with knee OA in the OAI cohort was 0.72 ± SD 0.30 g/kg. The current RDA for protein intake in adults is 0.8 g/kg [16], which is met by only 34.9% of the study population. However, it has been suggested that the current advised RDA is not sufficient for preserving muscle mass and quality at older age, or in patients with acute or chronic diseases [16, 20]. This may be the result of the dampened response in muscle protein synthesis on dietary protein intake (anabolic resistance) [16, 19]. Protein intake of 1.0–1.2 g/kg/day has been recommended for the preservation of muscles in healthy older adults, while 1.2–1.5 g/kg/day of protein may be necessary in older patients with acute or chronic diseases [16, 20]. Since the majority of the subjects of the study population is of an older age and all are diagnosed with knee OA (a chronic disease), these higher intakes may apply for this study population. Although more studies are needed on dietary protein intake in knee OA to strengthen our findings, the results of this study suggest that insufficient dietary protein intake is present in a large part of the knee OA population.
Lower daily dietary protein intake (both as a continuous and a dichotomized variable) was associated with lower muscle strength in subjects with knee OA. This association remained significant after adjusting for potential confounders. These results indicate that the association between daily dietary protein intake and muscle strength not only holds in older adults but also in subjects with knee OA [31, 39, 40]. This is a relevant finding, because of the important role of muscle strength in patients with knee OA [1,2,3,4,5]. To the best of our knowledge, no other studies have reported on the association between daily dietary protein intake and upper leg muscle strength in subjects with knee OA.
We did not find an interaction effect between dietary protein intake and physical activity on muscle strength. In a study on older adults, a synergistic effect of protein intake and exercise on muscle strength has been reported [41]. These contradictory findings may be explained by the rather general measurement used for physical activity in the present study, namely the physical activity scale for elderly (PASE). It could be that strenuous physical activity and exercise, especially resistance training, does moderate the impact of protein intake on muscle strength. In older adults protein supplementation in addition to resistance training was shown to be more effective in increasing muscle strength than resistance training alone [42, 43]. A daily supplementation of 1.6 g/kg was found to be sufficient to optimize the resistance training-induced changes in strength in healthy adults [43]. In knee OA a similar effect may be expected. Future research is needed to confirm this hypothesis.
The findings in the present study show that daily dietary protein intake in patients with knee OA is lower than the RDA. This suggests that daily dietary protein intake needs to be enhanced and that dietary protein intake could be an intervention target in subjects with knee OA. Of note, improving muscle protein synthesis by daily dietary protein intake is more complex than increasing the daily dietary intake. Previous studies in healthy adults have shown that the type of protein (animal or plant based), the quality of the protein and the timing of ingestion are important to optimally promote muscle protein synthesis [16]. In addition, since overweight is prevalent in knee OA and weight management is recommended [44], increasing daily dietary protein intake should not result in higher levels of total energy intake. Future research should address these issues.
The strength of the present study is the large sample size of the studied cohort. The study also has several limitations. First, daily dietary protein intake was measured with the Block 2000 FFQ. In general, retrospective food frequency questionnaires are less accurate in assessing food intake compared to a nutrition diary [25]. However, previous studies have shown that protein intake as measured with the Block FFQ was not significantly different from protein intake measured with a nutrition diary [26, 27]. Therefore, the Block 2000 FFQ seems to constitute a valid measure of protein intake. Second, although the relationship between protein intake and muscle strength is biologically plausible, we cannot exclude the possibility that total energy intake may be (partly) responsible for the association found between dietary protein intake and muscle strength. Because of multicollinearity (r = 0.822, p < 0.001), we could not adjust for energy intake in the evaluation of the association between protein intake and muscle strength. Third, no data were available on the type and quality of protein intake [16]. Fourth, the data of this study are cross-sectional, which precludes any conclusion regarding a causal relation between dietary protein intake and muscle strength. Fifth, the OAI provides data on protein intake and muscle strength of only 33 healthy (control) subjects; this sample is too small to compare protein intake and its relation with muscle strength between healthy subjects and subjects with knee OA. Sixth, high-quality data on physical activity and, more specific, on strenuous activities or resistance training were missing. Finally, this study was performed in a subgroup of an American cohort study, similar studies should be performed in other cohorts to be able to generalize our findings.
In conclusion, the results of this study suggest that the majority of subjects with knee OA have a dietary protein intake lower than the currently recommended daily allowance of 0.8 g/kg bodyweight [16]. In addition, lower protein intake was associated with lower upper leg muscle strength in subjects with knee OA, after adjusting for relevant confounders. Longitudinal observational and interventional studies are needed to establish whether dietary protein intake has a causal effect on muscle strength in subjects with knee OA.
References
Glass NA, Torner JC, Frey Law LA, Wang K, Yang T, Nevitt MC et al (2013) The relationship between quadriceps muscle weakness and worsening of knee pain in the most cohort: a 5-year longitudinal study. Osteoarthr Cartil 21:1154–1159. https://doi.org/10.1016/j.joca.2013.05.016
Oiestad BE, Juhl CB, Eitzen I, Thorlund JB (2015) Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthr Cartil 23:171–177. https://doi.org/10.1016/j.joca.2014.10.008
Segal N, Glass N (2011) Is quadriceps muscle weakness a risk factor for incident or progressive knee osteoarthritis? Phys Sportsmed 39:44–50
Emrani A, Bagheri H, Hadian MR, Jabal-Ameli M, Olyaei GR, Talebian S (2006) Isokinetic strength and functional status in knee osteoarthritis. J Phys Ther Sci 18:107–114. https://doi.org/10.1589/jpts.18.107
Culvenor AG, Ruhdorfer A, Juhl C, Eckstein F, Øiestad BE (2017) Knee extensor strength and risk of structural, symptomatic and functional decline in knee osteoarthritis: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 69:649–658. https://doi.org/10.1002/acr.23005
De Zwart AH, Dekker J, Lems WF, Roorda LD, Van Der Esch M, Van Der Leeden M (2018) Factors associated with upper leg muscle strength in knee osteoarthritis: a scoping review. J Rehabil Med Suppl 50:140–150
Baum JI, Wolfe RR (2015) The link between dietary protein intake, skeletal muscle function and health in older adults. Healthcare 3:529–543. https://doi.org/10.3390/healthcare3030529
Houston D, Nicklas B, Ding J, Harris T, Tylavsky F, Newman AB et al (2008) Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the health, aging, and body composition (health abc) study. Am J Clin Nutr 87:150–155
Beasley JM, Shikany JM, Thomson CA (2013) The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract 28:684–690. https://doi.org/10.1177/0884533613507607
Trumbo P, Schlicker S, Yates AA, Poos M, Food, Nutrition Board of the Institute of Medicine TNA (2002) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc 102:1621–1630
Deutz NE, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A et al (2014) Protein intake and exercise for optimal muscle function with aging: recommendations from the espen expert group. Clin Nutr 33:929–936. https://doi.org/10.1016/j.clnu.2014.04.007
Wolfe RR (2002) Regulation of muscle protein by amino acids. J Nutr 132:3219S–3219S24S
Breen L, Phillips SM (2011) Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond) 8:68. https://doi.org/10.1186/1743-7075-8-68
Wolfe RR, Miller SL, Miller KB (2008) Optimal protein intake in the elderly. Clin Nutr 27:675–684. https://doi.org/10.1016/j.clnu.2008.06.008
Kumar S (2004) Muscle strength. CRC Press, Boca Raton
Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE et al (2013) Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the prot-age study group. J Am Med Dir Assoc 14:542–559. https://doi.org/10.1016/j.jamda.2013.05.021
Kerstetter JE, O’Brien KO, Insogna KL (2003) Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr 133:855S–855S61S
Fulgoni VL 3rd (2008) Current protein intake in America: analysis of the national health and nutrition examination survey, 2003–2004. Am J Clin Nutr 87:1554S–1554S7S
Wall B, Gorissen S, Pennings B, Koopman R, Groen B, Verdijk L et al (2015) Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One 10:e0140903
Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR (2008) Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr 87:1562S–1562S6S
Herbolsheimer F, Schaap LA, Edwards MH, Maggi S, Otero A, Timmermans EJ et al (2016) Physical activity patterns among older adults with and without knee osteoarthritis in six european countries. Arthritis Care Res (Hoboken) 68:228–236. https://doi.org/10.1002/acr.22669
Scanzello CR (2017) Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol 29:79–85. https://doi.org/10.1097/BOR.0000000000000353
Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M et al (2014) The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73:1323–1330. https://doi.org/10.1136/annrheumdis-2013-204763
Nevitt M, Felson D, Lester G (2009) The osteoarthritis initiative. Protocol for the cohort study. Retrieved from: https://oai.epi-ucsf.org/datarelease/docs/studydesignprotocol.pdf
Block G, Hartman AM, Naughton D (1990) A reduced dietary questionnaire: development and validation. Epidemiology 1:58–64
Block G, Woods M, Potosky A, Clifford C (1990) Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43:1327–1335
Delgado C, Ward P, Chertow GM, Storer L, Dalrymple L, Block T et al (2014) Calibration of the brief food frequency questionnaire among patients on dialysis. J Ren Nutr 24:151–156
Rantanen T, Era P, Heikkinen E (1994) Maximal isometric strength and mobility among 75-year-old men and women. Age Ageing 23:132–137
Rantanen T, Era P, Heikkinen E (1997) Physical activity and the changes in maximal isometric strength in men and women from the age of 75 to 80 years. J Am Geriatr Soc 45:1439–1445
Bennell KL, Wrigley TV, Hunt MA, Lim BW, Hinman RS (2013) Update on the role of muscle in the genesis and management of knee osteoarthritis. Rheum Dis Clin North Am 39:145–176. https://doi.org/10.1016/j.rdc.2012.11.003
Sahni S, Mangano K, Hannan M, Kiel D, McLean R (2015) Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. J Nutr 145:1569–1575
Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10:37–48
Katz J, Chang L, Sangha O, Fossel A, Bates D (1996) Can comorbidity be measured by questionnaire rather than medical record review? Med Care 34:73–84
Charlson M, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47:1245–1251
Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA (1999) The physical activity scale for the elderly (pase): evidence for validity. J Clin Epidemiol 52:643–651
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of womac: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Hoaglin DC, Iglewicz B (1987) Fine-tuning some resistant rules for outlier labeling. J Am Stat Assoc 82:1147–1149
Kleinbaum D, Kupper L, Nizam A, Rosenberg E (2013) Applied regression analysis and other multivariable methods. Nelson Education, Toronto
McLean R, Mangano K, Hannan M, Kiel D, Sahni S (2015) Dietary protein intake is protective against loss of grip strength among older adults in the framingham offspring cohort. J Gerontol A Biol Sci Med Sci 71:356–361. https://doi.org/10.1093/gerona/glv184
Isanejad M, Mursu J, Sirola J, Kröger H, Rikkonen T, Tuppurainen M et al (2016) Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br J Nutr 115:1281–1291
Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ (2012) Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 96:1454–1464. https://doi.org/10.3945/ajcn.112.037556
Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC et al (2012) Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc 13:713–719. https://doi.org/10.1016/j.jamda.2012.05.020
Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E et al (2018) A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med 52:376–384
McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM et al (2014) Oarsi guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartil 22:363–388. https://doi.org/10.1016/j.joca.2014.01.003
Acknowledgements
The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Funding
This study was funded by the Dutch Arthritis Association (Grant number 13-1-401).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
AH de Zwart declares that he has no conflict of interest, M van der Leeden declares that she has no conflict of interest, LD Roorda declares that he has no conflict of interest, M Visser declares that she has no conflict of interest, M van der Esch declares that he has no conflict of interest, WF Lems declares that he has no conflict of interest, J Dekker declares that he has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Appendix
Appendix
OAI is a prospective cohort study focusing on studying biomarkers for the progression or development of knee OA. On entry, all included participants were aged 45–79 years old. Participants were excluded from the study in case of rheumatoid arthritis or inflammatory arthritis, unlikely to demonstrate measurable loss of joint space, bilateral total knee joint replacement or planned bilateral knee replacement in the next 3 years, unable to undergo a 3.0 Tesla MRI exam, positive pregnancy test, unable to provide a blood sample for any reason, use of ambulatory aids other than single straight cane, Co-morbid condition that might interfere with the ability to participate in a 4-year study, unlikely to reside in the clinical area for at least 3 years, current participation in a double-blind randomized controlled trial or unwilling to sign informed consent [24].
Rights and permissions
About this article
Cite this article
de Zwart, A.H., van der Leeden, M., Roorda, L.D. et al. Dietary protein intake and upper leg muscle strength in subjects with knee osteoarthritis: data from the osteoarthritis initiative. Rheumatol Int 39, 277–284 (2019). https://doi.org/10.1007/s00296-018-4223-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4223-x