Abstract
SLE is a disease that mainly affects women of childbearing age, however, a total of 15–20% of cases present in children. Although adult onset SLE (aSLE) and childhood onset SLE (cSLE) share the same diagnostic criteria, differences have been identified. The aim of this study is to compare the similarities and differences in between cSLE and aSLE in an Arab population from Oman. We evaluated 225 SLE patients, 139 adults and 86 children, who fulfilled the criteria for diagnosis. At disease onset, 99% of SLE cohort fulfilled the SLICC criteria; however the ACR 1997 criteria were fulfilled in 66% aSLE and 80% cSLE. The clinical features of SLE in cSLE showed higher frequency of renal (50 vs 19%; p < 0.001), musculoskeletal (67 vs 53%; p = 0.036) and pulmonary involvement (13 vs 2.9%, p = 0.005); while aSLE showed higher frequency of hematological (64 vs 49%; p = 0.25) and mucocutaneous (24 vs 10%; p = 0.13) involvement. The mean disease activity score at disease onset and during disease course was also higher in cSLE (13 vs 8.5; p < 0.0005) (16 vs 11.8; p < 0.0005), respectively. Differences in autoantibody profile were also noted in cSLE with higher positivity of anti-dsDNA and antiphospholipid antibody (94 vs 84%; p = 0.027) (53 vs 37%; p = 0.25), respectively. cSLE patients were more likely than aSLE to be treated with immunosuppressant such as cyclophosphamide (51 vs 22%; p < 0.001) and MMF (70 vs 54%; p = 0.019). Similarities and differences between aSLE and cSLE in a cohort from Oman of Arab ethnicity were identified. It appears that individual races and ethnicities may exhibit differences in disease susceptibility and manifestations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is a complex autoimmune disease. The clinical heterogeneity of the disease mirrors its complex immune and genetic pathogenesis. Many factors contribute to the diversity of clinical phenotypes of SLE including race, ancestry, ethnicity, gender and age at disease presentation [1,2,3,4,5,6]. Although SLE mainly affects women of childbearing age, however, its prevalence is not confined within this population. A total of 15–20% of SLE cases present in children < 16 years of age. Disease onset in children most frequently occurs between the ages 12 and 16 years, while it is uncommon before the age of 10 years and very rare before 5 years. Childhood onset SLE (cSLE) is in fact the same disease that occurs in adult onset SLE (aSLE); however, substantial differences in the initial presentation, disease severity, serology, treatment response and outcome have been reported [5,6,7,8,9,10,11].
Several reports demonstrate that cSLE has a more severe disease onset with higher disease activity, and higher major organ involvement, namely renal, neuropsychiatric and hematological [5, 6]. A recent meta-analysis demonstrated that cSLE has a different autoantibody profile than aSLE with increased anti-dsDNA and anticardiolipin antibody, and decreased rheumatoid factor positivity [12]. Comparative studies indicate that cSLE is more often treated with higher doses of corticosteroids and immunosuppressive medications than aSLE [6, 13, 14]. In addition, comparative long-term follow-up studies demonstrate that a significantly higher percentage of cSLE are on oral steroids compared to aSLE [15]. Furthermore, statistically significant differences in cumulative organ damage and mortality between cSLE and aSLE were noted [6, 14,15,16]. Although the age at diagnosis has a strong modulating effect on clinical presentation, disease course, response to treatment and prognosis, the findings are not always consistent across studies due to geographic and ethnic variations.
There is a scarcity of published literature on clinical phenotype of SLE in Oman. However, previous studies have demonstrated that both cSLE and aSLE have clinical characteristics that are unique to the region [17,18,19,20]. Given the complexity of clinical features of SLE which is influenced by racial, ethnic, genetic and environmental factors, this study aims to compare the differences and similarities in demographic, clinical presentation, disease severity, serology and treatment between cSLE and aSLE in an Omani population.
Methods
Patients
A cross-sectional study was conducted at Sultan Qaboos University Hospital, a tertiary hospital affiliated to an academic institute. We included patients with the diagnosis of SLE who were followed at the pediatric and adult rheumatology department from January 2006 to July 2016. cSLE was defined as onset of SLE in children at ≤ 16 years of age, while those diagnosed above the age of 16 were classified as having aSLE. All patients who were included in the study met the 1997 American College of Rheumatology (ACR) revised criteria or the Systemic Lupus International Collaborating Clinics Classification criteria (SLICC) for SLE or both [21, 22]. Patients were excluded from the study if they did not satisfy any of the SLE classification criteria, had other autoimmune rheumatological disorders or had missing data at disease onset. Ethical approval was obtained from the local research and ethics review committee.
Study variables
Demographic, clinical and immunological data were retrospectively collected from the hospital information system. The following variables were analyzed: demographic characteristics, clinical manifestation of disease at onset and during course of illness, serology, disease severity and treatment. Demographic characteristics included age at disease onset, gender, disease duration, and geographical regional origin. Clinical data collected included constitutional symptoms such as fever and weight loss, cutaneous manifestations (malar rash, photosensitivity, discoid rash and cutaneous vasculitis), mucosal involvement (oral or nasal ulcers), articular manifestation (arthritis), renal involvement (haematuria, proteinuria and casts), central nervous system manifestations (seizures, psychosis and headache), hematological involvement (leucopenia, lymphopenia, haemolytic anemia and thrombocytopenia), cardiovascular manifestations (pericarditis, myocarditis and hypertension), pulmonary involvement (pneumonitis and pleural effusion), gastrointestinal symptoms (abdominal pain, hepatomegaly, hepatosplenomegaly) and lymphadenopathy. Serological parameters, including antinuclear antibodies (ANA), were determined by immunofluorescence using Hep-2 cells as substrate with a cut of > 1:80. Autoantibodies including anti-double-stranded DNA (anti-dsDNA), anti-extractable nuclear antigen (anti-ENA) profile including (Ro, La, Smith, and ribonuclear proteins (RNP), as well as antiphospholipid antibodies) were measured qualitatively using enzyme-linked immunosorbent assay (ELISA) technique. Autoantibodies were considered positive if the value was above the cut-offs for the laboratory at least in one determination during the follow-up period, except for anticardiolipin antibodies, which were considered present if there was two positive occasions 12 weeks apart.
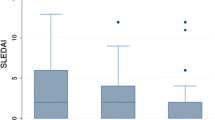
Pathological classifications of renal biopsy specimens in patients with lupus nephritis were according to International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003 [23]. Disease activity at onset and during the course of disease was assessed using SLE disease activity index (SLEDAI) [24]. Treatment with the following medications was recorded: non-steroidal anti-inflammatory drugs (NSAID), hydroxycloroquine, prednisolone, azathioprine, methotrexate, mycophenolate mofetil, cyclophosphamide, cyclosporin, rituximab, intravenous immunoglobulin, calcium and vitamin D.
Statistical analysis
Descriptive statistics were used to describe the data, and for categorical variables, frequencies and percentages were reported, with differences between groups analyzed using Pearson’s chi-square test (or Fisher’s exact test for cells < 5). For continuous variables, mean and standard deviation (median and interquartile range when not normally distributed) were used to summarise the data. Analyses were performed using a Student’s t test (or Wilcoxon–Mann–Whitney test as appropriate). An a priori two-tailed level of significance was set at 0.05 and statistical analyses were performed using STATA version 13.1 (STATA Corporation, College Station, Texas, USA).
Results
A total of 225 patients with the diagnosis of SLE were included in the study comprising 139 (62%) aSLE and 86 cSLE (38%). The demographic data of the patients are summarized in Table 1. cSLE cohort were 79% (n = 68) girls with a mean age at diagnosis of 9.5 ± 4.2 and with a median disease duration of 8 (5–13) years. The overall female:male (F:M) ratio in cSLE was 4:1; however, differences in ratios were noted across different age groups. The F:M ratio in children diagnosed < 5 years was 2.5:1, 5–12 years was 3.5:1, and 13–16 years old was 6.7:1. On the other hand, aSLE cohort consisted of 89% (n = 124) females, had a mean age at diagnosis of 28 ± 8.8 years with median disease duration of 5 (3–8) years. The overall F:M ratio was 8:1; however, differences in ratios were also noted across different groups. The ratio was highest when the diagnosis was made between the ages of 17–25 years 11:1; in the intermediate in age group of 25–50 years the ratio was 6.5:1 and least after the age of 50 years was 3:1.
Interestingly, there was a difference in geographical distribution of disease among cSLE and aSLE (p = 0.002). Geographical clustering of cSLE was identified in Sharqiya region (38%), while aSLE had a more equal distribution of disease among residents from Al Batinah (30%) and Muscat (26%) regions.
The clinical features at time of diagnosis of cSLE and aSLE are described in Table 1. At disease onset 99% of SLE cohort fulfilled the SLICC criteria; however, a significant difference was noted in fulfilling the ACR criteria (66% aSLE vs 80% cSLE) at disease onset. Similarly, at disease onset, cSLE had significantly higher frequency of major organ involvement including renal (50 vs 19%; p < 0.001), neuropsychiatric (17 vs 8.6%; p = 0.048), pulmonary (13 vs 4%; p = 0.005), hemolytic anemia (27 vs 16%; p = 0.045) and arthritis (67 vs 53%; p = 0.036). While aSLE had significantly higher involvement of cutaneous manifestations (malar, 24 vs 10%; p = 0.013; discoid rash, 5 vs 0%; p = 0.045; photosensitivity, 12 vs 0%; p < 0.001), lymphopenia (45 vs 22%; p < 0.001) as well as constitutional symptoms (60 vs 12%; p < 0.010).
The clinical features of SLE throughout the disease course showed a similar trend to the clinical features at disease onset in the majority of clinical presentation between cSLE and aSLE with renal, pulmonary, hemolytic and arthritis being predominant in cSLE while cutaneous manifestation and hematological manifestation being predominant in aSLE. However, it was interesting to note that neuropsychiatric manifestations during disease course in aSLE matched cSLE while constitutional manifestation in cSLE matched aSLE showing no difference during disease course (Table 2).
As shown in Table 1, disease activity at disease onset, measured by SLEDAI, was higher in cSLE (13 vs 8.5; p < 0.001). Similarly, as shown in Table 2, the highest mean SLEDAI score measured during disease course was also highest among cSLE (16 vs 11.8; p < 0.001).
At disease onset and during disease course, there was higher tendency of renal involvement in cSLE compared to aSLE. However, despite the difference in frequency of renal involvement, the clinical presentation of renal manifestation was similar. Hematuria, proteinuria and active sedimentation rate occurred in similar frequency (data not shown). Similarly, there was comparable distribution of lupus nephritis histological classes among cSLE compared to aSLE with diffuse lupus nephritis (class IV) occurring in highest frequency followed by focal lupus nephritis (class III) as shown in Table 3. Interestingly, there was higher occurrence of minimal mesangial lupus nephritis (class I) in cSLE as (8.6 vs 0%), while there was higher occurrence of membranous lupus nephritis (class V) in aSLE (15.6 vs 6.4%).
The relative incidence of autoantibodies detected in SLE is summarized in Table 4. ANA and Anti-dsDNA antibodies were the most common autoantibodies detected with a higher frequency of anti-dsDNA positivity in cSLE (94 vs 84%). Additionally, antiphospholipid antibodies (lupus anticoagulant, anticardiolipin antibodies, antiglycoprotein antibodies) were more often detected in children (54 vs 37%; p = 0.026) while anti-smith antibodies occurred more commonly among adults (37 vs 21%; p = 0.025). The incidence of presence of other antibodies was the same both in children and in the adult SLE population.
The prescribed treatment of SLE at disease onset and during course of illness is described in Table 5. Glucocorticoid was the most frequently prescribed treatment without a significant difference between the two groups. Cyclophosphamide and mycophenolate mofetil were more often administered to children with SLE. While exposure to other immunosuppressant drugs, including azathioprine, methotrexate, and biological therapies were similar between the two groups.
Discussion
This study compares childhood and adult onset SLE patients from similar geographical background in Oman of Arab ethnicity. Comparing SLE phenotypes across different age groups revealed similarities but also important differences in demographic features, clinical manifestation, disease activity, serology as well as treatment in our cohort.
We found the female predominance of disease is less pronounced in both age spectrums, < 5 years and > 50 years with a female to male ratio of 3:1. The highest female predominance was between the ages of 18–25 years with a ratio of 11:1. This distinction between the two groups supports the role of hormonal factors, including sex hormones in modifying the disease either in facilitating or suppressing symptoms [4, 25]. The age of onset of cSLE in our cohort was 9.5 ± 4.2 years, which is consistent with age of onset of cSLE in other Arab ethnicity [22], yet younger than Caucasian ethnicity (11.5–13.1 years) [26, 27]. The difference could be partly due to the lack of consensus regarding the cut-off age for defining cSLE, which varies from 13 to 18 years in the literature. In most Arab health care facilities, the transitional age between children to adults is 13 years, however, in our studies, we considered 16 years as the upper limit to define cSLE.
Geographical distribution of aSLE in Omani regions follows the population density of Oman as most of the aSLE being from Al Batinah (n = 42, 30%) and Muscat (n = 36, 26%) region, which together constitute 50% of population of Oman. However, there was geographical clustering of cSLE with 38% of cSLE originating from Al Sharqyiah region, which constitutes 15% of the population of Oman. This could be explained by higher frequency of familial SLE in the subgroup of cSLE patients from Al Sharqiya region [18].
The results of our study confirms the results of earlier studies that demonstrate that SLICC-2012 classification criteria to be more sensitive than ACR-1997 criteria for the diagnosis of SLE [22]. At disease onset, 99% of our SLE cohort fulfilled the SLICC criteria; however, a significant difference was noted in fulfilling the ACR criteria (66% aSLE vs 80% cSLE). Petri et al., demonstrated that SLICC had higher sensitivity (97 vs 83%; p < 0.001) but a lower specificity (84 vs 96%; p < 0.001) than ACR criteria [22]. In another large study multicenter study from Spain and Portugal comparing the two classification criteria in 2044 SLE patients, it demonstrated that the differences in sensitivity is highest when disease duration is < 5 years (76 vs 90%; p < 000.1), hence the SLICC criteria may allow patients with SLE to be diagnosed earlier in the disease course [28]. Similarly, the results of UK JSLE cohort study, which included 225 cSLE patients demonstrated that SLICC-2012 was more sensitive than ACR-1997 at diagnosis (93 vs 84%; p < 0.001) and after follow-up (100 vs 92%; p < 0.001) [29].
Comparing the clinical manifestation of SLE in our study, cSLE experienced a higher frequency of renal, neurological, hemolytic anemia, alopecia, arthritis and pulmonary involvement than their adult counterparts, while aSLE experienced higher frequency of photosensitivity and lymphopenia. A recent systematic review and meta-analysis comparing 1560 cSLE with 8701 aSLE demonstrated similarities as well as differences to the results of our study [30]. Our results were similar to the meta-analysis with higher frequency of renal and neurological manifestation in cSLE and with a higher frequency of cutaneous manifestation such as photosensitivity in aSLE. However, aSLE had a higher likelihood of developing arthritis and pulmonary manifestation in the meta-analysis, which is different from our results. Furthermore, in contrast to meta-analysis, our cSLE cohort did not display a higher tendency of constitutional manifestation; they were equal to aSLE with no significant difference.
Over disease course, the difference in frequency of clinical manifestation did not change between aSLE and cSLE compared to disease onset except for neurological manifestation. It is interesting to note that during the disease course in aSLE, there was increased frequency of neurological manifestations to match cSLE at disease onset. Our result are similar to previous studies that demonstrate that the neuropsychiatric involvement with SLE is at least as common in children as it is in adults, with the former experiencing symptoms more commonly at diagnosis SLE (70 vs 28%) [31, 32].
Generally, renal involvement in SLE, detected by abnormal urinalysis and/or renal function, occurs more frequently in cSLE than in aSLE [11]. Although the frequency of renal involvement in our SLE cohort was higher in children than adults, however the overall frequency was lower than reported at disease onset occurring in 49% of cSLE and 20% of aSLE. Previous studies support a similar distribution of lupus nephritis (LN) histological classes in cSLE compared to aSLE, with diffuse proliferative LN (Class IV) occurring in 40–60% of the patients, focal proliferative LN (Class III) in 10–20%, and membranous LN (Class V) in 3–18% [5, 33, 34]. Similarly, in our cohort of patients with lupus nephritis, Class IV was the most frequent glomerulonephritis (50% aSLE vs 61.7% cSLE) followed by Class III (21.9% aSLE vs 14.9% cSLE) as shown in Table 3. When investigating the clinical characteristics of LN in patients before and after puberty, Torr et al. [35] concluded that the post-pubertal form of SLE is similar to adult one, while pre-pubertal form of SLE had different histological LN profile. Although, the majority of our cohort had disease onset in the pre-pubertal period before the age of 12 years (n = 65; 76%), they had similar distribution of LN as aSLE.
Similar to recent meta-analysis, cSLE patients in our cohort had increased anti-dsDNA antibodies and anticardiolipin antibody compared to aSLE [12]. Infectious processes may result in transient and nonpathogenic anticardiolipin antibody positivity, which occurs more commonly in children. In general, antiphospholipid antibody-related thrombosis is rare in children, and prothrombotic or vasculopathic factors are more frequent in adults. A recent review demonstrated an association of positive RF with Sjogren’s syndrome and inflammatory arthritis [36]. Interestingly, our cohort showed that cSLE were more commonly affected with arthritis, although there was no difference in occurrence of RF between the two groups [12]. Additionally, our cohort showed increased anti-Smith antibody in aSLE. The frequency of anti-Smith antibody in our aSLE cohort (37%) was comparable to other Asian countries (30%), higher than Caucasians (26%) and Latin Americans (11%) but much lower than African Americans (494%) [36,37,38,39]. However, our results were similar to recent meta-analysis and reviews as there was no predilection of other autoantibodies to either age groups (ANA, anti-Smith, anti-RNP, anti- U1RNP, anti-Ro and anti-La, antiphospholipid, lupus anticoagulant, complements, dsDNA, and Coomb’s test).
This study is not without limitations. As this was a retrospective study, missing data from chart review may affect the overall results. While cSLE share many similarities with aSLE, awareness of its differences is crucial, namely higher disease activity and a more aggressive clinical course. The result of our study on SLE patients of Arab ethnicity from Oman confirms the results of other studies that emphasize the importance of ethnicity on disease burden [40]. The current treatment strategies in cSLE are hampered by lack of robust evidence-based studies. To establish optimum care and improve long-term outcomes, we need streamline treatments and improved transition programs from pediatric to adult care.
Conclusion
In conclusion, the results of our study showed similarities and differences between aSLE and cSLE. Our results were similar to other studies reporting cSLE as having a more severe disease onset with higher major organ involvement and having a more severe disease course requiring more aggressive treatment than aSLE. The overall treatment approach in SLE is similar in children to adults. However, as cSLE is a more aggressive disease, the requirements of high doses of corticosteroids and immunosuppressive treatment may be greater than adults to control disease. In our cohort, cSLE received more myocophenolate mofetil and cyclophosphamide therapy than aSLE, two drugs commonly used to treat severe lupus manifestations including lupus nephritis and central nervous system involvement.
References
Lewis MJ, Jawad A (2017) The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematous. Rheumatology 56:67–77
González LA, Toloza SMA, McGwin GJ, Alarcón GS (2013) Ethnicity in systemic lupus erythematous (SLE): its influence on susceptibility and outcomes. Lupus 22:1214–1224
Schwartzman-Morris J, Putterman C (2012) Gender differences in the pathogenesis and outcome of lupus and of lupus nephritis. Clin Dev Immunol 2012:604892. https://doi.org/10.1155/2012/604892
Grainne Murphy G, David Isenberg D (2013) Effect of gender on clinical presentation in systemic lupus erythematosus. Rheumatology 52:2108–2115
Brunner HI, Gladman DD, Ibañez DD, Urowitz MD, Silverman ED (2008) Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum 58:556–562
Brunner HI, Mina R (2013) Update of differences between childhood onset systemic lupus erythematosus and adult onset systemic lupus erythematosus. Arthritis Res Ther 15:218. https://doi.org/10.1186/ar4256
Abdel-Nabi HH, Abdel-Noor RA (2018). Comparison between disease onset patterns of Egyptian juvenile and adult systemic lupus erythematosus (single centre experience). Lupus https://doi.org/10.1177/0961203318760208
Torrente-Segarra V, Salman Monte TC, Rúa-Figueroa I et al (2017) Juvenile- and adult-onset systemic lupus erythematosus: a comparative study in a large cohort from the Spanish Society of Rheumatology Lupus Registry (RELESSER). RELESSER Study Group of the Spanish Society of Rheumatology (SER) and the Study Group of Systemic Autoimmune Diseases of the SER (EAS-SER). Clin Exp Rheumatol 35:1047–1055
Hartman EAR, van Royen-Kerkhof A, Jacobs JWG, Welsing PMJ, Fritsch-Stork RDE (2018) Performance of the 2012 Systemic Lupus International Collaborating Clinics classification criteria versus the 1997 American College of Rheumatology classification criteria in adult and juvenile systemic lupus erythematosus. A systematic review and meta-analysis. Autoimmun Rev 17:316–322
Kang JH, Park DJ, Lee KE, Lee JS, Choi YD, Lee SS (2017) Comparison of clinical, serological, and prognostic differences among juvenile-, adult-, and late-onset lupus nephritis in Korean patients. Clin Rheumatol 36:1289–1295
Malattia C, Martini A (2013) Pediatric onset systemic lupus erythematosus. Best Pract Res Clin Rheumatol 27:351–362
Livingston B, Bonner A, Pope J (2012) Differences in autoantibody profiles and disease activity and damage scores between childhood- and adult-onset systemic lupus erythematosus: a meta-analysis. Semin Arthritis Rheum 42:271–280
Hersh AO, von Scheven E, Yazdany J et al (2009) Differences in long-term disease activity and treatment of adult patients with childhood- and adult-onset systemic lupus erythematosus. Arthritis Rheum 61:13–20
Tucker LB, Uribe AG, Fernandez M et al (2008) Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 17:314–322
Hersh AO, Trupin L, Yazdany J et al (2010) Childhood-onset disease predicts mortality in an adult cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 62:1152–1159
Tektonidou MG, Lewandowski LB, Jinxiang H, Dasgupta A, Ward MM (2017) Survival in adults and children with systemic lupus erythematosus: a systematic review and Bayesian meta-analysis of studies from 1950 to 2016. Ann Rheum Dis 76:2009–2016
Abdwani R, Rizvi SG, El-Nour I (2008) Childhood onset systemic lupus erythematosus: demographics and clinical analysis. Lupus 17:683–686
Abdwani R, Al-Abrawi S, Sharef SW, Al-Zakwani I (2013) Geogrpahical clustering of juvenile onset systemic lupus erythematosus in the Sultanate of Oman. Oman Med J 28:199–203
Al-Maini MH, El-Ageb EM, Al-Wahaibi SS et al (2003) Demographic, autoimmune, and clinical profiles of patients with systemic lupus erythematosus in Oman. Rheumatol Int 23:186–191
Al-Maini M, El-ageb El-N, Al-shukaily AK, Al-wahaibi S, Richens E (2002) Tribal and geographical variations of lupus in the Sultanate of Oman: a hospital-based study. Rheum Int 21:141–145
Hochberg MC (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40:1725
Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT et al (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686
Weening JJ, Agati VD, Schawrtz MM et al (2004) Classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65:521–530
Bombardier C, Gladman D, Urowitz M, Vhang CH; Committee on Prognosis Studies in SLE (1993) Derivation of SLEDAI. A disease activity index for lupus patients. Arthritis Rheum 35:630–640
Margery-Muir A, Bundell C, Nelson D, Groth D, Wetherall J (2017) Gender balance in patients with systemic lupus erythematous. Autoimmun Rev 16:258–268
Hiraki L, Benseler S, Tyrrell P, Hebert D, Harevey E, Silverman E (2008) Clinical and laboratory characteristics and long term outcome of pediatric systemic lupus erythematosus: a longitudinal study. J Pediatr 152:550–556
Bader-Meunier B, Armengaud JB, Haddad E, Salomon R, Deschenes G et al (2005) Initial presentation of childhood-onset systemic lupus erythematosus: a French multicenter study. J Pediatr 146:648–653
Ines L, Silva C, Galindo M et al (2015) Classification of systemic lupus erythematosus: systemic lupus international collaborating clinics versus American college of rheumatology criteria. A comparative study of 2,055 patients from a real-life, international systemic lupus erythematosus cohort. Arthrits Care Res (Hoboken) 67:1180–1185
Lythgoe H, Morgan T, Heaf E et al (2017) Evaluation of ACR and SLICC classification criteria in juvenile-onset systemic lupus erythematous; a longitudinal analysis. Lupus 26:1285–1290
Bundhund PK, Kumari A, Huang F (2017) Medicine (Baltimore) 96(37):e8086
Benseler SM, Silverman ED (2007) Neuropsychiatric involvement in pediatric systemic lupus erythematosus. Lupus 16:564–571
Hanly JG, Urowitz MB, Sanchez-Guerrero J et al (2007) Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum 56:265–273
Marks SD, Sebire NJ, Pilkington C, Tullus K (2007) Clinicopathological correlations of paediatric lupus nephritis. Pediatr Nephrol 22:77–83
Zappitelli M, Duffy CM, Bernard C, Gupta IR (2008) Evaluation of activity, chronicity and tubulointersitial indices for childhood lupus nephritis. Pediatr Nephrol 23:83–91
Tarr T, Derfalvi B, Gyori N et al (2015) Similarities and differences between pediatric and adult patients with systemic lupus erythematosus. Lupus 24:796–803
Merkel PA (2004) Systemic lupus erythematosus. In: Andreoli TE, Carpenter CCJ, Griggs RC, Loscalzo J (eds) Cecil essentials of medicine, 6th edn. W. B. Saunders, Philadelphia (PA, pp 745–749
Ni JD, Yao X, Pan HF, Li XP, Xu JH, Ye DQ (2009) Clinical and serological correlates of anti-Sm autoantibodies in Chinese patients with systemic lupus erythematosus: 1,584 cases. Rheumatol Int 29:1323–1326
Pons-Estel BA, Catoggio LJ, Cardiel MH et al (2004) The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among Hispanics. Medicine (Baltimore) 83:1–17
Arnett FC, Hamilton RG, Roebber MG, Harley JB, Reichlin M (1988) Increased frequencies of Sm and nRNP autoantibodies in American blacks compared to whites with systemic lupus erythematosus. J Rheumatol 15:1773–1776
Ale’ed A, Al-Mayouf S (2014) Systemic lupus erythematosus in Arab children. Differences and similarities with different ethnicities. Saudi Med J 35:566–571
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al Rasbi, A., Abdalla, E., Sultan, R. et al. Spectrum of systemic lupus erythematosus in Oman: from childhood to adulthood. Rheumatol Int 38, 1691–1698 (2018). https://doi.org/10.1007/s00296-018-4032-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-018-4032-2