Abstract
To investigate the influence of fibromyalgia (FM) on achieving remission defined on the basis of the Simplified Disease Activity Index (SDAI) remission criteria in patients with long-standing rheumatoid arthritis (RA). This observational longitudinal cohort consisted of long-standing RA patients being treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) or biological DMARDs (bDMARDs). After 6 months of follow-up, the patients fulfilling or not fulfilling the remission criteria were identified and compared with each other in terms of the presence of FM, neuropathic pain, and other comorbidities. At the end of the 6-month observation period, 24 of the 117 patients (20.4%) met the SDAI remission criteria. Logistic regression analysis showed that the modified Rheumatic Disease Comorbidity Index (mRDCI) (p = 0.0001), the FM presence (p = 0.0001), and the 36-item short-form health survey Mental Component Summary (SF-36 MCS) Score (p = 0.0088) were the strongest predictors of not being in SDAI remission. None of the patients with concomitant FM (17.1%) achieved SDAI remission. In comparison with the non-FM patients, the patients with RA and FM patients had worse scores on the SF-36 MCS (p = 0.011), on the sleep Visual Analogue Scale (VAS) (p = 0.018), on the self-counts of tender joints (p = 0.039), and on the PainDetect Questionnaire (PDQ) (p = 0.001). To avoid over treatment, an assessment of FM should be considered in RA patients who do not fulfil the remission criteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic and disabling inflammatory disease with an unpredictable course and wide variations in severity that affects about 0.5% of the population [1]. It has been accepted that the main target of treatment in RA patients is disease remission [2], which can be defined on the basis of criteria such as the 28-joint Disease Activity Score (DAS-28), a Boolean-based definition of remission simplified criteria, or the Simplified Disease Activity Index (SDAI) [3]. All of these indices consider patient global assessment (PtGA), a variable that can be influenced by various patient-derived, non-inflammatory factors such as fatigue or the concomitant presence of fibromyalgia (FM) [4].
FM often co-exists with RA: it has a prevalence of 2.7–5.1% in the general population, but 10–20% among RA patients [5,6,7]. RA is more severe in patients with concomitant FM in terms of subjective and objective measures, greater medical costs, poorer outcomes, more comorbidities, greater social disadvantages, and a worse quality of life [8, 9].
Pain is the most important independent determinant of PtGA and the patients’ perception of disease activity [10, 11], and the fact that it persists in a substantial proportion of patients even if inflammation seems to be well controlled [12] suggests that inflammation and subsequent joint damage may not be the only causative factors. RA-related pain is often described as ‘gnawing’ or ‘aching’, descriptors that are typically associated with nociceptive pain. However, some patients report qualities typically associated with neuropathic pain (NP) such as ‘burning’ or ‘prickling’ [13,14,15], and neuropatic-like pain symptoms independently correlate with poorer self-reported physical and mental health [16].
The aim of this cross-sectional study was to explore how the presence of FM, and comorbidities can affect the achievement of SDAI remission criteria in patients with RA.
Materials and methods
Patients
RA patients with long-standing disease being treated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs: methotrexate, leflunomide, sulfasalazine, or hydroxychloroquine) or biological DMARDs (bDMARDs) were recruited at the outpatient clinics of two Italian tertiary rheumatology centres between June 2014 and September 2016. The selection criteria were an age of 18–75 years, and the presence of active disease fulfilling the revised ACR/EULAR criteria for RA [17]. Active disease was defined as at least three of the following: an erythrocyte sedimentation rate (ESR) of ≥28 mm/h or C-reactive protein (CRP) levels of >19 mg/dl, morning stiffness for ≥30 min, >5 swollen joints, and >6 tender joints.
Drugs started during study
Of the 27 patients who started csDMARDs, 81.5% began methotrexate, 11.1% sulphasalazine and 7.4% leflunomide. Of the 90 who commenced anti-TNF, 36.5% commenced adalimumab, 31.7% etanercept, 15.8% infliximab, 9.5% golimumab, and 6.3% certolizumab pegol. Further, 11.9% started abatacept, and 11.1% tocilizumab. Forty-eight patients (41.0%) were taking oral corticosteroids at a mean prednisone or equivalent dose of 6.5 mg/day (range 2.5–30).The planned study clinical follow-up was ‘per routine care’, with clinical assessment at baseline and 6 months. All of the patients required an escalated treatment strategy, and the therapy was modified by each rheumatologist with the aim of achieving remission. When possible, the biological drugs were changed, csDMARD treatments were intensified and/or the oral corticosteroid doses were increased.
Assessment of variables
The subjects were evaluated at baseline and after 6 months. Data concerning current and previous treatments were collected from their medical records, and confirmed by the patients themselves during their clinical visits. Their Body Mass Index (BMI) was recorded, and comprehensive questionnaire package was administered that included sociodemographic data, functional measures, and disease-related variables, including disease duration.
The clinical assessment consisted of 28-joint swollen and tender joint counts (SJC and TJC), PtGA, physician global assessment (PhGA), and the determination of the ESR, and CRP, rheumatoid factor (RF), and anti-citrullinated protein antibody (ACPA) levels. The presence of IgM-RF determined by nephelometric method (Image Beckman) and of ACPA determined by ImmunoFluoroMetric Assay (IFMA) (EliA CCP, ImmunoCAP 250, Phadia S.r.l, Italy). The cut-off point for the ACPA positivity was >10 IU/ml, according to the manufacturer’ s instructions, whereas a titre of IgM-RF >40 UI/ml was considered as positive.
The clinical variables and the acute phase reactants were used to calculate the composite DAS28-ESR and DAS28-CRP, the Simplified Disease Activity Index (SDAI), and the Clinical Disease Activity Index (CDAI). For SDAI, remission is defined as values ≤3.3, and low disease activity is defined as values >3.3 and ≤11, moderate disease activity as values >11 and ≤26, and high disease activity as values >26 [18].
The questionnaires were designed to assess patient-reported RA, the presence of FM, NP, the health-related quality of life (HRQoL) and comorbidities. The patient-reported evaluations of RA were obtained using the clinical arthritis activity (PRO-CLARA) [19] and the Recent-Onset Arthritis Disability (ROAD) Questionnaires [20].
The PRO-CLARA is a short and easy to complete self-administered index, without formal joint counts, combining three items on patient’s physical function (as measured by ROAD) [20], self-administered TJC and PGA into a single measure of disease activity. The self-administered TJC was evaluated according to joint list of the RADAI [21]. The RADAI joint mannequin list queries pain “today” in 16 joints or joint groups, including left and right shoulders, elbows, wrists, fingers, hips, knees, ankles, and toes. The self-administered TJC weighted the degree of tenderness of each joint on the following scale: 0 = none; 1 = mild; 2 = moderate; 3 = severe. The self-administered TJC is scored as 0–48; the raw 0–48 score may be recoded to 0–10. The PGA, is scored 0–10 on an 11-point NRS, with the follow question: “How would you describe your general health today? (0 = very well to 10 = very poorly)”. The total score of the PRO-CLARA was completed by summing the scores of the three individual measures and dividing this by three, and range from 0 to 10.
The ROAD questionnaire, is a reliable, valid and responsive tool for measuring physical functioning in patients with RA, and it is suitable for use in clinical trials and daily clinical practice [20, 22, 23]. The ROAD consists of 12 items assessing a patient’s level of functional ability and includes questions related to fine movements of the upper extremity, activities of the lower extremity, and activities that involve both upper and lower extremities. For each item, patients are asked to rate the level of difficulty over the past week on a 5-point scale, which ranges from 0 (without any difficulty) to 4 (unable to do). The ROAD ranges from 0 to 48. To express these scores in a more clinically meaningful format, a simple mathematical normalization procedure was then performed so that all the scores could be expressed in the range 0–10, with 0 representing better status and 10 representing poorer status.
The presence of FM was classified on the basis of the 2010 American College of Rheumatology criteria, which include the Widespread Pain Index (WPI) and a Symptom Severity (SS) Scale [24]. The sum of the WPI and the SS scores was used as a measure of FM [25].
The self-administered PainDetect Questionnaire (PDQ) was used to identify the presence of NP. It consists in nine questions concerning the quality of NP symptoms; no physical examination is required. The first seven questions concern the degree of pain, and are scored from 0 to 5 (0 = never to 5 = very strongly); question eight concerns the pattern of the course of pain, and is scored from −1 to 1 depending on which course pattern diagram is selected; and question nine concerns the presence of radiating pain (“yes” is scored 2 and “no” is scored 0. The final score ranges from −1 to 38, and indicates the likelihood of a neuropathic component: a score of ≤12 indicates a low likelihood (NoP), and a score of ≥19 suggests a high likelihood (NP); an intermediate score indicates the possibility of a neuropathic component [26].
The comorbidity data (self-reported and confirmed or revealed by medical records) were assessed to compute a modified Rheumatic Disease Comorbidity Index (mRDCI), which was specifically created for use in evaluating patients with rheumatic diseases, and has been developed to predict outcomes such as death, physical functioning, direct medical costs, working and social security disability, and hospitalisation [27]. The RDCI was developed initially by Michaud and Wolfe [28] as a self-report instrument among patients with RA to assess the influence of comorbidity on HRQoL, physical disability and costs. The proposed comorbidity index can be calculated by scoring 11 weighted comorbid conditions. Recently, the RDCI was further modified by including obesity (BMI > 30 kg/m2) and kidney disease (defined as a glomerular filtration rate <60 ml/min/1.73 m2) [27]. Finally, a simple comorbidity count (COUNT; range 0-–13), which incorporated all the comorbidities included in the RDCI and its proposed modification, was computed. The formula of the mRDCI is as follows = 1* lung disease and [2* (MI, other CV, or stroke) or 1* Hypertension] and 1* (ulcer or other GI) and 2* kidney disease and 1* if BMI > 30 or 2* if BMI > 35 and 1 for each of the following conditions: diabetes, fracture, depression and cancer [27].
Finally, the study participants were asked to complete the EuroQoL-5 dimensions (EQ-5D) and 36-item short-form health survey (SF-36) as generic HRQoL instruments.
The EQ-5D is divided into two sections: a descriptive system that assesses HRQoL in the five dimensions of mobility, self-care, usual activities, pain/discomfort and anxiety/depression, each with three levels of severity (1 = no problems, 2 = some problems, 3 = severe problems); and a Visual Analogue Scale (VAS) in the form of a thermometer (0 = worst imaginable and 100 = best imaginable health status) that describes the self-perception of health in each dimension on the day of administration [29].
The SF-36 is a generic measure that is designed to capture health status in many different conditions [30]. The SF-36 contains 36 items, organized into eight scales covering the dimension’s physical functioning (PF), role limitations due to physical function (RP), bodily pain (BP), general health (GH), mental health (MH), role limitations due to emotional health (RE), social functioning (SF), and vitality (VT). One additional item pertains to health transition. Raw domain scores are converted to a 0–100 scale, with higher scores indicating better health. These scores are Z transformed and weighted to yield values used to calculate Physical (PCS) and Mental Component Summary (MCS) Scores [30].
Statistical analysis
Descriptive statistics were used to describe the sample, and are given as mean values ± SD or median values and interquartile range (IQR) depending on the distribution (skewness) of continuous data. The two groups were compared using Student’s t test or the Mann–Whitney U test for continuous variables, and the Chi-squared test for categorical variables. If the numbers were <5 in any of the cells of the 2 × 2 analysis, Fisher’s exact test was used.
The statistically significantly variables related to SDAI remission in the univariate analyses (p ≤ 0.05) were included in the multivariate binary logistic regression analysis to determine the independent predictors of SDAI remission.
The statistical analyses were made using SPSS Statistics 19.0 (IBM, Armonk, NY, USA), and MedCalc 7.1.02 (MedCalc Software, Ostend, Belgium) statistical software packages for Windows XP.
Results
The study was completed by 117 RA patients (77.2% females) with a mean age of 59 years (range 25–83), a mean disease duration of 11.2 years, a mean BMI of 25.8 (range 18.5–44.1). Their serology was characterised by the presence of RF in 82 patients (70.1%) and ACPA in 71 (60.6%). The mean mRDCI was 2.16 ± 1.32. Table 1 shows the demographic and disease-related characteristics of the cohort as a whole, and the results of the comprehensive baseline clinimetric evaluation.
After 6-month follow-up, 24 of the 117 patients (20.4%) fulfilled the SDAI remission criteria.
Comparison of the patients in or not in SDAI remission showed that the only statistically significant differences were baseline PDQ score (p = 0.011), mRDCI values (p = 0.021), and the FM presence (p = 0.001).
The logistic regression analysis used SDAI remission as the dependent variable, and age, gender, ACPA and RF titres, BMI, disease duration, years of education, the PDQ, mRDCI, sum of the WPI and SS scales, and the SF-36 MCS and PCS values as independent variables. The results showed that the strongest predictors of not being in SDAI remission were the mRDCI (odds ratio 9.593, p = 0.0001), the FM presence (odds ratio 9.147, p = 0.0001), and the SF-36 MCS score (odds ratio 2.358, p = 0.0088) (Table 2).
Twenty patients (17.1%) had concomitant FM, and worse PDQ (p = 0.001), SF-36 MCS (p = 0.011), sleep VAS (p = 0.018), self-TJC (p = 0.039), TJC (p = 0.041), GH (p = 0.035), and WPI + SS values (p = 0.001). Table 3 shows the baseline differences in all of the parameters between the patients with and without concomitant FM.
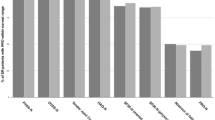
Analysis of the individual SF-36 domains showed that the patients with FM had worse VT, SF, RE and MH scores (p < 0.01) (Fig. 1). None of the patients with FM had achieved remission by the end of the 6-month observation period (contingency coefficient 0.199; p = 0.0285).
Box-and-whisker plot of the 36-item short-form health survey domains in rheumatoid arthritis patients with (dashed box and lines) and without fibromyalgia (blue box and lines). BP bodily pain, GH general health perceptions, RP role limitations due to physical health, PF physical functioning, RE role limitations due to emotional problems, MH general mental health, SF social functioning, VT vitality, PCS physical component summary, MCS mental component summary
Discussion
The findings of this longitudinal prospective study show that only a minority of patients with long-standing RA achieved the SDAI remission criteria 6 months after the escalation of treatment.
Logistic regression analysis revealed how the strongest independent predictors of a SDAI remission failure were the presence of FM, the presence of the other comorbidities evaluated using the mRDCI, and poor mental health as assessed by means of the SF-36.
It has been shown that concomitant FM may explain why patients give higher disease activity ratings than their physicians. FM is more prevalent among RA patients than in the general population, and RA patients with concomitant FM give higher disease activity ratings than their counterparts without FM [31]. FM was present in 17.1% of our RA patients as against its 2.2% prevalence among controls in the Italian population [1], and none of these patients achieved remission. Similarly, Inanc et al. found that their patients’ Polysymptomatic FM Distress Scores were higher in those who did not achieve remission than in those who did [32]. Data from the ESPOIR cohort confirmed these findings in patients with early RA: the achievement of remission or low disease activity was significantly less likely in subjects with concomitant FM [33]. This could lead to strategies of escalating the intensity of RA treatment. The aim of RA treatment is to control inflammation, and composite disease activity indices may be affected by non-inflammatory pain in RA patients with co-existing FM. One possibility in such subjects is to establish a less stringent target but, given the cost and potential toxicity of DMARDs, it is crucial to determine who will truly benefit from intensive therapy.
It has been demonstrated that the presence of FM in RA patients leads to higher medical costs, worse outcomes, more comorbidities, sociodemographic disadvantages, and a worse quality of life [8], and concomitant FM in our RA patients was associated with worse PDQ, SF-36 MCS and sleep VAS scores, and worse self-TJCs. Lee et al. have shown that inflammation, psychosocial factors, and peripheral and central pain processing are intricately interrelated in RA patients [34]. As pain is the most important determinant of a patient’s perception of RA disease activity [11], it is essential that patients and physicians recognise the causes of non-inflammatory pain [35].
The administration of the PDQ could be an interesting means of identifying the presence of neuropatic-like pain. A previous study has used the PDQ to assess the occurrence and associations of NP-like symptoms in a cohort of patients with relatively well-controlled RA. Although almost 75% of the patients were in DAS28 remission, 44% still reported clinically significant pain, 17% had probable NP, and 21% had the features of possible NP: the patients all had more severe pain, used pain medications more frequently, and reported a poorer HRQoL [16]. However, patients with FM usually record high PDQ scores, and the results of PDQ should be carefully interpreted as Gauffin et al. found that only 34% of patients with primary FM and a high PDQ score had clinically verifiable neuropathic pain, and compromised central pain control is a major problem [36].
This study has some limitations that should be taken into account when interpreting the findings. First of all, as it was carried out in a tertiary referral setting, patients with more severe RA may be over-represented and the results may not be generalisable to all RA patients in the community. Second, the recall periods of the various measures were different as is usual when using multiple self-report measures, however, self-reported data are a valuable resource and the problems encountered are similar to those associated with other forms of data collection. Third, we used self-report questionnaires to evaluate NP instead of a clinical diagnostic examination.
In conclusion, RA patients frequently have associated FM, and therefore report poorer mental health and persisting pain even if the inflammation is well controlled. Fibromyalgic symptoms, pain, and poorer mental health are all associated with increased disease activity scores. In RA patients who do not fulfil the remission criteria, the assessment of other comorbidities (especially FM) and neuropathy-like pain must be considered as separate pain sources to avoid improperly targeted treatment.
References
Salaffi F, De Angelis R, Grassi W (2005) Prevalence of musculoskeletal conditions in an Italian population sample: results of a regional community-based study. The MAPPING study. Clin Exp Rheumatol 23:819–828
Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G et al (2010) Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis 69:631–637
Makinen H, Hannonen P, Sokka T (2006) Definitions of remission for rheumatoid arthritis and review of selected clinical cohorts and randomized clinical trials for the rate of remission. Clin Exp Rheumatol 24:S22–S28
Masri KR, Shaver TS, Shahouri SH, Wang S, Anderson JD, Busch RE et al (2012) Validity and reliability problems with patient global as a component of the ACR/EULAR remission criteria as used in clinical practice. J Rheumatol 39:1139–1145
Wolfe F, Cathey MA, Kleinheksel SM (1984) Fibrositis (fibromyalgia) in rheumatoid arthritis. J Rheumatol 11:814–818
Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L (1995) The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 38:19–28
Bazzichi L, Giacomelli C, Consensi A, Atzeni F, Batticciotto A, Di Franco M, Casale R, Sarzi-Puttini P (2016) One year in review 2016: fibromyalgia. Clin Exp Rheumatol 34:S145–S149
Wolfe F, Michaud K (2004) Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize RA patients with fibromyalgia. J Rheumatol 31:695–700
Wolfe F, Michaud K (2009) Outcome and predictor relationships in fibromyalgia and rheumatoid arthritis: evidence concerning the continuum versus discrete disorder hypothesis. J Rheumatol 36:831–836
Khan NA, Spencer HJ, Abda E, Aggarwal A, Alten R, Ancuta C et al (2012) Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res 64:206–214
Studenic P, Radner H, Smolen JS, Aletaha D (2012) Discrepancies between patients and physicians in their perceptions of rheumatoid arthritis disease activity. Arthritis Rheum 64:2814–2823
Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA et al (2011) Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther 13:R83
Burckhardt CS (1984) The use of the McGill Pain Questionnaire in assessing arthritis pain. Pain 19:305–314
Charter RA, Nehemkis AM, Keenan MA, Person D, Prete PE (1985) The nature of arthritis pain. Br J Rheumatol 24:53–60
Roche PA, Klestov AC, Heim HM (2003) Description of stable pain in rheumatoid arthritis: a 6 year study. J Rheumatol 30:1733–1738
Koop SM, ten Klooster PM, Vonkeman HE, Steunebrink LM, van de Laar MA (2015) Neuropathic-like pain features and cross-sectional associations in rheumatoid arthritis. Arthritis Res Ther 17:R237
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 62:2569–2581
Mierau M, Schoels M, Gonda G, Fuchs J, Aletaha D, Smolen JS (2007) Assessing remission in clinical practice. Rheumatology 46:975–979
Salaffi F, Migliore A, Scarpellini M, Corsaro SM, Laganà B, Mozzani F et al (2010) Psychometric properties of an index of three patient-reported outcome (PRO) measures, termed the CLinical ARthritis Activity (PRO-CLARA) in patients with rheumatoid arthritis. The NEW INDICES study. Clin Exp Rheumatol 28:186–200
Salaffi F, Bazzichi L, Stancati A, Neri R, Cazzato M, Consensi A et al (2005) Development of a functional disability measurement tool to assess early arthritis: the Recent-Onset Arthritis Disability (Road) Questionnaire. Clin Exp Rheumatol 23:628–636
Stucki G, Liang MH, Stucki S, Brühlmann P, Michel BA (1995) A self-administered rheumatoid arthritis disease activity index (RADAI) for epidemiologic research: psychometric properties and correlation with parameters of disease activity. Arthritis Rheum 38:795–798
Salaffi F, Stancati A, Neri R, Grassi W, Bombardieri S (2005) Measuring functional disability in early rheumatoid arthritis: the validity, reliability and responsiveness of the Recent-Onset Arthritis Disability (ROAD) index. Clin Exp Rheumatol 23:S31–S42
Salaffi F, Franchignoni F, Giordano A, Ciapetti A, Gasparini S, Ottonello M (2013) Classical test theory and Rasch analysis validation of the Recent-Onset Arthritis Disability questionnaire in rheumatoid arthritis patients. Clin Rheumatol 32:211–217
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P et al (2010) The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 62:600–610
Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS et al (2011) Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 38:1113–1122
Freynhagen R, Baron R, Gockel U, Tölle TR (2006) PainDetect: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 22:1911–1920
Spaetgens B, Wijnands JM, van Durme C, Boonen A (2015) Content and construct validity of the rheumatic diseases comorbidity index in patients with gout. Rheumatology (Oxford) 54:1659–1663
Michaud K, Wolfe F (2007) Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheum 21:885–906
Group EQ (1990) EuroQol—a new facility for the measurement of health-related quality of life. Health Pol 16:199–208
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Ranzolin A, Brenol JC, Bredemeier M, Guarienti J, Rizzatti M, Feldman D et al (2009) Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short-form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum 61:794–800
Inanc N, Yilmaz-Oner S, Can M, Sokka T, Direskeneli H (2014) The role of depression, anxiety, fatigue, and fibromyalgia on the evaluation of the remission status in patients with rheumatoid arthritis. J Rheumatol 41:1755–1760
Durán J, Combe B, Niu J, Rincheval N, Gaujoux-Viala C, Felson DT (2015) The effect on treatment response of fibromyalgic symptoms in early rheumatoid arthritis patients: results from the ESPOIR cohort. Rheumatology (Oxford) 54:2166–2170
Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH et al (2009) The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross- sectional study. Arthritis Res Ther 11:R160
Ablin JN, Efrati S, Buskila D (2016) Building up the pressure on chronic pain. Clin Exp Rheumatol 34:S3–S5
Gauffin J, Hankama T, Kautiainen H, Hannonen P, Haanpää M (2013) Neuropathic pain and use of PainDETECT in patients with fibromyalgia: a cohort study. BMC Neurol 13:21
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have not received any financial support or other benefits from commercial sources for the work described in this paper. They also declare that they have no other financial interests that could create a potential conflict of interest or the appearance of a conflict of interest with regard to this work.
Funding
None.
Ethical approval
All the procedures in this work were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Salaffi, F., Gerardi, M.C., Atzeni, F. et al. The influence of fibromyalgia on achieving remission in patients with long-standing rheumatoid arthritis. Rheumatol Int 37, 2035–2042 (2017). https://doi.org/10.1007/s00296-017-3792-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3792-4