Abstract
An imbalance in pro- and anti-inflammatory cytokines is suggested to contribute to tissue damage in rheumatoid arthritis (RA). This study was aimed at investigating profiles of cytokines in circulation and cytokines produced by mitogen-stimulated peripheral blood mononuclear cells (PBMC) in RA patients and healthy controls, and to explore correlations of cytokines with disease activity. Our aim was to identify patterns of cytokine expression as possible indicators of disease activity. Levels of plasma cytokines and PBMC-secreted cytokines were estimated in 26 female RA patients and 28 controls. Five pro-inflammatory cytokines (IFN-γ, TNF-α, IL-6, IL-17, IL-12) and three anti-inflammatory cytokines (IL-4, IL-10, IL-13) were assayed in a multiplex ELISA. RA patients had significantly higher plasma levels of TNF-α, IL-12, and IL-4 compared to healthy controls. On the other hand, mitogen-activated PBMC secreted significantly higher levels of the pro-inflammatory cytokines TNF-α, IFN-γ, IL-17, and IL-12, but lower levels of the anti-inflammatory cytokine IL-10 in RA compared to healthy subjects. The ratios TNF-α/IL-10, IFN-γ/IL-10, IL-17/IL-10, IL-12/IL-10, and IFN-γ/IL-13 were significantly higher in RA patients compared to healthy controls. The range and expression of cytokines were higher in PBMC than in the plasma in all the groups studied. Multivariate pattern analysis of eight cytokines revealed a prediction accuracy of 69% in differentiating RA patients from healthy controls, and of 73% in classifying patients as in remission or active RA. Our data suggest that it is worthwhile to explore ratios of pro- to anti-inflammatory cytokines produced by mitogen-stimulated PBMC in RA, and the use of multivariate cytokine pattern and algorithms for better delineation of this condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While the etiology of rheumatoid arthritis (RA) continues to be unexplained, cytokines are known to be major contributors to its pathophysiology [1,2,3]. Several reports have demonstrated critical roles for pro-inflammatory cytokines, such as tumour necrosis factor-alpha (TNF-α), interferon-gamma (IFN-γ), and interleukin (IL)-1 in the pathogenesis of RA, while other cytokines of the pro-inflammatory axis including IL-12, IL-15, IL-17, and IL-18 have been shown to be essential in sustaining the inflammation [2]. On the other hand, the compensatory anti-inflammatory responses of IL-4, IL-10, IL-13, and TGF-α [3] are known to decrease the formation of pro-inflammatory cytokines and regulate the pathophysiology of RA [1,2,3]. Given the fact that cytokines generally function as part of a large and complex network of other cytokines, chemokines, receptors, and antagonists, it has been suggested that the pathology underlying RA, to a significant extent, is due to the imbalance between the pro- and anti-inflammatory cytokines that leads to subsequent tissue damage [2].
Numerous researchers have focused their attention on identifying candidate biomarkers that correlate accurately with rheumatoid arthritis, as this will assist clinicians in pretreatment prediction of therapy effectiveness, monitoring treatment outcome, and assessing disease activity [2]. Thus, there is a need to search for new biochemical predictors or indicators that better reflect the disease status, not only in terms of clinical features but also to mirror the overall inflammatory immune status. While finding a single marker would be ideal, a ratio of two or profile of a group may be more informative and predictive.
Several previous studies have reported contradictory results while using single or multiple serum levels of cytokines as potential biomarkers for RA [4]. It has been suggested that measuring cytokines in plasma is more accurate than that in serum as the clotting process may contribute or induce cytokine production [5,6,7]. Another approach to assess the systemic inflammatory status would be to measure cytokines produced by mitogen-stimulated peripheral blood mononuclear cell (PBMC) cultures ex vivo. Cytokine production patterns of PBMC are likely to provide more information on the immune reactivity and bias as compared to serum or plasma levels alone. Although this approach has been reported by some researchers [8, 9], further work is needed to compare these approaches in the same subjects.
In this study, we report the simultaneous measurement of multi-cytokine profiles in plasma and culture supernatants of mitogen-stimulated PBMC from patients with RA as compared to age- and sex-matched healthy controls. We further attempt to correlate the levels of these cytokines to disease activity as measured by various standardized clinical indices. Our aim was to identify patterns of cytokine expression as possible indicators of disease activity and compare their levels in plasma versus culture supernatants.
However, the absolute levels of cytokines per se may not be as informative as the relative levels of pro- to anti-inflammatory cytokines, as this will provide a better picture of the overall dominance of one group of cytokines over another. As anti-inflammatory cytokines may neutralize or antagonize the effect of pro-inflammatory cytokines, it is logical to calculate the ratios of pro- to anti-inflammatory cytokines as an indicator of the balance of cytokines or the overall cytokine bias, and thus the overall immune reactivity. Keeping this in mind, we calculated the ratios of pro- to anti-inflammatory cytokines in different permutations.
Given that cytokines form a network of interacting entities and that a single cytokine or a ratio of two may not provide sufficient information about the overall immune reactivity, it is of interest to study the combined levels of several cytokines as this may provide a better picture of immune reactivity. The multivariate cytokine profile may also suggest a pattern that can be used as a predictive tool to follow the progression and prognosis of the disease. We therefore tested our data using multivariate predictive modeling statistical methods that can detect more complex differences in patterns of cytokine interrelationships than simple ratios or tests of correlations. We also made a classification model to classify patients with RA as compared to normal healthy controls. Moreover, we studied possible correlation of cytokine production patterns to disease activity.
Methods
Patients and controls
This is an observational case–control study comparing rheumatoid arthritis patients to healthy controls. The study group consisted of female patients (n = 26) with RA disease as per the definition of the American College of Rheumatology [10]. These subjects were recruited from the Rheumatology Unit of Mubarak Al Kabeer Hospital, Kuwait, and underwent clinical assessment by a single recruiter. All patients were treated with disease-modifying anti-rheumatic drugs (DMARDs), and non-steroidal anti-inflammatory drugs (NSAIDs) were added when appropriate. Complete clinical and laboratory evaluations were conducted for the patients and accordingly categorized as active RA (n = 14) or remission RA (n = 12), using conventional clinical tools and indices for assessment of disease activity [10]. The indices used were standard 28-joint disease activity score (DAS-28) [11], clinical disease activity index (CDAI) [12], and simple disease activity index (SDAI) [13].
The control group consisted of age-matched healthy female volunteers with no history of inflammatory or autoimmune diseases, who were recruited by advertisements on campus (n = 28). All clinical and laboratory evaluations for these subjects were within the normal healthy limits. The study was granted ethical approval from the Health Sciences Center, Kuwait University and all participants gave written informed consent prior to participating in the study.
Patients on systemic corticosteroids were excluded due to possible effects of these drugs on immune function and cytokine production. Women who were experiencing any infectious disease were also excluded, due to possible systemic changes in cytokine profiles. Power analysis was conducted using the G*Power statistics software [14] (www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3). Based on the means and SEM observed by us in previous studies on cytokines in pregnancy [15, 16], the tested sample numbers were adequate to demonstrate differences at the 95% confidence interval.
Blood sampling and measurement of CRP
The procedures for blood sampling and transport were similar for all subjects. Whole blood samples were collected in EDTA on the day of clinical assessment, and processed for erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Plasma and sera samples were separated and stored at −80 °C until analysis was done.
C-Reactive protein (CRP) was measured in the sera by rate nepholometry using the IMMAGE Immunochemistry Systems from Beckman Coulter (Galway, Ireland). The detection sensitivity of the assay was 0.1 mg/dL.
Mitogen-induced stimulation of PBMC
Peripheral blood mononuclear cells (PBMC) were separated from blood by Ficoll-hypaque density gradient centrifugation, suspended in RPMI medium containing 10% fetal calf serum, aliquoted into 96-well tissue culture plates at a density of 105 cells per well, and then challenged with the mitogen phytohemagglutinin (PHA) at a concentration of 5 μg/ml for a period of 96 h. Supernatants were collected, centrifuged, and stored frozen till analysis.
Estimation of cytokine levels in plasma and culture supernatants
A multiplex ELISA-based immunoassay, containing dyed microspheres conjugated with monoclonal antibodies specific for target cytokines, was used according to the manufacturer’s instructions (Merck Millipore, Darmstadt, Germany). Soluble molecules were measured using human cytokine commercially available kits for an 8-plex panel (IFN-γ, TNF-α, IL-6, IL-12, IL-17, IL-4, IL-10, and IL-13) (HCYTOMAG-60 K). Concentrations of all analytes were determined using a MAGPIX array reader (Luminex, Austin, TX) that quantitates multiplex immunoassays in a 96-well format with very small sample volumes. Analyte concentrations were calculated using standard curves, with software provided by the manufacturer (Luminex Manager Software). The sensitivity of each of the assays (minimum detectable concentrations, pg/ml) was as follows: TNF-α: 0.7, IL-6: 0.9, IL-12P70: 0.6, IL-17: 0.6, IL-4: 4.5, IL-10: 1.1, IL-13: 1.3, and IFN-γ: 0.7. Quality control measures were followed as per the recommendation of the manufacturers. The range of intra-assay coefficient of variation was 1.6–2.9%, while that of inter-assay was 9.2–18.3%. The accuracy in terms of recovery in matrix ranged from 94.5 to 104%.
Statistical methods
Normality of distribution of data was first determined by Kolmogorov–Smirnov test and groups were compared with one-way ANOVA, Student’s t test or Mann–Whitney U test. Categorical variables were compared using Pearson’s Chi-square test, while Spearman’s rank correlation coefficient was calculated to determine correlations between different measures and cytokine levels. p value of less than 0.05 was considered statistically significant for all tests. For testing differences between multivariate groups, the nonparametric multivariate Cramer Test [17] was run in RStudio [18]. This test gives a p-value for testing whether two dataset samples are from the same multivariate distribution. The test statistic is the difference of the sum of all the Euclidean interpoint distances between the random variables from the two different samples, and half of the two corresponding sums of distances of the variables within the same sample. Thus if samples within a group are closer to each other than samples between groups, on an average, then the two data samples may have been generated by different distributions. This is a nonparametric test, and hence is suited to our cytokine data. Because the test is based on distances, we first log-transformed the cytokine, and then centered and scaled the variables in the usual manner using the mean and standard deviation. By multivariate cytokine pattern, we mean the typical cytokine values/combinations associated to the condition.
For the classification algorithm, classification and regression trees (CART), the default gini method was used for creating trees and finding the accuracy [19]. By “accuracy” we mean the fraction of correct classification predictions. The data are repeatedly and randomly split into a ‘training set’ and ‘testing set’, and the training set is used to predict the classes (e.g., whether active or remission) of the testing samples. The fraction of correct predictions is the accuracy we refer to. One advantage of using trees for classification is that trees can give a fairly clear interpretation as to why a given sample was classified into one group or the other. The reason is that the tree is designed so that the uppermost splits of the tree (the first few splits) are for the cytokine that most cleanly separates the groups. Hence the relative importance of each cytokine for separating the groups—cytokine importances—can be extracted by combining factors, such as how often and how high in the tree the cytokine appears. The final cytokine importance value is a kind of average of these factors. Variable importances are a standard and commonly used way to measure the relative influence of each variable in separating groups. Hyper parameters were optimized by cross-validation, while searching over these values: Max tree depth 1, 2, 3, 4; minimum samples per leaf 5, 6, 7; the number of features to consider when looking for the best split was 2, 3, 4,.., 9. Cross-validation was undertaken by 500 random stratified samples, splitting the data into train/test sets. For the normal vs. RA data, test sets of size 5 were used (from 54 total data samples). For the active vs. remission data, test sets of size 2 were used (from 26 total data samples). Data and statistical analysis were done using SPSS 23 software (SPSS Inc., Chicago, IL, USA) and Scikit-learn [20] using the iPython interface [21].
Results
Demographic characteristics of subjects are as shown in Table 1. Patients (i.e., all RA) and healthy control subjects as well as active and remission RA groups were similar with respect to age. Patients in the active group had significantly higher values of all clinical and laboratory measures with the exception of CRP.
Plasma and PBMC-secreted cytokine levels in patients and controls
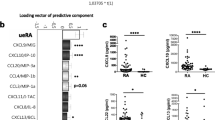
Patients with RA showed significantly higher plasma levels of the pro-inflammatory cytokines TNF-α, IL-12, and the anti-inflammatory cytokine IL-4 as compared to healthy controls (p = 0.019, 0.042, and 0.013, respectively) (Fig. 1a). There were no significant differences in the levels of other pro-inflammatory or anti-inflammatory cytokines.
On the other hand, the mitogen-stimulated PBMC of the same groups produced significantly higher levels of the pro-inflammatory cytokines TNF-α, IFN-γ, IL-12, and IL-17 in RA patients as compared to the control group (p = 0.044, 0.004, 0.046, and 0.002, respectively). Further, the median level of the anti-inflammatory cytokine IL-10 was significantly lower in RA patients as compared to the control group (p = 0.025) (Fig. 1b).
Ratios of pro- to anti-inflammatory cytokines in RA patients and healthy controls
Interestingly, none of the cytokine ratios in plasma were statistically significantly different between the two groups (RA vs. healthy controls). However, in mitogen-induced PBMC cultures, ratios of TNF-α/IL-10, IFN-γ/IL-10, IL-17/IL-10, IL-12/IL-10, and IFN-γ/IL-13 were statistically significantly higher in RA patients as compared to healthy controls (p = 0.002, 0.0001, 0.0001, 0.001, and 0.014 respectively) (Table 2).
Multivariate cytokine profiles in patients and controls
The multivariate Cramer test showed no significant difference in plasma levels of all the tested cytokines in patients with RA as compared to normal healthy controls. Looking into prediction accuracy using the CART method, and as this study included 28 healthy volunteers and 26 RA patients, the trivial classification rule which assigns all subjects as normal would accurately predict 28/(28 + 26) = 52% of patients. Hence 52% is the no-information rate (NIR) [22], which is a baseline to compare classification accuracies. Using the CART method, we obtained a prediction accuracy of 64%. This is better than the NIR of 52%. The algorithm also provides information on the cytokines used to classify the two groups, as well as a value between 0 and 1 indicating their importance (higher values meaning higher importance). In this case, the cytokines and values were: TNF-α (0.41), IL-4 (0.29), IL-12 (0.21), and IL-6 (0.07).
However, the multivariate Cramer test showed significant differences in all the PBMC-secreted cytokines measured in patients with RA as compared to normal healthy controls (p = 0.007). Looking into prediction accuracy using the CART method for the eight cytokines measured in the PBMC culture supernatants, we obtained a prediction accuracy of 69%. This accuracy is better than 52%, and more accurate than that obtained using the plasma samples. The most important cytokines were: IFN-γ (0.32), IL-17 (0.24), and IL-10 (0.33), where only cytokines with an importance value >0.05 are given. In other words, according to this algorithm, the most important cytokines in distinguishing the two groups were IFN-γ, IL-17, and IL-10.
Plasma and PBMC-secreted cytokine levels in RA patients with active disease and remission
Patients with remission showed statistically significantly higher production of IL-6 in mitogen-stimulated PBMC cultures as compared to those with active disease (p = 0.027). However, the differences in levels of other cytokines were not statistically different (Fig. 2b). Similarly, plasma cytokine levels were not statistically significantly different between the two groups (Fig. 2a). The pro- to anti-inflammatory cytokine ratios in plasma and PBMC cultures were also not significantly different between patients with active disease as compared to those in remission (data are not shown). We also tested for multivariate differences between groups of active and remission patients in plasma or culture samples using the Cramer test, but did not find any statistically significant differences.
Turning to the classification algorithm, we attempted to classify patients into active vs. remission based only on their cytokine levels using the same approach as for the normal vs. RA groups. Because this study included 12 patients with remission vs. 14 patients with active disease, the NIR baseline is 14/(14 + 12) = 54%. Using cytokine values from plasma, we obtain an accuracy of 66%. The most important cytokines were IL-12 (0.41), IFN-γ (0.35), and IL-6 (0.14). Using cytokine values from mitogen-induced PBMC cultures, we obtain classification accuracy of 73%, which is higher than the plasma value. The most important cytokines were IL-6 (0.58) and IL-4 (0.37). In other words, RA patients at active or remission state of the disease showed a similar circulatory and culture cytokine pictures with respect to individual levels (with the exception of IL-6 levels in culture), pro- to anti-inflammatory ratios as well as similar multivariate cytokine patterns.
Correlations between parameters and clinical measures of rheumatoid arthritis patients
Table 3 presents the significant correlations between cytokine levels and clinical indicators in patients with rheumatoid arthritis. It is worth mentioning that although some cytokines showed significant p values, the correlation values (rho) were in the range 0.4–0.6 indicating weak associations. Among the circulatory plasma cytokine levels, IFN-γ correlated only with CDAI, IL-6 correlated with DAS-28, while levels of IL-12 and IL-13 correlated with all three clinical indicators, such as DAS-28, CDAI, and SDAI (Table 3). The plasma multivariate cytokine pattern further correlated significantly with DAS, SDAI, and CDAI (rho = 0.6). However, neither of the individual cytokine levels in mitogen-stimulated PBMC culture nor the multivariate pattern correlated with any of the clinical measures (DAS-28, CDAI, and SDAI) (data not shown).
Discussion
To the best of our knowledge, this is the first study to report simultaneous measurement of multiple cytokines from plasma and cell culture supernatants followed by comparisons of individual levels, ratios of pro- to anti- inflammatory cytokines, and investigation of the differences in multivariate cytokine profile patterns in RA patients as compared to healthy controls, and between RA patients in remission and active disease.
There are several studies reporting contradictory results on circulating levels of cytokines. We reported higher plasma levels of TNF-α, IL-12, and IL-4 in patients with RA as compared to healthy controls. However, the pro- to anti-inflammatory cytokine ratios in plasma were comparable, and similarly there was no significant difference between groups using the multivariate analysis for all cytokines tested. Other studies have reported a range from no detectable levels of cytokines in sera or plasma to no significant differences between the healthy and RA groups, and up to a wide range of significant differences in a variable number of individuals or combinations of cytokines (reviewed in [4]). Such discrepancies highlight the importance of analyzing a wider panel of cytokines and examining the balance of several cytokines rather than single differences. On the other hand, our study demonstrates that cytokine production patterns of peripheral blood mononuclear cells are more discriminatory than plasma measurements of cytokine profiles; which provide optimism for the identification of potential biomarkers. As compared to healthy controls, PBMC from patients with RA produced statistically significantly different levels of multiple cytokines, supported by differences in ratios of pro- to anti-inflammatory cytokines and multivariate pattern with reasonable prediction accuracy.
It is interesting that the multivariate pattern of all tested cytokines was discriminatory in PBMC cultures but not in circulatory cytokine levels. The observation of plasma samples having less accurate predictions as compared to culture supernatants could be partly explained by the fact that many of the cytokines were undetectable in the plasma, which typically means that there is less information available in plasma measurements to differentiate between groups.
Distinguishing active and remission on the basis of cytokines is more challenging than distinguishing healthy individuals from patients with rheumatoid arthritis [4, 9, 23]. It would be of great clinical use to develop a tool to classify patients into active/remission by measuring a single or couple of blood markers, such as cytokines. However, with the exception of culture levels of IL-6, there were no differences in levels of other cytokines or ratios. IL-6 has several contradictory activities—while mainly recognized as a pro-inflammatory molecule; under some circumstances, it can exhibit anti-inflammatory properties suppressing other pro-inflammatory cytokines, such as IL-1, TNF-α, and IL-12 [3]. Also, while many pro-inflammatory cytokines are categorized as Th1-type cytokines, IL-6 is classified as a Th2-type cytokine [1,2,3]. Davis et al. [9] studied cytokine profiles in early and late RA, but could not distinguish between these groups based on cytokines profiles. Our data showed that neither individual levels, ratios of pro- to anti-inflammatory cytokines nor the multivariate patterns from plasma or culture supernatants were statistically significantly different in patients with active or remission RA. This may help explain the discrepancy between the clinical evaluation and inflammatory status of the disease.
Clinical indices were reported to have limited sensitivity in the detection of ongoing subclinical systemic inflammation which may explain in part the increased prevalence of atherosclerosis and cardiovascular complications in patients with rheumatic diseases [24, 25]. A patient classified as being in “remission” by a clinical index may still have subclinical systemic inflammation which may continue to contribute to joint deterioration [26].
Although we observed significant correlations between plasma levels of a few cytokines and some clinical indices, the associations were weak. Furthermore, neither the individual cytokine levels in mitogen-stimulated PBMC culture nor the multivariate pattern correlated with any of the clinical assessment indices of disease activity. While some earlier reports showed a positive correlation between plasma levels of IL-6 and disease activity [23], others reported no such relation [27]. This may further suggest that the changes in cytokine balance in these groups are more complex than just a simple linear pattern. Thus we suggest that it might be worthwhile to use multivariate analysis and define parameters with most discrepant contribution between groups as a better approach to discriminate patients into active and remission stage of the RA disease. However, despite the limited data, the developed algorithm using the multivariate pattern of 8 cytokines revealed a prediction accuracy of up to 73% in classifying patients as in remission or active RA using cytokine production patterns in mitogen-induced PBMC culture supernatants.
We recognize that this study has limitations. The statistical power of multivariate analysis is dependent on sample numbers, and there is an increased risk of “over-fitting” or observing false positive results when analyzing high dimensional data with just a few samples (many cytokines, but few samples). Further, cytokine importance values are sensitive to the idiosyncrasies of the particular dataset being studied. Given our dataset of only a few samples, and especially because the data have several variables, i.e., several cytokines, both the accuracies and cytokine importance values should be taken as tentative results, subject to future corroboration or adjustment. Having said that, our analysis and findings do point to several interesting avenues to be explored further.
Multivariate cytokine analysis and cytokine ratios may well prove to be valuable in devising strategies for immunomodulatory intervention in rheumatoid arthritis. It would be interesting to develop a multivariate model to predict changes in cytokine balance and to evaluate the progression of patient disease status accordingly. The accuracy of such a model would certainly imply the use of extensive data at different stages of RA disease.
In conclusion, compared to normal healthy females, RA patients had cytokine levels, ratios, and production patterns that are markedly different in mitogen-stimulated PBMC cultures than those seen in plasma. Our data further suggest that it would be sensible to explore ratios of pro- to anti-inflammatory cytokines produced by mitogen-stimulated PBMC, and to use multivariate cytokine patterns and algorithms for better prediction of RA disease.
References
Mateen S, Zafar A, Moin S, Khan AQ, Zubair S (2016) Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin Chim Acta 455:161–171. doi:10.1016/j.cca.2016.02.010
Brzustewicz E, Bryl E (2015) The role of cytokines in the pathogenesis of rheumatoid arthritis—practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 76(2):527–536. doi:10.1016/j.cyto.2015.08.260
Vervoordeldonk MJ, Tak PP (2002) Cytokines in rheumatoid arthritis. Curr Rheumatol Rep 4(3):208–217
Burska A, Boissinot M, Ponchel F (2014) Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm 2014:545493. doi:10.1155/2014/545493
Whiteside TL (1994) Cytokines and cytokine measurements in a clinical laboratory. Clin Diagn Lab Immunol 1(3):257–260
Tworoger SS, Hankinson SE (2006) Collection, processing, and storage of biological samples in epidemiologic studies: sex hormones, carotenoids, inflammatory markers, and proteomics as examples. Cancer Epidemiol Biomarkers Prev 15(9):1578–1581. doi:10.1158/1055-9965.EPI-06-0629
Hosnijeh FS, Krop EJ, Portengen L, Rabkin CS, Linseisen J, Vineis P, Vermeulen R (2010) Stability and reproducibility of simultaneously detected plasma and serum cytokine levels in asymptomatic subjects. Biomarkers 15(2):140–148. doi:10.3109/13547500903340570
Uppal SS, Raghupathy R, Hayat SJ, Chowdhury RI, Abraham M, Rawoot P (2008) Patient demographics and disease variables correlate with distinct cytokine patterns in mitogen-stimulated peripheral blood mononuclear cells from rheumatoid arthritis patients. Rheumatol Int 28(6):533–539. doi:10.1007/s00296-007-0495-2
Davis JM 3rd, Knutson KL, Strausbauch MA, Crowson CS, Therneau TM, Wettstein PJ, Matteson EL, Gabriel SE (2010) Analysis of complex biomarkers for human immune-mediated disorders based on cytokine responsiveness of peripheral blood cells. J Immunol 184(12):7297–7304. doi:10.4049/jimmunol.0904180
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31(3):315–324
van der Heijde DM, van’t Hof MA, van Riel PL, Theunisse LA, Lubberts EW, van Leeuwen MA, van Rijswijk MH, van de Putte LB (1990) Judging disease activity in clinical practice in rheumatoid arthritis: first step in the development of a disease activity score. Ann Rheum Dis 49(11):916–920
Aletaha D, Smolen J (2005) The simplified disease activity index (SDAI) and the clinical disease activity index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 23(5 Suppl 39):S100–S108
Smolen JS, Breedveld FC, Schiff MH, Kalden JR, Emery P, Eberl G, van Riel PL, Tugwell P (2003) A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology (Oxford) 42(2):244–257
Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39(2):175–191
Raghupathy R, Makhseed M, Azizieh F, Omu A, Gupta M, Farhat R (2000) Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum Reprod 15(3):713–718
Makhseed M, Raghupathy R, Azizieh F, Al-Azemi MM, Hassan NA, Bandar A (1999) Mitogen-induced cytokine responses of maternal peripheral blood lymphocytes indicate a differential Th-type bias in normal pregnancy and pregnancy failure. Am J Reprod Immunol 42(5):273–281
Baringhaus L, Franz C (2004) On a new multivariate two-sample test. J Multivar Anal 88(1):190–206. doi:10.1016/S0047-259X(03)00079-4
Team R (2015) RStudio: integrated development for R. RStudio Inc, Boston
Praagman J (1985) Classification and regression trees: Leo BREIMAN, Jerome H. FRIEDMAN, Richard A. OLSHEN and Charles J. STONE The Wadsworth Statistics/Probability Series, Wadsworth, Belmont, 1984, x + 358 pages. North-Holland
Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V (2011) Scikit-learn: Machine learning in Python. J Mach Learn Res 12:2825–2830
Pérez F, Granger BE (2007) IPython: a system for interactive scientific computing. Comput Sci Eng 9(3):21–29
Kuhn M, Johnson K (2013) Applied predictive modeling. Springer, New York
Milman N, Karsh J, Booth RA (2010) Correlation of a multi-cytokine panel with clinical disease activity in patients with rheumatoid arthritis. Clin Biochem 43(16–17):1309–1314. doi:10.1016/j.clinbiochem.2010.07.012
Ku IA, Imboden JB, Hsue PY, Ganz P (2009) Rheumatoid arthritis: model of systemic inflammation driving atherosclerosis. Circ J 73(6):977–985
Sattar N, McCarey DW, Capell H, McInnes IB (2003) Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation 108(24):2957–2963. doi:10.1161/01.CIR.0000099844.31524.05
Brown AK, Conaghan PG, Karim Z, Quinn MA, Ikeda K, Peterfy CG, Hensor E, Wakefield RJ, O’Connor PJ, Emery P (2008) An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 58(10):2958–2967. doi:10.1002/art.23945
Chung SJ, Kwon YJ, Park MC, Park YB, Lee SK (2011) The correlation between increased serum concentrations of interleukin-6 family cytokines and disease activity in rheumatoid arthritis patients. Yonsei Med J 52(1):113–120. doi:10.3349/ymj.2011.52.1.113
Acknowledgements
This study is supported by Kuwait Foundation of Advancement of Science (KFAS), Project no. 2013-1302-02.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Azizieh, F.Y., Al Jarallah, K., Shehab, D. et al. Patterns of circulatory and peripheral blood mononuclear cytokines in rheumatoid arthritis. Rheumatol Int 37, 1727–1734 (2017). https://doi.org/10.1007/s00296-017-3774-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3774-6