Abstract
The objective is to establish recommendations, based on evidence and expert opinion, for the identification and management of comorbidities in patients with psoriatic arthritis (PsA). The following techniques were applied: discussion group, systematic review, and Delphi survey for agreement. A panel of professionals from four specialties defined the users, the sections of the document, possible recommendations, and what systematic reviews should be performed. A second discussion was held with the results of the systematic reviews. Recommendations were formulated in the second meeting and voted online from 1 (total disagreement) to 10 (total agreement). Agreement was considered if at least 70% voted ≥7. The level of evidence and grade of recommendation were assigned using the Oxford Centre for Evidence-Based Medicine guidance. The full document was critically appraised by the experts, and the project was supervised at all times by a methodologist. In a final step, the document was reviewed and commented by a patient and a health management specialist. Fourteen recommendations were produced, together with a checklist to facilitate the implementation. The items with the largest support from evidence were those related to cardiovascular disease and risk factors. The panel recommends paying special attention to obesity, smoking, and alcohol consumption, as they are all modifiable factors with an impact on treatment response or complications of PsA. Psychological and organizational aspects were also deemed important. We herein suggest practical recommendations for the management of comorbidities in PsA based on evidence and expert opinion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psoriatic disease is an inflammatory condition that affects skin and joints, among other organs and tissues. Since rheumatologists refer to it as psoriatic arthritis (PsA), we will use this latter term throughout the document [1]. Patients with PsA show a high prevalence of comorbidities and risk factors, specially cardiovascular (CV) disease and metabolic syndrome (MetS) [2, 3]. Other frequent comorbidities are hyperuricemia and gout [4], liver steatosis, and mood disorders; in addition, patients with PsA consume more tobacco and alcohol than healthy controls, with all its implications [5].
An adequate management of comorbidities is of great importance for the specialist: comorbidity influences diagnosis, prognosis, and treatment decisions, and has a great impact on health care resources [6]. In situations of time constraint, such as in busy clinics, attending adequately complex patients may be a challenge. This may even pose an inequity or harm to the patient—if, for instance, the specialist fails to identify a potential and preventable harm. As a response to this problem, a group of Spanish rheumatologists, all with renowned interest in psoriatic arthritis, gathered with a common goal, to develop practical materials and recommendations that may aid rheumatologists, and other health professionals, in the identification and management of comorbidities in patients with PsA.
Methods
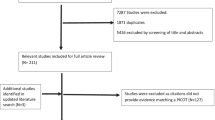
Figure 1 shows the methods and processes followed in this document. The panellists were selected by the taskforce chair (JTA) and the methodologist (LC) on the basis of their expertise in PsA, or specific aspects related to its comorbidity. The panel included ten rheumatologists, two dermatologists, two family doctors, and an internist—(CS) who is also a well renowned expert in hypertension and hyperlipidemia—from various geographic areas and levels of care to ensure representativeness. They are all well-respected professionals in Spain and considered expert clinicians in their field by their peers. This group established the scope, users, and process to develop the recommendations. The topics for the recommendations were decided by discussion among the panellists, as well as the needs for systematic reviews (SR). Four rheumatologists among the panellists (EG, RA, JB, and MM), who were specifically trained in the methodology of SR, searched systematically the literature in controversial aspects: weight and response to treatment, effect of PsA treatments on CV outcomes, and hepatic steatosis and adverse effects of treatments. In addition, each panellist was in charge of a specific aspect and of searching for supporting evidence. All searches, whether these were for systematic or for scoping reviews, followed the PICO approach and were supervised by experienced methodologists. In addition, the SR followed the usual procedures with ascertainment of biases and data synthesis by PICO. Agreement was explored through a two-round Delphi process among the panellists via SurveyMonkey©; the final round agreement is shown as the mean with standard deviation (SD), and minimum and maximum (min and max). All recommendations with an agreement of less than 75% were excluded from the list. The methodologist assigned the levels of evidence (LE) and grade of recommendation (GR) for each statement in accordance with the Oxford Centre for Evidence-based Medicine Levels of Evidence [7]. Finally, the document was reviewed by a patient and a health management specialist and corrected as suggested.
Results
The panel focused on prevalent comorbidities as well as on those that could interfere the most with decision-making. Initially, dermatological and rheumatic comorbidity were included in the list—e.g., onychomycosis, or hand osteoarthritis. However, to make the list of comorbidities simpler, and assuming that dermatologists and rheumatologists perform differential diagnoses routinely, these were deleted. The list of recommendations is presented in Table 1, and explanatory text for each recommendation together with the supporting evidence. Table 2 displays suggested periodicity of actions. A checklist based on the recommendations is available as supplementary material.
The optimal management of patients with PsA needs a holistic perspective whoever the coordinator of care: preferably a rheumatologist or dermatologist (LE: 5; A: 9.8; SD: 0.4; min: 9-max: 10)
Management responsibilities of PsA may change over time between dermatologists, rheumatologists, and family doctors. Ideally, the coordinator of care should provide an integrated report reflecting not only the skin and articular status, but also indicating recommendations on healthy lifestyle and guidance for family doctors on how to monitor certain parameters [5]. Many of these latter aspects could be covered by a nurse, at Primary Care or at the hospital, depending on resources and competence.
It is essential to involve the family doctor and other specialists in the control and monitoring of comorbidities associated with PsA, as well as to promote the active participation of the patient (LE:5; A: 9.3; SD:0.8; min:8-max:10)
Health care should be delivered as a coordinated process with clear aims, a sensitive budget, and comprehensible information circuits, with minimal disturbance for the patient; this is of uttermost importance for systemic diseases, such as PsA. By involving the family doctor, trips to the hospital and duplication of tests are avoided, and they can promote shared decisions in adjustment to the chronic-care model [8]. Patients must be informed of the need to inform about PsA in any medical encounter apart from the specific care of rheumatologists and dermatologists, and they should be instructed on prognostic signs and medication-related matters. As many patients will seek information at internet, it may be worth recommending trustable pages. Additionally, patients’ associations can become a good reference for completing patient education and promoting self-care, as well as provide support for patients and their families.
The panel recommends to assess comorbidities at least at the time of diagnosis, and at every treatment change (LE: 5; A: 8.8; SD: 1.4; min: 5-max: 10)
Comorbidities at the time of diagnosis will determine prognosis and therapeutic decisions, and thus, it is a critical point to consider them. In addition, comorbidities need to be ruled-out or considered when changing treatment [9, 10]. Specific comorbidities may need varying periodicity (Table 2). In any case, follow-up should be customized depending on risk profile, comorbidities, and patient preferences.
The risk/benefit ratio of drugs with potential negative effect on comorbidities should be taken into account when initiating treatments for PsA (LE: 5; A: 9.5; SD: 0.8; min: 8-max: 10)
All therapeutic options in PsA have potential adverse events that should be considered prior and during administration. In Table 3, we disclose the guidelines in place in our setting for managing risks of adverse events. Special attention should be paid to (1) NSAIDs and glucocorticoids if hypertension is present and the renin–angiotensin–aldosterone blockers are being used [11]; (2) risk of infection and of non-melanoma skin cancer with systemic treatment, including biologics [10]; and (3) hepatic steatosis and methotrexate [12]. To provide clear information to patients and professionals who will monitor these therapies is critical for safety [10].
Early and periodic screening for CV risk factors should be performed; if present, risk factors should be controlled and monitored (LE: 3, A: 9.8, SD: 0.6; min: 8-max: 10)
PsA is associated with ischemic heart disease, cerebrovascular disease, left ventricular diastolic dysfunction, and subclinical atherosclerosis with increased carotid intima-media thickness, peripheral vascular disease, and CV mortality [2, 13–16]. Not only traditional risk factors, such as hypertension, obesity, diabetes mellitus, dyslipemia, MetS, and tobacco, use are increased in PsA patients, but evidence supports accelerated atherogenesis related to persistent inflammation [2, 17]. If the patient has risk factors present, or a history of CV disease, he or she should be referred to a specialist in this area for appropriate treatment. Periodic screening is recommended at least once every 5 years, as suggested by EULAR [18]; however, in patients with high CV risk, the assessment should be reconsidered during the course of the disease, depending on the level of inflammation and treatment, as suggested by Simplified Preventive Activities Program and Health Promotion (PAPPS), 2014 [19] (see supplementary material). In a systematic review, disease-modifying treatment in PsA showed a protective effect on CV risk [20], likely due to appropriate control of inflammation.
The panel recommends informing about the high CV risk even in the absence of risk factors (LE: 5; A: 8.4; SD: 1.2; min: 6-max: 10)
Patients with PsA show increased CV risk even in the absence of risk factors [21]. It is essential that both the patient and the family doctor take part in preventing and controlling all CV risk factors (see supplementary material on monitoring CV risk in Primary Care).
Adequate control of obesity and overweight should be a priority (LE: 2; A: 9.3; SD: 1.0; min: 7- max: 10)
Obesity and its complications—i.e., diabetes, fatty liver, hypertension and CV events—are common in patients with PsA [22–24]. The adipose tissue produces proinflammatory factors—namely, adipokines—that could influence PsA [25]. Both obesity and MetS appear to be predisposing factors for severe PsA [26–29]. Likewise, response to treatment may be impaired in overweight patients [30] and weight loss, by means of diet coupled or not with exercise, has a marginal but beneficial effect on treatment response [31]. There is no consistent evidence to support any specific diet, such as those enriched in delta-3-omega fatty acids, antioxidants, vitamin D, or gluten-free [32]. The panel, however, advises to adhere as much as possible to the Mediterranean diet, as evidence supports CV benefits in population at risk [33] (see supplementary material for the Mediterranean diet score).
Regular aerobic exercise should be recommended (LE: 2, A: 9.1; SD: 0.8; min: 7-max: 10)
Although most studies examining the effect of exercise on different aspects of PsA are of low quality—e.g., lack of control group, short follow-up periods, or small sample size—they clearly suggest an improvement on physical condition and psychological well-being [34–36]. Moreover, exercise enhances the effect of weigh loss by diet on treatment response in patients with PsA [31, 37].
Smoker patients should be encouraged to cease smoking on each visit (LE: 3; A: 9.8; SD: 0.5; min: 9-max: 10)
Smoking is associated with worse health status, high risk of CV death, and lung cancer. Furthermore, smoking has been shown associated with a higher incidence of PsA [38] and poor physical function in patients with PsA [39, 40]. Tobacco use attenuates treatment response very plausibly via decreasing pain thresholds; in addition, adherence is poorer in smokers [41]. Finally, smoking causes chronic obstructive pulmonary disease, a risk factor for infections and for cancer, in particular in patients treated with biological therapies [10, 42].
Patients should be encouraged to cease or reduce alcohol intake (LE: 4; A: 9.1; SD: 1.2; min: 6-max: 10)
It is unclear whether alcohol consumption is a cause of PsA, or if, on the contrary, it is the disease that favours the habit [43]. Nevertheless, alcohol has a negative impact on PsA, as it may worsen hepatic steatosis secondary to obesity, interfere with methotrexate liver-metabolism, increase blood pressure, triglycerides and uric acid, and favour the arousal of psycho-affective disorders. Alcohol intake should be investigated, either directly or through questionnaires, such as CAGE [44] or AUDIT [45]. Patients with alcohol-related problems should be advised or referred to the appropriate specialist.
Psycho-affective disorders should be investigated (LE: 2b; A: 8.9; SD: 1.2; min: 6-max: 10)
PsA not only affects patients by rendering loss of functional capacity, but can also have a significant impact on the psycho-social sphere, with nearly a quarter of patients showing signs of anxiety or depression [46]. Psycho-affective disorders may impair not only quality of life, but also self-assessment, treatment adherence [47] and the willingness to observe a healthy lifestyle [48]. Furthermore, chronic pain and fatigue in patients with PsA could be potentially related to a psycho-affective disorder or to concurrent fibromyalgia; it is of upmost importance to rule out such possibilities to avoid over-exposure to ineffective systemic treatments [49, 50].
There are questionnaires with reasonable sensitivity and feasibility for the identification of psycho-affective disorders that may be worth using in patients with PsA [51, 52], two of which are attached as supplementary material. When a psycho-affective disorder is suspected, the patient should be referred to a specialist in psychology or psychiatry if possible.
Inquiring about sexual dysfunctions is recommended (LE: 5, A: 7.7; SD: 0.9, min: 5-max: 10)
Between 20 and 70% of patients with PsA present some type of sexual dysfunction [53], related or not to mood disorders or alcohol abuse. Most frequently reported dysfunctions are erectile dysfunction, under-satisfaction, and reduced number of partners or frequency of intercourse or oral sex. Predisposing factors are: age, feminine gender, severity of skin lesions, psoriatic lesions in genital areas, and psycho-affective disorders [53, 54]. Erectile dysfunction may be reflecting subclinical endothelial dysfunction and vascular disease [55, 56]. After being inquired about sexual problems, the patient with dysfunction should be referred to the appropriate specialist (psychologist or urologist).
Patients with ocular symptoms, especially if PsA had a juvenile onset or there is suspicion of uveitis, should be evaluated as soon as possible by an ophthalmologist (LE:5; DA:8.8; SD:1.2; min:6-max:10)
Many eye conditions can affect patients with PsA, being uveitis the most severe form [57, 58]. Uveitis should be investigated actively by inquiring the patient about visual disturbances, especially in young patients, and with dactylitis [59]. Unlike uveitis in spondylitis, uveitis in PsA is usually bilateral, chronic, with greater involvement of the posterior pole, and have more complications at diagnosis and poor prognosis, requiring extensive systemic treatment [60].
The panel recommends to consider non-melanoma skin cancer when non-psoriatic skin lesions appear, especially in patients who have received PUVA, or cyclosporine or biological treatment (LE: 3; A: 8.5; SD: 0.7; min: 8- max: 10)
It is unclear whether patients with PsA are at higher risk of developing a cancer [61]. The risk may be similar to other chronic inflammatory diseases, such as RA [62], especially at the expense of non-melanoma skin cancer [63, 64]. Some studies conclude that prolonged treatment with PUVA may increase melanoma risk [65, 66]; however, the association of UV-B narrowband with skin cancer is unclear [67].
Discussion
After a comprehensive review of the literature, voting, and discussions, the multidisciplinary panel issued 14 recommendations, practical tables, and a checklist, with the intention to facilitate the management of comorbidities in patients with PsA. With this goal, different aspects have been incorporated, including CV risk factors, psycho-affective disorders, and management in routine clinical practice.
These recommendations provide a different view in regard to previous recommendations for patients with PsA [5, 68]. Previous guidelines evaluate only specific aspects of the comorbidities of patients with PsA, such as how to manage CV comorbidity, or algorithms to deal with specific comorbidities. This document is more practical and based on a holistic perspective.
An early detection and management are desirable to reduce comorbidity negative effects. However, it could be argued that our approach—which implies rheumatologists needing more time and forces close connection with other specialists and family doctors—may be time-consuming, and even costly. However, the use of checklists, as we endorse, has demonstrated improved teamwork and safety in more complicated settings, such as in operating rooms [69] and they do not automatically imply more people or more time. A trained nurse could pass the checklist, for instance, or it could be implemented in the electronic medical records. In any case, preventing comorbidities is, at large, safer and less costly than having to treat them.
The panel decided to focus on selected comorbidities and not being comprehensive. The decision implied a critical discussion on frequency and impact on treatment and prognosis of a large list of comorbidities; finally, the group acknowledged that being too comprehensive would hamper practicability (see overarching principles in supplementary material), and included comorbidities that were prevalent or would affect therapeutic decisions. Notwithstanding, other comorbidities may co-exist in the same patient, and they need to be managed properly.
Noteworthy, the panel had more rheumatologists than other specialists to ensure that these recommendations reflected what is possible to do at the rheumatology office. In this sense, the initial recommendations included areas, such as onychomicosis, and osteoporosis or gout. Being these conditions frequent and important, they do not pose a great challenge to the rheumatologist or the dermatologist as they are used to manage them. Although osteoporosis is not as common in PsA as in other arthritides, it is important to determine the risk of fracture in patients receiving glucocorticoids. The risk can be assessed either by specific indices, with or without bone densitometry and there are appropriate guidelines [70]. In addition, hyperuricemia and gout are common findings in patients with PsA, especially if obese or with MetS [71, 72], and gout symptoms can overlap those of PsA [73]. In addition, chronic deposits of uric acid crystals are associated with subclinical atherosclerosis and death by CV cause [74, 75]. Again, rheumatologists are aware of this, and although we included it in the checklist, we decided to take any specific recommendation out. Furthermore, other rheumatic diseases should be taken into account in the differential diagnosis, and they can co-exist. Hand osteoarthritis may affect the same anatomical territories as PsA [76]; knee osteoarthritis, common in obese people, must be taken into consideration in patients with PsA and overweight [76]. A misdiagnosis of osteoarthritis may lead to an apparent failure of disease-modifying agents.
As previously pointed-out, the recommendations underscore the need for a systematic and detailed CV risk assessment. With this aim, a CV risk index, e.g., SCORE, Framingham adapted, HUNT, etc., could be of use. EULAR encourages the assessment of CV risk factors in PsA every 5 years and upon treatment changes with SCORE [18]. However, compared to controls, patients with PsA have similar SCORE levels despite clearly increased CV risk profile [77]. This is so because patients with PsA usually have CV risk factors that are not included in the SCORE algorithm, such as obesity, hypertriglyceridemia, or elevated C-reactive protein levels, or even carotid plaques in absence of all of the above [15, 78]. Our panel signifies their preference in terms of motivating the patient towards healthy lifestyle habits, e.g., quitting tobacco and alcohol, exercising regularly, reaching a normal body mass index, etc, more than in specifying an index for assessing CV risk.
Some remarks on the recommendations that showed the lowest agreement, which we believe will be the hardest to implement. Investigating problems of the psycho-affective and sexual spheres was not well supported, mainly because it puts doctors and patients away from the comfort zone. A correct communication between professionals and between patients and doctors was not well supported either [79, 80]. Although many rheumatologists and dermatologist are not comfortable inquiring about personal aspects—as reflected in the agreement with these recommendations, lower than with others—the discussion brought up a likely positive response to the inquiry from the patients, which in turn may have an impact on medical trust, and thus on adherence to treatment [47]. In addition, any effort made towards a truly patient-centered care, such as working for better information systems and for better use of them, and to increase communication between professional at all care levels, will clearly benefit outcomes by improving satisfaction both of patients and health care professionals.
Shortcomings of these recommendations are not being the panel from a specific society, but a self-selected group of professionals, or not engaging patient partners from the beginning or other specialists, such as psychologists. Regarding the group selection, we really believe that the endorsement from a society does not necessarily make any recommendations better. We are not producing recommendations that could be prone to conflicts of interest, and our sole interest was to produce a practical document. As for patient participation, our experience with patient-partners with PsA in ongoing studies makes us think that we touched aspects fundamental for them. As an additional shortcoming, it may be posed that these recommendations do not deal specifically how to treat comorbidity. There are plenty recommendations for the specific problems detected by our checklist and we did not think that was our task. Each problem detected should be pin-pointed to a specific treatment guideline.
It could be argued that some of the recommendations would not likely work in every health setting. For example, it may not be feasible to refer every patient with a suspected psycho-affective disorder to a specialist. In most healthcare settings, it would be appropriate for these to be managed within the context of primary care. We encourage, in fact, the adaptation of the recommendations and checklist to the setting.
Finally, being a principle of the document its practicality, an implementation strategy is already in place. The document and checklist will be subject to a large Delphi survey, together with other consensus documents produced in the field of comorbidity in RMD. The checklist (supplementary material) will be distributed, explained, and discussed in regional meetings.
To summarize, when caring for persons with PsA, we will not success if comorbidities and other sensitive and organizational aspects are not included as a routine part of the integrative care process. These recommendations were issued to facilitate such aim: the detection, monitoring, and integration of selected comorbidities to ensure optimal management of patients with PsA, and are based on the best evidence available and expert opinion.
References
Scarpa R, Ayala F, Caporaso N, Olivieri I (2006) Psoriasis, psoriatic arthritis, or psoriatic disease? J Rheumatol 33(2):210–212
Jamnitski A, Symmons D, Peters MJ, Sattar N et al (2013) Cardiovascular comorbidities in patients with psoriatic arthritis: a systematic review. Ann Rheum Dis 72(2):211–216
Khraishi M, MacDonald D, Rampakakis E, Vaillancourt J et al (2011) Prevalence of patient-reported comorbidities in early and established psoriatic arthritis cohorts. Clin Rheumatol 30(7):877–885
Oliviero F, Scanu A, Galozzi P, Gava A et al (2013) Prevalence of calcium pyrophosphate and monosodium urate crystals in synovial fluid of patients with previously diagnosed joint diseases. Jt Bone Spine 80(3):287–290
Dauden E, Castaneda S, Suarez C, Garcia-Campayo J et al (2013) Clinical practice guideline for an integrated approach to comorbidity in patients with psoriasis. J Eur Acad Dermatol Venereol 27(11):1387–1404
Lee S, Mendelsohn A, Sarnes E (2010) The burden of psoriatic arthritis: a literature review from a global health systems perspective. P T 35(12):680–689
Howick J, Chalmers I, Glasziou P, Greenhalgh T et al (2011) Explanation of the 2011 Oxford Centre for Evidence-Based Medicine (OCEBM) Levels of Evidence (Background Document). Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653. Accessed 02 Nov 2015
Rubiera Lopez G, Riera Velasco JR (2004) Program to improve care in chronic illnesses: application of the model of care in chronic illness. Aten Primaria 34(4):206–209
Peters MJ, Symmons DP, McCarey D, Dijkmans BA et al (2010) EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 69(2):325–331
Gomez Reino J, Loza E, Andreu JL, Balsa A et al (2011) Consensus statement of the Spanish Society of Rheumatology on risk management of biologic therapy in rheumatic patients. Reumatol Clin 7(5):284–298
Bori Segura G, Hernandez Cruz B, Gobbo M, Lanas Arbeloa A et al (2009) Appropriate use of non-steroidal anti-inflammatory drugs in rheumatology: guidelines from the Spanish Society of Rheumatology and the Mexican College of Rheumatology. Reumatol Clin 5(1):3–12
Langman G, Hall PM, Todd G (2001) Role of non-alcoholic steatohepatitis in methotrexate-induced liver injury. J Gastroenterol Hepatol 16(12):1395–1401
Shang Q, Tam LS, Yip GW, Sanderson JE et al (2011) High prevalence of subclinical left ventricular dysfunction in patients with psoriatic arthritis. J Rheumatol 38(7):1363–1370
Shang Q, Tam LS, Sanderson JE, Sun JP et al (2012) Increase in ventricular-arterial stiffness in patients with psoriatic arthritis. Rheumatology (Oxford) 51(12):2215–2223
Eder L, Jayakar J, Shanmugarajah S, Thavaneswaran A et al (2013) The burden of carotid artery plaques is higher in patients with psoriatic arthritis compared with those with psoriasis alone. Ann Rheum Dis 72(5):715–720
Lin YC, Dalal D, Churton S, Brennan DM et al (2014) Relationship between metabolic syndrome and carotid intima-media thickness: cross-sectional comparison between psoriasis and psoriatic arthritis. Arthritis Care Res (Hoboken) 66(1):97–103
Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P et al (2011) Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med 124(8):775 e771–776
Agca R, Heslinga SC, Rollefstad S, Heslinga M et al (2017) EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 76(1):17–28
Maiques Galan A, Brotons Cuixart C, Villar Alvarez F, Martin Rioboo E et al (2014) Recomendaciones preventivas cardiovasculares. Aten Primaria 46(Suppl 4):3–15
Roubille C, Richer V, Starnino T, McCourt C et al (2015) The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 74(3):480–489
Kristensen SL, McInnes IB, Sattar N (2015) Psoriasis, psoriatic arthritis and cardiovascular risk: are we closer to a clinical recommendation? Ann Rheum Dis 74(2):321–322
Labitigan M, Bahce-Altuntas A, Kremer JM, Reed G et al (2014) Higher rates and clustering of abnormal lipids, obesity, and diabetes mellitus in psoriatic arthritis compared with rheumatoid arthritis. Arthritis Care Res (Hoboken) 66(4):600–607
Love TJ, Qureshi AA, Karlson EW, Gelfand JM et al (2011) Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003–2006. Arch Dermatol 147(4):419–424
Haroon M, Gallagher P, Heffernan E, FitzGerald O (2014) High prevalence of metabolic syndrome and of insulin resistance in psoriatic arthritis is associated with the severity of underlying disease. J Rheumatol 41(7):1357–1365
Wang Y, Chen J, Zhao Y, Geng L et al (2008) Psoriasis is associated with increased levels of serum leptin. Br J Dermatol 158:1134–1135
Li W, Han J, Qureshi AA (2012) Obesity and risk of incident psoriatic arthritis in US women. Ann Rheum Dis 71(8):1267–1272
Versini M, Jeandel PY, Rosenthal E, Shoenfeld Y (2014) Obesity in autoimmune diseases: not a passive bystander. Autoimmun Rev 13(9):981–1000
Setty A, Curhan G, Choi H (2007) Obesity, waist circumference, weight change, and the risk of psoriasis in women. Nurses’ Health Study II. Arch Intern Med 167:1670–1675
Love TJ, Zhu Y, Zhang Y, Wall-Burns L et al (2012) Obesity and the risk of psoriatic arthritis: a population-based study. Ann Rheum Dis 71(8):1273–1277
Galindez E, Carmona L (2016) Is obesity in psoriatic arthritis associated with a poorer therapeutic response and more adverse effects of treatment with an anchor drug? Reumatol Clin 12(6):307–312
Almodovar R, Zarco P, Oton T, Carmona L (2017) Effect of weight loss on activity in psoriatic arthritis: a systematic review. Reumatol Clin. doi:10.1016/j.reuma.2017.01.010
Debbanech M, Millsop J, Bhatia B, Koo J et al (2014) Diet and psoriasis, part I: Impact of weigt loss interventions. J Am Acad Dermatol 71:133–140
Martinez-Gonzalez MA, Garcia-Arellano A, Toledo E, Salas-Salvado J et al (2012) A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 7(8):e43134
Frankel H, Han J, Li T, Qureshi A (2012) The association between physical activity and the risk of incident psoriasis. Arch Dermatol 148(8):918–924
Lubrano E, Spadaro A, Parsons W, Atteno M et al. (2009) Rehabilitation in psoriatic arthritis. J Rheumatol 2009(Suppl (83)):81–82
Hagel S, Lindqvist E, Bremander A, Petersson I (2010) Team-based rehabilitation improves long-term aerobic capacity and health-related quality of life in patients with chronic inflammatory arthritis. Disabil Rehabil 32:1686–1696
Naldi L, Conti A, Cazzaniga S, Patrizi A et al (2014) Diet and physical exercise in psoriasis: a randomized controlled trial. Br J Dermatol 170:634–642
Li W, Han J, Qureshi AA (2012) Smoking and risk of incident psoriatic arthritis in US women. Ann Rheum Dis 71(6):804–808
Bremander A, Jacobsson LT, Bergman S, Haglund E et al (2015) Smoking is associated with a worse self-reported health status in patients with psoriatic arthritis: data from a Swedish population-based cohort. Clin Rheumatol 34(3):579–583
Tillett W, Jadon D, Shaddick G, Cavill C et al (2013) Smoking and delay to diagnosis are associated with poorer functional outcome in psoriatic arthritis. Ann Rheum Dis 72(8):1358–1361
Hojgaard P, Glintborg B, Hetland ML, Hansen TH et al (2015) Association between tobacco smoking and response to tumour necrosis factor alpha inhibitor treatment in psoriatic arthritis: results from the DANBIO registry. Ann Rheum Dis 74(12):2130–2136
Carmona L, Abasolo L, Descalzo MA, Perez-Zafrilla B et al (2011) Cancer in patients with rheumatic diseases exposed to TNF antagonists. Semin Arthritis Rheum 41(1):71–80
Wu S, Cho E, Li WQ, Han J et al (2015) Alcohol intake and risk of incident psoriatic arthritis in women. J Rheumatol 42(5):835–840
Ewing JA (1984) Detecting alcoholism. The CAGE questionnaire. JAMA 252(14):1905–1907
Babor TB, Higgins-Biddle JC, Saunders JB, Monteiro MG (2001) AUDIT: the alcohol use disorders identification test: guidelines for use in primary care, 2nd edn. World Health Organization, Department of Mental Health and Substance Dependence
Freire M, Rodriguez J, Moller I, Valcarcel A et al (2011) Prevalence of symptoms of anxiety and depression in patients with psoriatic arthritis attending rheumatology clinics. Reumatol Clin 7(1):20–26
DiMatteo MR, Lepper HS, Croghan TW (2000) Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 160(14):2101–2107
Sumlin LL, Garcia TJ, Brown SA, Winter MA et al (2014) Depression and adherence to lifestyle changes in type 2 diabetes: a systematic review. Diabetes Educ 40(6):731–744
Magrey MN, Antonelli M, James N, Khan MA (2013) High frequency of fibromyalgia in patients with psoriatic arthritis: a pilot study. Arthritis 2013:762921
Husted JA, Thavaneswaran A, Chandran V, Gladman DD (2013) Incremental effects of comorbidity on quality of life in patients with psoriatic arthritis. J Rheumatol 40(8):1349–1356
Ortega Orcos R, Salinero Fort MA, Kazemzadeh Khajoui A, Vidal Aparicio S et al (2007) Validation of 5 and 15 items Spanish version of the geriatric depression scale in elderly subjects in primary health care setting. Rev Clin Esp 207(11):559–562
Goldberg DP, Hillier VF (1979) A scaled version of the General Health Questionnaire. Psychol Med 9(1):139–145
Molina-Leyva A, Jimenez-Moleon JJ, Naranjo-Sintes R, Ruiz-Carrascosa JC (2015) Sexual dysfunction in psoriasis: a systematic review. J Eur Acad Dermatol Venereol 29(4):649–655
Meeuwis KA, de Hullu JA, van de Nieuwenhof HP, Evers AW et al (2011) Quality of life and sexual health in patients with genital psoriasis. Br J Dermatol 164(6):1247–1255
Billups KL (2005) Erectile dysfunction as a marker for vascular disease. Curr Urol Rep 6(6):439–444
Gonzalez-Juanatey JR, Alegria Ezquerra E, Gomis Barbera R, Salvador Taboada MJ et al (2009) Erectile dysfunction as a marker of silent cardiovascular disease in type-2 diabetic patients in Spain. The DIVA (DIabetes and VAscular disease) study. Med Clin (Barc) 132(8):291–297
Paiva ES, Macaluso DC, Edwards A, Rosenbaum JT (2000) Characterisation of uveitis in patients with psoriatic arthritis. Ann Rheum Dis 59(1):67–70
Queiro R, Torre JC, Belzunegui J, Gonzalez C et al (2002) Clinical features and predictive factors in psoriatic arthritis-related uveitis. Semin Arthritis Rheum 31(4):264–270
Niccoli L, Nannini C, Cassarà E, Kaloudi O et al (2012) Frequency of iridocyclitis in patients with early psoriatic arthritis: a prospective, follow up study. Int J Rheum Dis 15(4):414–418
Durrani K, Foster CS (2005) Psoriatic uveitis: a distinct clinical entity? Am J Ophthalmol 139(1):106–111
Rohekar S, Tom BD, Hassa A, Schentag CT et al (2008) Prevalence of malignancy in psoriatic arthritis. Arthritis Rheum 58(1):82–87
Gross RL, Schwartzman-Morris JS, Krathen M, Reed G et al (2014) A comparison of the malignancy incidence among patients with psoriatic arthritis and patients with rheumatoid arthritis in a large US cohort. Arthritis Rheumatol 66(6):1472–1481
Margolis D, Bilker W, Hennessy S, Vittorio C et al (2001) The risk of malignancy associated with psoriasis. Arch Dermatol 137(6):778–783
Husni ME, Mease PJ (2010) Managing comorbid disease in patients with psoriatic arthritis. Curr Rheumatol Rep 12(4):281–287
Naldi L (2010) Malignancy concerns with psoriasis treatments using phototherapy, methotrexate, cyclosporin, and biologics: facts and controversies. Clin Dermatol 28(1):88–92
Stern RS, Lunder EJ (1998) Risk of squamous cell carcinoma and methoxsalen (psoralen) and UV-A radiation (PUVA). A meta-analysis. Arch Dermatol 134(12):1582–1585
Patel RV, Clark LN, Lebwohl M, Weinberg JM (2009) Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol 60(6):1001–1017
Roubille C, Richer V, Starnino T, McCourt C et al (2015) Evidence-based recommendations for the management of comorbidities in rheumatoid arthritis, psoriasis, and psoriatic arthritis: expert opinion of the canadian dermatology-rheumatology comorbidity initiative. J Rheumatol 42(10):1767–1780
Treadwell JR, Lucas S, Tsou AY (2014) Surgical checklists: a systematic review of impacts and implementation. BMJ Qual Saf 23(4):299–318
Grossman JM, Gordon R, Ranganath VK, Deal C et al (2010) American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 62(11):1515–1526
Gisondi P, Targher G, Cagalli A, Girolomoni G (2014) Hyperuricemia in patients with chronic plaque psoriasis. J Am Acad Dermatol 70(1):127–130
Merola JF, Wu S, Han J, Choi HK et al (2015) Psoriasis, psoriatic arthritis and risk of gout in US men and women. Ann Rheum Dis 74(8):1495–1500
Lopez-Reyes A, Hernandez-Diaz C, Hofmann F, Pineda C (2012) Gout mimicking psoriatic arthritis flare. J Clin Rheumatol 18(4):220
Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Gomez-Acebo I et al (2009) Asymptomatic hyperuricemia and serum uric acid concentration correlate with subclinical atherosclerosis in psoriatic arthritis patients without clinically evident cardiovascular disease. Semin Arthritis Rheum 39(3):157–162
Clarson L, Chandratre P, Hider S, Belcher J et al (2015) Increased cardiovascular mortality associated with gout: a systematic review and meta-analysis. Eur J. Prev Cardiol 22(3):335–343
McGonagle D, Hermann KG, Tan AL (2015) Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era. Rheumatology (Oxford) 54(1):29–38
Gulati AM, Semb AG, Rollefstad S, Romundstad PR et al (2016) On the HUNT for cardiovascular risk factors and disease in patients with psoriatic arthritis: population-based data from the Nord-Trondelag Health Study. Ann Rheum Dis 75(5):819–824
Eder L, Chandran V, Gladman DD (2014) The Framingham Risk Score underestimates the extent of subclinical atherosclerosis in patients with psoriatic disease. Ann Rheum Dis 73(11):1990–1996
Berdal G, Smedslund G, Dagfinrud H, Hagen KB et al (2015) Design and effects of supportive followup interventions in clinical care of patients with rheumatic diseases: a systematic review with meta-analysis. Arthritis Care Res (Hoboken) 67(2):240–254
Betteridge N, Boehncke WH, Bundy C, Gossec L et al (2016) Promoting patient-centred care in psoriatic arthritis: a multidisciplinary European perspective on improving the patient experience. J Eur Acad Dermatol Venereol 30(4):576–585
Acknowledgements
The panel appreciated the critical revision of the recommendations and thoughtful suggestions made by Dr Silvia Rodríguez Sopena, Hospital manager at Ferrol Hospital and Miguel Martínez Villafranca, informatics engineer with PsA. We also want to acknowledge the invaluable help of Teresa Otón with the translation and checklist preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study is based on the review of the literature and on the opinion of the authors and collaborators. No human subject was recruited for research. Due to the nature of the study, an Ethical Review Board was not consulted.
Conflict of interest
The authors declare no conflicts of interest in relation to the contents of this consensus.
Funding
This Project was funded by a non-restricted grant by Merck Sharp & Dohme of Spain. Merck Sharp & Dohme did not influence the conduct of the project nor the final content of the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Torre-Alonso, J.C., Carmona, L., Moreno, M. et al. Identification and management of comorbidity in psoriatic arthritis: evidence- and expert-based recommendations from a multidisciplinary panel from Spain. Rheumatol Int 37, 1239–1248 (2017). https://doi.org/10.1007/s00296-017-3702-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3702-9