Abstract
To evaluate whether C-reactive protein (CRP), including variation within the normal range, is predictive of long-term disease outcome in Juvenile Idiopathic Arthritis (JIA). Consecutive patients with newly diagnosed JIA were included prospectively from defined geographic areas of the Nordic countries from 1997 to 2000. Inclusion criteria were availability of a baseline serum sample within 12 months after disease onset and 8-year clinical assessment data. Systemic onset JIA was not included. CRP was measured by high-sensitive ELISA (detection limit of 0.2 mg/l). One hundred and thirty participants with a median follow-up time of 97 months (range 95–100) were included. At follow-up, 38% of the patients were in remission off medication. Absence of remission was associated with elevated level of CRP at baseline (odds ratio (OR) 1.33, confidence interval (CI) 1.08–1.63, p = 0.007). By applying a cutoff at the normal upper limit (>10 mg/l), the risk of not achieving remission was increased to an OR of 8.60 (CI 2.98–24.81, p < 0.001). Variations of CRP within the normal range had no predictive impact on disease activity at follow-up. Baseline levels of ESR were available in 80 patients (61%) and elevated ESR was associated with absence of remission in a multivariable logistic regression analysis (OR 2.32, CI 1.35-4.00, p = 0.002). This results of this study indicate that baseline CRP concentrations above 10 mg/l are predictive of a poor outcome at 8-year follow-up. We could not demonstrate any predictive value of CRP variations within the normal range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile idiopathic arthritis (JIA) is a group of chronic rheumatic diseases in childhood with an incidence in the Nordic countries of 9–23/100.000 children [1, 2]. For some children, the disease has a mild course, but for others it is a long-lasting and potentially disabling disease [3–6]. We have previously shown a highly variable outcome of the disease with more than 50% of patients having persistent disease 8 years after onset [3]. Unfortunately, our insight into prognostic risk factors is still limited and although several predictive markers of outcome have been proposed, results have been inconsistent [5, 7–12].
In a large proportion of patients, the level of the standard inflammatory markers, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) is within the normal range despite clinical signs of disease activity [13, 14]. Thus, JIA is for a major group of the patients a low-grade inflammatory disease, and there is a need to develop reliable prognostic markers for this subgroup of JIA patients.
High-sensitive CRP assays have been developed to differentiate between patients with low-grade inflammatory conditions having CRP values within the normal range [15]. Interestingly, inter-individual differences within the normal range of CRP have been associated with the risk of low-grade inflammatory conditions such as atherosclerosis, coronary events [16–18], and the metabolic syndrome [19, 20]. Furthermore, there is evidence that differences in CRP within the normal range is an early predictive marker prior to the onset of rheumatoid arthritis (RA) and for the response to treatment [21, 22]. Recently, a small study indicated that children with active JIA have higher levels of IL-6 and CRP than children with inactive JIA and healthy controls, although still within the normal range [23]. In addition, studies in JIA have suggested an association between CRP levels in the upper end of the normal range and the risk of flare after treatment withdrawal during remission [24].
We hypothesized that an increased level of inflammation within the first year of disease onset is associated with prolonged active disease with reduced chance of achieving remission off medication.
The aim of this study was to investigate whether variations in baseline CRP and ESR, including differences within the normal range, are predictive of long-term disease outcome in JIA.
Materials and methods
Patients
Between 1997 and 2000, patients with newly diagnosed JIA were consecutively included in the Nordic JIA cohort study from defined geographic areas of Sweden, Norway, and Denmark [2, 3]. Blood samples from the initial visit were collected and stored for later analysis and a uniform, detailed, and systematic clinical assessment was performed at baseline and at an 8-year follow-up visit performed in relation to a clinical follow-up study [3]. Blood were taken at the initial visit at the pediatric rheumatology department prior to treatment with disease modifying anti-rheumatic drugs and steroids. However, an unspecified proportion of the patients were treated with non-steroid anti-rheumatic drugs prior to the blood sampling.
The inclusion criteria for this study were JIA according to the ILAR classification system [1], availability of clinical data from the time of inclusion and at the 8-year follow-up and access to a baseline serum sample taken within 1 year after onset of JIA. Patients with systemic onset JIA were excluded from the study (n = 17), because this entity constitutes a minor group with a different immune-inflammatory pathology [25–27]. Three hundred and two patients were included in the Nordic JIA cohort and had an 8-year follow-up assessment. Of these, 130 patients fulfilled the inclusion criteria and had no exclusions criteria. Inability to meet the inclusion criteria was due to absence of a stored serum sample within the first year after JIA. ESR values from within 1 year after disease onset were available in 80 of included patients.
Outcome measures
All outcome variables were evaluated at the 8-year follow-up visit. The primary outcome was remission off medication at 8-year follow-up. Remission was defined according to the validated Wallace criteria [7, 28] as inactive disease off medication for at least 12 months [8, 29].
As secondary outcomes, damage-scores according to the validated Juvenile arthritis damage index, articular (JADI-A) and extra-articular (JADI-E), were applied. JADI-A evaluates 36 joints and scores each of those from 0 to 2 giving a maximum score of 72. JADI-E scores 13 items in five organ systems with a differentiated score, giving a total score of 0–17 [29]. The JADI scores were analyzed dichotomized as no damage (=0) or damage (>0).
Finally, JIA-associated uveitis assessed and analyzed dichotomized as presence or absence of uveitis during the follow-up period.
Analyses of biomarkers
Serum samples were stored at minus 80 degrees for later analysis. Stored samples were in 2014 analyzed by a ROCHE® CRPHS COBAS-kit, with a CRP measuring range of 0.2–20 mg/l. Samples with a concentration >20 mg/l were reanalyzed with a standard immunoturbidometric assay in a COBAS® INTEGRA system. For samples with a CRP concentration below the detection limit (0.2 mg/l) a value of 0.1 mg/l was applied in the statistical analysis (11/130 patients). Samples were analyzed in uniplicate.
ESR was available from the records of the baseline visit and were based on use of the Westergren method [30] according to the local biochemical department guidelines. Values of zero were, for the statistical analysis, set at 1 mm/hour (7/80 patients).
Statistical methods
Descriptive statistics were presented as absolute numbers, percentages and absolute frequencies, and as median and interquartile range (IQR) as appropriate.
The biomarkers were analyzed as continuous variables using non-parametric Mann–Whitney U test, since CRP and ESR levels did not follow a normal distribution. Significant results (p < 0.05) were subjected to conditional logistic regression analysis using dichotomized outcome variables for the regression models. For these analyses the data were logarithmically transformed, as the residuals of the variable did not follow a normal distribution.
The relationship between outcome and co-variables was analyzed with Fisher’s exact test. As potential co-variables gender, age at disease onset (0–4 years/5–9 years/10–16 years), anti-nuclear-antibodies (ANA), HLA-B27, rheumatoid factor (RF), and cumulative number of active inflamed joints within the first six months of disease onset (dichotomized into ≤4 joints or >4 joints) were analyzed in an initial univariable analysis.
If associations (p < 0.1) were found between these co-variables and the outcomes, the co-variables were applied in a multivariable logistic regression analysis. In the multivariable analysis, p values in two-tailed tests of <0.05 were considered statistically significant, and odds ratio (OR) and 95% confidence intervals (CI) were calculated to evaluate the predictive ability of the markers. Correlation analysis was performed using Spearman’s Rho-correlation. Sensitivity, specificity, and likelihood ratio of the biomarkers were calculated for selected concentrations. For CRP, dichotomized at ≤10 mg/l and >10 mg/l, the Chi-square test was performed for the dichotomized outcome variables.
Analyses were carried out using SAS v9.3 (SAS Institute Inc., Cary, NC, USA) and figures were made using Graphpad Prism 6.0 (GraphPad Software Inc. La Jolla, CA, USA).
Ethical issues
The study was conducted in accordance with the Declaration of Helsinki and Research Ethics Committees according to national practice and legislation. Written informed consent was obtained from children and parents of children aged <16 years.
Results
Study population
One hundred and thirty patients met the inclusion criteria and this study cohort did not differ significantly from the total population of patients with respect to gender, age at onset, ANA, HLA-B27, RF or JIA category (Table 1). The patients were followed prospectively for 97 months (95–100). At the 8-year follow-up assessment, 38% were in remission off medication (Table 1 ).
Baseline CRP measurements
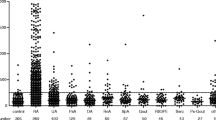
The time from onset of disease to baseline CRP blood sampling was 5.53 months (2.60–7.37). Baseline CRP concentrations were 1.75 mg/l (0.36–10.86) and 96 patients (74%) had CRP below the normal threshold (10 mg/l). Baseline levels of CRP according to the specific JIA category are shown in Fig. 1. JIA categories were used as co-variables in the multivariable analysis.. CRP correlated modestly with clinical activity parameters at the time of sampling, while the correlations between CRP and ESR were stronger (Table 2).
Baseline CRP according to JIA category. CRP C-reactive protein, ESR erythrocyte sedimentation rate, JIA Juvenile idiopathic arthritis, pOJIA Persistent oligoarticular JIA, eOJIA extended oligoarticular JIA, RF-PJIA rheumatoid factor negative polyarticular JIA, RF + PJIA rheumatoid factor positive polyarticular JIA, PsoA psoriatic JIA, ERA entesitis-related JIA, UnDJIA undifferentiated JIA
Elevated CRP (>10 mg/l) was significantly associated with absence of remission (OR 8.60, CI 2.98–24.81, p < 0.001) (Table 3 ) and the median CRP concentration of 0.77 mg/l (0.24–5.12) in the remission group and a median concentration of 4.47 mg/l (0.48–16.92) in the non-remission group were significantly different (p ≤ 0.001) (Fig. 2). Adjusting for oligoarticular or polyarticular disease onset in a multivariable logistic regression analysis, elevated CRP remained significantly associated with absence of remission at 8-year follow-up (OR 1.33, CI 1.08–1.63, p = 0.007) (Table 4 ).
To explore the informative value of CRP within the normal range, we investigated the predictive value of baseline CRP below 10 mg/l in a separate analysis. No significant associations between CRP and outcomes were found within this subgroup (Table 4 ).
Baseline ESR and associations with outcome
In 80 patients, a baseline ESR value was available at 5.8 months (3.13–7.35) after disease onset.
ESR correlated significantly with CRP (rho = 0.44, p < 0.001) and higher ESR at baseline was associated with a reduced chance of remission (6 mm/h (4–13) in the remission group and 15 mm/h (6–41) in the non-remission group) (Fig. 2b). These findings remained significant in the multivariable analysis (OR 2.32, CI 1.35–4.00, p = 0.002) (Table 4 ). Elevated ESR was also associated with increased risk of developing extra-articular damage (JADI-E) (OR 7.14, CI 1.79–28.53, p = 0.005) (Table 4 ).
Sensitivity and specificity of CRP and ESR with respect to achievement of remission
To evaluate the prognostic potential of the two biomarkers, sensitivity, specificity, and likelihood ratios were calculated for selected values of CRP and ESR (Table 5 ). The specificity was high (>0.9) for values above the normal threshold (CRP: 10 mg/l, ESR: 20 mm/h), implying that 90% of the patients who went into remission after 8 years had a baseline biomarker below the normal threshold. Importantly, the sensitivity of the biomarkers was poor (<0.4), implying that only about 40% of patients who did not achieve remission had increased CRP at baseline. For CRP > 10 mg/l, the positive likelihood ratio was 3.7. Using the Fagan nomogram [31], this corresponds to an increase in the risk of not achieving remission when having a CRP > 10 mg/l, from 62% in the cohort as a pre-test risk, to approximately 85% post-test risk.
Calculations of relative risks (RR) of not achieving remission off medication at the 8-year follow-up depending on whether inflammatory markers were elevated are shown in Table 6. The table also describes the RR of not achieving inactive disease at the 8-year follow-up depending on the JIA category. The RR is around 1.5 if the baseline level of CRP or ESR is elevated, resembling what is found for the JIA categories other than persistent oligoarticular and undifferentiated JIA. However, for most JIA categories, the results are insignificant.The calculated RR demonstrates the superiority of the inflammatory markers to the specific JIA categories, as predictive markers.
Uveitis
We analyzed associations between inflammatory markers and the risk of uveitis. In univariate analysis, CRP was slightly associated with the risk of uveitis, although with borderline significance (p = 0.044), and this was insignificant in the multivariable analysis that included the presence of antinuclear antibodies (ANA) (OR 1.25, CI 0.98–1.60, p = 0.069) (Table 4 ). In accordance, ESR was not associated with uveitis in the univariable analysis (OR 1.59, CI 0.91–2.79, p = 0.10) (Table 4 ).
Discussion
This study demonstrates, in accordance with previous studies [8, 32, 33], that a large group of JIA patients exhibits acute phase reactants within normal range early after disease onset. This indicates that JIA in many patients is a low-grade inflammatory disease in which current inflammatory biomarkers may be of limited value. During the last decade, several studies have investigated possible predictors of JIA outcome, and there is indications that an increased number of active joints at disease onset [8, 10, 11], an increased baseline JADAS score [12] and involvement of specific “risk joints” (e.g., hip and ankle) [34, 35] may be predictive of a poor outcome, although the findings are not consistent between the studies [5]. Likewise, increased levels of ESR at baseline have appeared predictive in some investigations [8], while other studies found no association [11].
We observed a predictive ability of both CRP and ESR dichotomized at the upper normal limit for remission off medication at the 8-year follow-up. Thus, patients with CRP levels above the upper normal range had about nine times higher risk of not being in remission. Of note, however, a low value of this biomarker was uninformative regarding the risk of a poor long-term outcome and similarly, we did not find any predictive value of CRP variations within the normal range.Thus, JIA patients with low levels of inflammatory markers at baseline remain a heterogenic group that cannot be sub-grouped in different risk profiles by high-sensitive CRP. This patient group with low-grade inflammation should be the focus of further investigations to identify prognostic biomarkers.
We could not convincingly demonstrate an association between CRP, ESR and the presence of uveitis as suggested in previous studies [36, 37], that both found an association with baseline ESR levels and the risk of uveitis.
The RR of not achieving remission off medication at follow-up according to JIA category tended to be elevated for all JIA categories, except the persistent oligoarticular JIA and undifferentiated JIA, although generally insignificant. In contrast, significantly increased RR was found for both elevated CRP and ESR.
Division of patients into oligoarticular vs. polyarticular patients (≤ 4/>4 active joints) within the first 6 month of disease onset did show significant association to the outcome and accordingly this variable was applied in the multivariable analysis.
We found only modest correlation between baseline CRP and clinical markers of JIA activity at baseline. This must be viewed in the light that more than 75% of patients had a baseline CRP within the normal range, despite active JIA.
A major limitation of the study is that the clinical disease status was only assessed at a single time-point during the 8-year of follow-up, missing the opportunity to evaluate time to onset of remission and fluctuations in disease activity over time. This is of particular importance given the fact that JIA patients often have a multi-phasic disease course, with periods of remission followed by disease-flair [7, 38]. Nevertheless, we were able to explore the degree of damage, which may reflect the total burden of the disease over time.
Furthermore, we were not able to differentiate patients according to treatment. Differences in levels of inflammatory markers and clinical presentation during the course of disease have most likely influenced the clinical decisions regarding the intensity of the treatment. Accordingly, a statistical correction for differences in the treatment may have resulted in stronger associations between biomarkers and outcome of disease than reported here. In line with this, a stratified analysis between JIA categories may have revealed stronger associations between elevated CRP and a poorer outcome, and we cannot exclude that this may also have revealed associations between outcome and CRP variations within the normal range, although this issue was addressed by multivariable analysis.
Patients were only treated with NSAIDs prior to blood sampling while DMARDs were introduced after sampling for this study. An ability of NSAIDs to influence CRP/ESR levels cannot be excluded, although it is most likely minor.
A strength of this study is the well-characterized population-based cohort with robust baseline and follow-up data performed in relation to a study assessment. The baseline visit and blood sampling were performed as soon as possible at the onset of disease, illustrating “real life settings”. Furthermore, our population-based cohort represents the total range of disease severities within the categories of JIA studied, and most importantly including the mild cases that are numerous in a population-based material. Therefore, the results of this study are relevant in relation to the broad field of pediatric rheumatology.
In this prospective long-term followed cohort, increased levels of baseline CRP and ESR were predictive of a poor disease outcome, but quantification of CRP within the normal range did not provide additional prognostic information.
Although only a minor part of our patients had baseline CRP above 10 mg/l, our data suggest that aggressive treatment at an early stage of disease should be considered for this group of patients.
It should be underlined that the major part of the JIA patients had normal levels of inflammatory markers, regardless of their long-term disease outcome. For this group of patients, we still need to identify predictive biomarkers of disease outcome to guide the clinical decisions regarding treatment intensity at the early stage.
References
Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J et al (2004) International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 31(2):390–392
Berntson L, Andersson GB, Fasth A, Herlin T, Kristinsson J, Lahdenne P et al (2003) Incidence of juvenile idiopathic arthritis in the Nordic countries. A population based study with special reference to the validity of the ILAR and EULAR criteria. J Rheumatol 30(10):2275–2282
Nordal E, Zak M, Aalto K, Berntson L, Fasth A, Herlin T et al (2011) Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum 63(9):2809–2818
Zak M, Pedersen FK (2000) Juvenile chronic arthritis into adulthood: a long-term follow-up study. Rheumatology (Oxf) 39(2):198–204
van Dijkhuizen EH, Wulffraat NM (2015) Early predictors of prognosis in juvenile idiopathic arthritis: a systematic literature review. Ann Rheum Dis 74(11):1996–2005
Adib N, Silman A, Thomson W (2005) Outcome following onset of juvenile idiopathic inflammatory arthritis: I. frequency of different outcomes. Rheumatology (Oxf) 44(8):995–1001
Wallace CA, Huang B, Bandeira M, Ravelli A, Giannini EH (2005) Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum 52(11):3554–3562
Flato B, Lien G, Smerdel A, Vinje O, Dale K, Johnston V et al (2003) Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol 30(2):386–393
Fantini F, Gerloni V, Gattinara M, Cimaz R, Arnoldi C, Lupi E (2003) Remission in juvenile chronic arthritis: a cohort study of 683 consecutive cases with a mean 10 year followup. J Rheumatol 30(3):579–584
Bertilsson L, Andersson-Gare B, Fasth A, Forsblad-D’elia H (2012) A 5-year prospective population-based study of juvenile chronic arthritis: onset, disease process, and outcome. Scand J Rheumatol 41(5):379–382
Minden K, Kiessling U, Listing J, Niewerth M, Doring E, Meincke J et al (2000) Prognosis of patients with juvenile chronic arthritis and juvenile spondyloarthropathy. J Rheumatol 27(9):2256–2263
Nordal EB, Zak M, Aalto K, Berntson L, Fasth A, Herlin T et al (2012) Validity and predictive ability of the juvenile arthritis disease activity score based on CRP versus ESR in a Nordic population-based setting. Ann Rheum Dis 71(7):1122–1127
Giannini EH, Brewer EJ (1987) Poor correlation between the erythrocyte sedimentation rate and clinical activity in juvenile rheumatoid arthritis. Clin Rheumatol 6(2):197–201
Petty RE, Laxer RM, Lindsley CB, Wedderburn LR. Juvenile Idiopathic Arthritis. Textbook of Pediatric Rheumatology. 7 edn. Elsevier-Saunders, Philadelphia; 2016. 188–204
Kushner I, Samols D, Magrey M. A unifying biologic explanation for “high-sensitivity” C-reactive protein and “low-grade” inflammation. Arthritis Care Res (Hoboken) 2010; 62(4):442–446.
Kushner I, Rzewnicki D, Samols D (2006) What does minor elevation of C-reactive protein signify? Am J Med 119(2):166-e17
Buckley DI, Fu R, Freeman M, Rogers K, Helfand M (2009) C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med 151(7):483–495
Musunuru K, Kral BG, Blumenthal RS, Fuster V, Campbell CY, Gluckman TJ et al (2008) The use of high-sensitivity assays for C-reactive protein in clinical practice. Nat Clin Pract Cardiovasc Med 5(10):621–635
Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW et al (2004) Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350(23):2362–2374
Devaraj S, Singh U, Jialal I (2009) Human C-reactive protein and the metabolic syndrome. Curr Opin Lipidol 20(3):182–189
Sennels H, Sorensen S, Ostergaard M, Knudsen L, Hansen M, Skjodt H et al (2008) Circulating levels of osteopontin, osteoprotegerin, total soluble receptor activator of nuclear factor-kappa B ligand, and high-sensitivity C-reactive protein in patients with active rheumatoid arthritis randomized to etanercept alone or in combination with methotrexate. Scand J Rheumatol 37(4):241–247
Hiura K, Iwaki-Egawa S, Kawashima T, Fujisawa S, Takeda T, Komori H et al (2013) The diagnostic utility of matrix metalloproteinase-3 and high-sensitivity C-reactive protein for predicting rheumatoid arthritis in anti-cyclic citrullinated peptide antibody-negative patients with recent-onset undifferentiated arthritis. Rheumatol Int 33(9):2309–2314
Jednacz E, Rutkowska-Sak L (2015) Assessment of the body composition and parameters of the cardiovascular risk in juvenile idiopathic arthritis. Biomed Res Int 2015:619023
Gerss J, Roth J, Holzinger D, Ruperto N, Wittkowski H, Frosch M et al (2012) Phagocyte-specific S100 proteins and high-sensitivity C reactive protein as biomarkers for a risk-adapted treatment to maintain remission in juvenile idiopathic arthritis: a comparative study. Ann Rheum Dis 71(12):1991–1997
De BF, Martini A (1998) Is systemic juvenile rheumatoid arthritis an interleukin 6 mediated disease? J Rheumatol 25(2):203–207
De BF, Martini A (2005) Targeting the interleukin-6 receptor: a new treatment for systemic juvenile idiopathic arthritis? Arthritis Rheum 52(3):687–693
Ravelli A, Martini A (2007) Juvenile idiopathic arthritis. The Lancet 369(9563):767–778
Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American college of rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011; 63(7):929–36.
Viola S, Felici E, Magni-Manzoni S, Pistorio A, Buoncompagni A, Ruperto N et al (2005) Development and validation of a clinical index for assessment of long-term damage in juvenile idiopathic arthritis. Arthritis Rheum 52(7):2092–2102
ICSH recommendations for measurement of erythrocyte sedimentation rate (1993) International Council for Standardization in Haematology (Expert Panel on Blood Rheology). J Clin Pathol 46(3):198–203
Fagan TJ (1975) Letter: nomogram for Bayes theorem. N Engl J Med 293(5):257
Oen K, Tucker L, Huber AM, Miettunen P, Scuccimarri R, Campillo S et al (2009) Predictors of early inactive disease in a juvenile idiopathic arthritis cohort: results of a Canadian multicenter, prospective inception cohort study. Arthritis Rheum 61(8):1077–1086
Hyrich KL, Lal SD, Foster HE, Thornton J, Adib N, Baildam E et al (2010) Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. Results from the Childhood Arthritis Prospective Study. Rheumatology (Oxf) 49(1):116–122
Esbjornsson AC, Aalto K, Brostrom EW, Fasth A, Herlin T, Nielsen S et al. Ankle arthritis predicts polyarticular disease course and unfavourable outcome in children with juvenile idiopathic arthritis. Clin Exp Rheumatol 2015
Flato B, Hoffmann-Vold AM, Reiff A, Forre O, Lien G, Vinje O (2006) Long-term outcome and prognostic factors in enthesitis-related arthritis: a case-control study. Arthritis Rheum 54(11):3573–3582
Haasnoot AJ, van Tent-Hoeve M, Wulffraat NM, Schalij-Delfos NE, Los LI, Armbrust W et al (2015) Erythrocyte sedimentation rate as baseline predictor for the development of uveitis in children with juvenile idiopathic arthritis. Am J Ophthalmol 159(2):372–377
Pelegrin L, Casaroli-Marano R, Anton J, Garcia de Vicuna MC, Molina-Prat N, Ignacio AJ et al (2014) Predictive value of selected biomarkers, polymorphisms, and clinical features for oligoarticular juvenile idiopathic arthritis-associated uveitis. Ocul Immunol Inflamm 22(3):208–212
Guzman J, Oen K, Huber AM, Watanabe DK, Boire G, Shiff N et al. The risk and nature of flares in juvenile idiopathic arthritis: results from the ReACCh-Out cohort. Ann Rheum Dis 2015
Acknowledgements
This work was supported by grants from the Department of Pediatrics, Naestved hospital, Region Zealand, the Research Department, Region Zealand, The Danish Arthritis foundation, and the Aase and Ejnar Danielsen Foundation.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Alberdi-Saugstrup, M., Zak, M., Nielsen, S. et al. High-sensitive CRP as a predictive marker of long-term outcome in juvenile idiopathic arthritis. Rheumatol Int 37, 695–703 (2017). https://doi.org/10.1007/s00296-017-3657-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-017-3657-x