Abstract
Takayasu arteritis (TA) is a rare autoimmune disease of unknown etiology. Genome-wide association studies (GWAS) have demonstrated association between genetic variants of IL12B and IL6 and TA. Since TA has been reported with ethnic heterogeneity, we sought to investigate whether the single-nucleotide-polymorphisms (SNPs) reported in these studies are associated with TA in the Chinese Han population. A multi-center study involving 412 patients with TA and 597 healthy controls was conducted. Sequenom MassArray iPLEX platform was used to determine the frequencies of SNPs in the IL12B and IL6 region. We demonstrated a allele association between the four SNPs of IL12B and TA (rs6871626: OR 1.52, 95% CI 1.26–1.83; rs4921492: OR 1.46, 95% CI 1.21–1.75; rs60689680: OR 1.41, 95% CI 1.17–1.69; rs4921493: OR 1.45, 95% CI 1.21–1.75, all P c < 10− 3 ). A meta-analysis consist of four populations showed rs6871626 was a susceptible locus of TA. Its OR was 1.51, and 95% CI was 1.31–1.74. The four SNPs were in strong linkage disequilibrium and two haplotypes were significantly different between patients and controls. Conditional analysis shows that these SNPs were not independent factors contributing to TA. Nevertheless, neither genotype nor allele frequencies of rs2069837 in IL6 showed significant between-group differences. Thus SNP of IL12B may be considered a high-risk factor for TA in Chinese Han population and provide further clues for research into the pathogenesis of TA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Takayasu arteritis (TA) is a rare large-vessel vasculitis of unknown etiology, characterized by inflammation of the aorta and its major branches such as subclavian and carotid artery, which may lead to vascular stenosis, occlusion or dilatation [1]. During early stages, patients present with non-specific symptoms such as fever, fatigue, weight loss, headache, and fainting spells. With disease progression, neck pain, claudication, bruits, difference in arterial pressure between bilateral limbs and hypotension may occur. Serious cases of aortic regurgitation, pulmonary stenosis or hypertension, cerebral infarction, coronary artery involvement, and renal artery stenosis are on record [2].
TA usually affects young women; with the age at onset generally being younger than 40 years. A higher prevalence has been reported in East Asia, Middle-East, and Latin America in comparison to Europe and North America. Given the differences in age, gender, and geographical location of the affected population, genetic factors are thought to be involved in the disease causation [3, 4]. Research into the genetic basis of TA has largely centered on the HLA region in which a series of associations having been identified. While several HLA alleles were proposed as susceptible genotype, notably only HLA-B*52:01 was proved to be associated with TA in multiple geographies, including Asians, Turks, and Americans [5].
Because TA is an autoimmune disease, multiple genetic components outside HLA region may also be involved in pathogenesis. Recent genome-wide association studies (GWAS) showed that the genetic variants of IL12B conferred a higher risk for TA in a Turkish, North American, and Japanese cohorts [6, 7], while IL6 (rs2069837) was associated with TA in a meta-analysis studies involving Turkish and North American populations [8]. A Turkish candidate gene association study also suggested the polymorphism of IL12 (1188 allele) and IL6 (IL6 −174 and − 598) may contribute to TA susceptibility, although this study has a small sample size [9].
IL12B plays a vital role in inflammation by encoding p40 subunit of the pro-inflammatory cytokines IL-12 and IL-23. IL12 promotes Th1 development and IL23 helps in Th17 differentiation; both are critical in the pathogenesis of various autoimmune diseases [10]. Notably, Sadoun et al. reported increased expression of IL12 and IL23 in TA patients [11]. Cytokine IL6 promotes the maturation of B cells and functioned in inflammation-associated disease including autoimmune thyroid disease and systemic sclerosis [12, 13]. Moreover, IL6 polymorphism (rs7805828 and rs1546766) has been identified as a risk factor for giant cell arteritis, which is also characterized by large-vessel vasculitis [14]. Importantly, Alibaz-Oner et al. reported significantly increased serum IL6 levels in patients with TA when compared with those in healthy controls [15]. Together, multiple lines of evidence indicate that polymorphism of IL12B and IL6 may be genetic factors underlying TA, which act by modulating cytokine production and inducing inflammation.
Considering that polymorphism of HLA region in different ethnicities may contribute to the heterogenous prevalence of TA, we hypothesized that genetic variants of IL12B and IL6 could also have ethnic differences. Thus we performed a case-control study among the Chinese Han population to validate the association reported in Turkish and North American populations in previous GWAS.
Materials and methods
Patients and controls
We recruited 412 patients diagnosed with TA and 597 Chinese Han healthy controls in this multi-center study. Of these, 230 patients and all healthy controls were recruited at the Peking Union Medical College Hospital (PUMCH, Beijing, China) between February 2013 and July 2015. The remaining 182 patients were enrolled at 23 centers across China, with support from the Research Special Fund for Public Welfare Industry of Health. All patients fulfilled the 1990 American College of Rheumatology (ACR) classification criteria for TA [16]. Patients diagnosed with Behcet′s disease, rheumatoid arthritis or any other co-existing autoimmune diseases were excluded.
Clinical data on the following variables were collected: hypertension (hypertension as presenting symptom or that developing during the disease course), coronary artery involvement (stenosis or dilatation detected on coronary arteriography), ischemic brain disease (transient ischemic attacks diagnosed on the basis of clinical history or cerebral infarction diagnosed on CT or MRI), renal artery stenosis (diagnosed by ultrasonography), and pulmonary artery involvement (pulmonary hypertension diagnosed by echocardiography or pulmonary stenosis diagnosed on pulmonary angiography). The study was approved by the Ethics Committee at the Peking Union Medical College Hospital and written informed consent was obtained from all participants prior to their enrollment in the study.
Selection of SNPs
According to previous reported GWAS on risk genetic variants of TA, five SNPs in IL12B (rs6871626, rs4921492, rs60689680, and rs4921493), and IL6 (rs2069837) in Han Chinese were selected.
Genotyping
Genomic DNA of each subject was extracted from 2 mL anticoagulated peripheral blood sample by using Tiangen DNA extraction kit (Beijing, China). SNPs of IL12B and IL6 were genotyped using Sequenom MassArray system (San Diego, CA, USA) according to the manufacturer’s protocol at the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. About 15–20 ng DNA sample was subjected to two amplified reaction steps:, first a multiplex polymerase chain reaction (PCR) and second the locus-specific single-base extension. Primers of the two procedures were designed by the MassArray Assay Design 4.0 software. After PCR in a 384-well plate, the products were digested with shrimp alkaline phosphatase, followed by locus-specific single-base extension reactions. The final products were then desalted and transferred to a 384-element SpectroCHIP array (Sequenom,CA) and detected by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF MS). The mass spectrogram data were then analyzed using MassArray Typer 4.0 software.
Statistical analysis
Data analyses were performed using PLINKv1.07 program (http://pngu.mgh.harvard.edu/purcell/plink/). Initially, the Hardy–Weinberg equilibrium (HWE) test was performed, and in the event of P value being <0.05 in the healthy controls, it was excluded. Subsequently, allele and genotype frequencies in the cases and controls were calculated using Chi-squared (χ 2) test. OR with 95% CI were calculated; P c values < 0.05 (corrected for multiple comparisons by Bonferroni adjustment) were considered statistically significant. Subsequently, three genetic models (additive, dominant, and recessive) were assessed using logistic regression analysis. Conditional analysis using PLINK was also performed to find out independent signals in IL12B. Finally, linkage disequilibrium(LD) and haplotype analysis were carried out using Haploview software v4.2 (http://www.broadinstitute.org/haploview). Data of Utah residents with Northern and Western European ancestry (CEU) which show positive association in previous reports and Han Chinese in Beijing (CHB) from 1000 Genome Project which contain the most detailed catalogue of human genetic variation were used to perform LD analysis.
Meta analysis
Meta analysis was conducted by STATA software, version 12.0 (Stata Corporation, College Station, USA). The associations between rs6871626 and TA were estimated by OR and 95% CI. The heterogeneity of the two studies was assessed by Q-test and I2 statistics [26]. If there was no heterogeneity detected, a fixed-effects model was used. Otherwise, a random effects model was used.
Results
Characteristics of the study population
A total of 412 (mean age 31.6 ± 10.8 years; 85.0% women) Chinese Han patients diagnosed with TA, and sex-ratio matched 597 Chinese Han healthy controls were enrolled in the study. The mean age at onset was 27 years. Hypertension, coronary artery involvement, ischemic brain disease, renal artery stenosis, and pulmonary artery involvement were detected in 27, 2.7, 16.3, 26.9, and 12.1% patients, respectively (Table 1). The statistical power was more than 80% (α = 0.05) for detecting association with an OR of 1.24–1.81, for both heterozygotes and homozygotes, at an estimated prevalence of 40 per million and the risk allele frequency of 0.329.
Allele and genotype frequencies
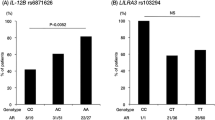
The primary information of seven SNPs in the same region of IL12B and IL6 (rs2069837) are summarized in supplementary table 1. The minor allele frequency (MAF) of IL12B SNPs in CHB, CEU and Turkish population were similar and MAF of rs2069837 in CEU was lower than CHB and Turks. Call rate of the five SNPs detected in our patients as well as healthy controls exceeded 97%, and these SNPs were in Hardy–Weinberg equilibrium (P > 0.05). Both genotype and allele frequencies of the four SNPs of IL12B were found to be significantly different between the cases and controls (all P < 10− 3, OR of allele frequency for rs6871626, rs4921492, rs60689680, and rs4921493 were 1.52, 1.46, 1.41, and 1.45, respectively, Table 2). However, no significant association was observed between rs2069837 of IL6 and TA. Moreover, no significant inter-group difference in genotype distribution was found between PUMCH and non-PUMCH patients; hence we believe that our cases can well reflect the whole Chinese Han TA patients (Supplementary table 2). In addition, we conducted association studies with patients and healthy controls only from PUMCH and reached consistent results with all patients from multicenter, which provide conclusive evidence of positive association in Han Chinese (Supplementary table 3).
Three genetic models based on multiple logistic regression were also analyzed. For rs6871626, rs4921492, and rs4921493, strong association with TA was detected in the additive, dominant, and recessive models. For rs60689680, strong association with TA was detected only in additive and recessive models (P c = 2.32 × 10− 3 and 8.4 × 10− 4, respectively). However, rs2069837 of IL6 did not show any association in any model (Table 3).
Meta analysis of two Chinese Han research
We did a meta-analysis of previous reports and our result of rs6871626 in four populations (Fig. 1). The P value by Q test was 0.068 and I 2 was 54.2% which showed heterogeneity among these studies. Thus we used a random effects model and found rs6871626 was associated with TA. The pooled OR of risk allele frequency was 1.51, 95% CI was 1.31–1.74. In the subgroup analysis, rs6871626 of IL12B was associated with TA in Chinese (OR was 1.35, 95% CI was 1.03–1.78).
Linkage disequilibrium, haplotype, and conditional analysis of IL12B
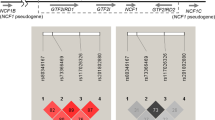
We used Haploview software to perform LD and haplotype analysis. The results of the LD analysis are shown in Fig. 2. Our study shows that rs6871626, rs4921492, rs60689680, and rs4921493 of IL12B were in strong LD (r 2 > 0.7, Fig. 2a). According to 1000 Genomes, six SNPs (rs5616732, rs755374, rs4921492, rs12374547, rs60689680 and rs4921493) in this region were in strong LD (r 2 > 0.7) of CHB (LD data of rs6871626 is not available in 1000 Genome, Fig. 2b). Comparing the two LD blocks, our result of three SNPs rs4921492, rs60689680, and rs4921493 was consistent with 1000 Genomes data. And they together indicated the seven SNPs are in strong LD (r 2 > 0.7). Six SNPs were also in LD (r 2 > 0.7) in CEU; the LD intensity seems weaker than CHB (Fig. 2c).
LD analysis of the SNPs in the IL12B gene region. a The data analysis of healthy controls from our study. b Data from 1000 Genome of CHB. c Data from 1000 Genome of CEU. The number the small square is r 2 value. LD linkage disequilibrium, SNP single nucleotide polymorphism, CEU Utah residents with Northern and Western European ancestry, CHB Han Chinese in Beijing, China
Then we performed haplotype analysis to assess its association with TA risk. Three major haplotypes, CCGT, AATC, and CATC (rs6871626, rs4921492, rs60689680 and rs4921493 of IL12B) were found. Of these, AATC was associated with a high risk of TA, while CCGT was found to be protective (P c <10− 4). No significant association of CATC with TA was found (Table 4).
Because of the high LD signal, conditional analysis among risk SNPs was performed to discuss the possibility of independent signals in IL12B. As a result, these SNPs were not considered independent factors contributing to TA.
Discussion
TA is a rare autoimmune disease with inter-ethnic variability in prevalence. Since previous GWAS reported genetic variants of IL12B and IL6 on susceptibility to TA, it was indeed worthwhile performing a large cohort study to identify and validate genes that confer risk in Chinese Han population. In this multi-center study, we demonstrated that the four SNPs of IL12B were significantly associated with TA in Han Chinese, and they were in strong LD. However, rs2069837 in IL6 was not found to be associated with an increased risk of TA.
In order to gain a greater understanding of the pathogenesis of TA, genetic factors have been extensively investigated. Two GWAS studies, along with our own, have shown that IL12B polymorphisms are related to TA in Turkish, North American [16], Japanese [7], and Chinese Han population. However, contrary to our result, rs6871626 and rs56167332 in the same LD block in IL12B were negatively related to TA in another Chinese study [17]. As the authors speculated, the discrepancy may due to their relatively small sample size. We then performed a meta-analysis of the two Chinese studies and found that the A allele of rs6871626 did correlate to TA in Chinese Han population. Thus, IL12B has now been shown to be positively associated with TA in multiple populations.
Further, in the GWAS conducted by a Japanese group, rs6871626 not only conferred a higher susceptibility to TA, but was also associated with higher rates of complications, such as severe aortic regurgitation and an aggravated disease course. Later, the group also showed that rs6871626 of IL12B was associated with severe TA defined as younger age at onset, more frequent relapses, and poor response to treatment [18]. Interestingly, rs6871626 of IL12B has been found to be also associated with ankylosing spondylitis [19], leprosy [20], Crohn’s disease (CD), and ulcerative colitis (UC) [21]. Terao et al. demonstrated that 6.4% of the patients with TA also had UC [22]. Therefore, shared IL12B polymorphism may play a role in the co-occurrence of the two diseases. Some SNPs that are in strong LD with rs6871626 have been reported to be associated with other diseases, such as rs4921492 with sarcoidosis [23], and rs4921493 with psoriasis [24]. In addition, polymorphism of IL12B increased risk of other autoimmune diseases, such as SLE [25] and Behcet’s disease [26]. Taken together, these multiple lines of evidence suggest that IL12B may be involved in the pathogenesis of TA as well as various autoimmune disorders and immune-mediated inflammatory disease.
Given the positive association between four IL12B SNPs and TA, and LD data from the 1000 Genome project, it was speculated that seven SNPs present in this region conferred susceptibility to TA, and this was demonstrated in the Turkish population. Haplotype AATC (rs6871626, rs4921492, rs60689680, and rs4921493 of IL12B) was found to be associated with an increased risk of TA in the Han Chinese population. However, five SNPs were dependent on rs6871626 or rs56167332 in the Turkish cohort while the SNPs were not independent factors in CHB, which further suggested ethnic difference in TA pathogenesis. Though seven SNPs were in LD, only three SNPs rs6871626, rs56167332, and rs755374 were associated with TA in the North American cohort. Considering the similar minor allele frequency among these populations, the discrepancy may result from ethnic heterogeneity which can present as different LD structure.
Notably, IL-12 3′UTR polymorphism is believed to affect cytokine production [27]. Though these SNPs were located 69–79 kb upstream of IL12B, the latest data from the ENCODE (Encyclopedia of DNA elements) program show that there were rich transcription regulatory sequences in the majority of intron and gene desert regions, which could regulate related gene expression leading to diseases/traits observed. It would be worthwhile to study whether the high-risk haplotype is associated with the expression of IL12B, which may provide clues for the development of specific treatment in these patients.
The present study showed a negative association of IL6 (rs2069837) with TA in Chinese Han population which was inconsistent with Turkish cohort. Though meta-analysis of studies conducted in Turkish and North American cohorts had found an association between the two, the North American cohort showed a weak association (P < 10− 3) at the GWAS level. One reason for this discrepancy is likely attributable to minor allele frequency such as North American vs CHB and Turks, and another may be due to ethnic differences between CHB and Turks.
Therefore, we demonstrated again TA is a complicated disease with ethnic heterogeneity. The difference should be validated in more ethnicities and the association of IL6 polymorphism and expression level should be further investigated.
TA is a rare disease and the sample size in this multi-center study was by far the largest used in candidate gene association research. We confirmed the fact that four SNPs of IL12B (rs6871626, rs4921492, rs60689680, and rs4921493) confer a genetic susceptibility to TA in the Chinese Han population and that IL6 (rs2069837) was of ethic heterogeneity which did not correlate to TA in Han Chinese. Yet there were certain limitations in our study which ought to be mentioned. First, healthy controls should be enrolled from multicenter as well as TA cases to reduce potential bias. Second, the association between expression levels and genetic variants is still not clear, and further functional investigations need to be conducted to understand the role of SNPs of IL12B and IL6 in the pathogenesis of TA.
References
Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M, Hoffman GS (1994) Takayasu arteritis. Ann Intern Med 120(11):919–929
Keser G, Direskeneli H, Aksu K (2014) Management of Takayasu arteritis: a systematic review. Rheumatology 53(5):793–801
Dreyer L, Faurschou M, Baslund B (2011) A population-based study of Takayasu’s arteritis in eastern Denmark. Clin Exp Rheumatol 29(1 Suppl 64):S40–S42
Alibaz-Oner F, Direskeneli H (2015) Update on Takayasu’s arteritis. La Presse Médicale 44 (6):e259–e265.
Terao C (2016) Revisited HLA and non-HLA genetics of Takayasu arteritis-where are we? J Hum Genet 61(1):27–32
Saruhan-Direskeneli G, Hughes T, Aksu K et al (2013) Identification of multiple genetic susceptibility loci in Takayasu Arteritis. Am J Hum Genet 93(2):298–305
Terao C, Yoshifuji H, Kimura A et al (2013) Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B Regions in a Japanese population. Am J Hum Genet 93(2):289–297
Renauer PA, Saruhan-Direskeneli G, Coit P et al (2015) Identification of susceptibility loci in IL6, RPS9/LILRB3, and an intergenic locus on chromosome 21q22 in Takayasu arteritis in a genome-wide association study. Arthritis Rheumatol 67(5):1361–1368.
Saruhan-Direskeneli G, Bicakcigil M et al (2006) Interleukin (IL)-12, IL-2, and IL-6 gene polymorphisms in Takayasu’s arteritis from Turkey. Hum Immunol 67(9):735–740
Raphael I, Nalawade S, Eagar TN, Forsthuber TG (2015) T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokin 74 (1):5–17.
Saadoun D, Garrido M, Comarmond C et al (2015) Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol 67 (5):1353–1360.
Duraes C, Moreira CS, Alvelos I et al (2014) Polymorphisms in the TNFA and IL6 genes represent risk factors for autoimmune thyroid disease. PLoS One 9 (8):e105492.
Cenit MC, Simeon CP, Vonk MC et al (2012) Influence of the IL6 gene in susceptibility to systemic sclerosis. J Rhumatol 39(12):2294–2302
Enjuanes A, Benavente Y, Hernandez-Rodriguez J et al (2012) Association of NOS2 and potential effect of VEGF, IL6, CCL2 and IL1RN polymorphisms and haplotypes on susceptibility to GCA—a simultaneous study of 130 potentially functional SNPs in 14 candidate genes. Rheumatology (Oxford) 51(5):841–851
Alibaz-Oner F, Yentur SP, Saruhan-Direskeneli G et al (2015) Serum cytokine profiles in Takayasu’s arteritis: search for biomarkers. Clin Exp Rheumatol 33(2 Suppl 89):S-32-35
Arend WP, Michel BA, Bloch DA et al (1990) The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 33(8):1129–1134
Yang K, Yang Y, Meng X et al (2016) Lack of association between polymorphisms in interleukin (IL)-12, IL-12R, IL-23, IL-23R genes and Takayasu arteritis in a Chinese population. Inflamm Res 65(7):543–550
Matsumura T, Amiya E, Tamura N et al (2016) A novel susceptibility locus for Takayasu arteritis in the IL12B region can be a genetic marker of disease severity. Heart Vessls 31(6):1016–1019.
Zhang L, Fan D, Liu L et al (2015) Association study of IL-12B polymorphisms susceptibility with ankylosing spondylitis in Mainland Han population. PLoS One 10 (6):e130982.
Liu H, Irwanto A, Tian H et al (2012) Identification of IL18RAP/IL18R1 and IL12B as leprosy risk genes demonstrates shared pathogenesis between inflammation and infectious diseases. Am J Hum Genet 91(5):935–941
Anderson CA, Boucher G, Lees CW et al (2011) Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 43(3):246–252
Terao C, Matsumura T, Yoshifuji H et al (2015) Brief report: Takayasu arteritis and ulcerative colitis: high rate of co-occurrence and genetic Overlap. Arthritis Rheumatol 67(8):2226–2232
Fischer A, Ellinghaus D, Nutsua M et al (2015) Identification of immune-relevant factors conferring sarcoidosis genetic risk. Am J Respir Crit Care Med 192(6):727–736
Yin X, Low HQ, Wang L et al (2015) Genome-wide meta-analysis identifies multiple novel associations and ethnic heterogeneity of psoriasis susceptibility. Nat Commun 6:6916
Sun C, Molineros JE, Looger LL et al (2016) High-density genotyping of immune-related loci identifies new SLE risk variants in individuals with Asian ancestry. Nat Genet 48(3):323–333
Li X, Bai L, Fang J et al (2014) Genetic variations of IL-12B, IL-12Rbeta1, IL-12Rbeta2 in Behcet’s disease and VKH syndrome. PLoS One 9(5):e98373
Yilmaz V, Yentur S, Saruhandireskeneli G (2005) IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine 30(4):188–194
Acknowledgements
We thank Lv Guanting in Beijing DNALead Co. LTD and all our colleagues who contributed to the TA study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there are no conflicts of interest.
Ethnical approval
The study was approved by the Ethics Committee at the Peking Union Medical College Hospital; all participants gave informed consent.
Funding
This work was supported by the Research Special Fund for Public Welfare Industry of Health (201202004); the National Natural Science Foundation of China Grants (81172857, 81373188); the Chinese National High Technology Research and Development Program, Ministry of Science and Technology Grants (2011AA02A113); the National Science Technology Pillar Program in the 12th 5-year Plan (2014BAI07B00), and the Capital Health Research and Development of Special (2014-1-4011).
Additional information
X. Wen, S. Chen and P. Li contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wen, X., Chen, S., Li, P. et al. Single nucleotide polymorphisms of IL12B are associated with Takayasu arteritis in Chinese Han population. Rheumatol Int 37, 547–555 (2017). https://doi.org/10.1007/s00296-016-3648-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3648-3