Abstract
Steroid treatment is commonly recommended for autoimmune disorders in rheumatology practice. While adrenal crisis may occur upon existence of an inducing factor in patients with known or unknown adrenal insufficiency as well as in those with a suppressed hypothalamic–pituitary–adrenal (HPA) axis due to chronic steroid use, addisonian crisis rarely develops in patients on supraphysiological doses of steroid and, when emerged, it might be very difficult to recognize. Here, we present a patient who developed adrenal crisis while receiving high-dose methylprednisolone treatment due to retroperitoneal fibrosis and we also discuss possible mechanisms with a brief literature review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In rheumatology practice, chronic steroid use is frequently recommended for the treatment of autoimmune diseases. When moderate or high-dose steroid treatment is stopped after a long-term use, addisonian crisis can take place in patients with a suppressed hypothalamic–pituitary–adrenal (HPA) axis [1]. While adrenal crisis may occur upon existence of an inducing factor in patients with known or unknown adrenal insufficiency as well as in those with a suppressed HPA axis due to chronic steroid use, it may be difficult to recognize an addisonian crisis when it develops in patients on supraphysiological doses of steroids. Here, we present a case who developed adrenal crisis receiving high-dose methylprednisolone for the treatment of retroperitoneal fibrosis due to tuberculosis (TBC) and we also discuss possible mechanisms along with a literature review.
Case report
A 55-year-old male was admitted to hospital with a lumbar pain and weight loss in March 2015. Laboratory findings were as follows: Erythrocyte sedimentation rate (ESR) 125 mm/h, C-reactive protein (CRP) 16.7 mg/dL, sodium 134 mEq/L, potassium 4.6 mEq/L, blood urea nitrogen 24 mg/dL, and creatinine 1.61 mg/dL. Serum immunoglobulins (Ig) and IgG4 levels were within normal levels. Antinuclear antibody, rheumatoid factor, antineutrophil cytoplasmic antibody tests, and complement C3 and C4 values were or within normal ranges. Purified protein derivative (PPD) skin test was found to be positive (13 mm). Thoracic computerized tomography (CT) showed fibrotic alterations at the apical segment of the lung with a size of 30 × 10 mm with irregular boundaries. Abdominal CT revealed increased nodular thickness of right suprarenal gland, and two nodular appearances at the lateral limb of left suprarenal gland, the bigger one with an approximate dimension of 13x15 mm, which also have central hypodense areas (Fig. 1). Positron emission tomography/computed tomography (PET-CT) disclosed enhanced activity uptake at the mass lesion of unspecific nature at the lung and at the soft tissue mass located to abdominal aortic bifurcation in a size of about 20 x 20 mm and increased activity uptake in aorta adjacent to mass. A double-J stent was inserted because of bilateral hydronephrosis demonstrated by ultrasonographic image of urinary tract. The biopsy samples obtained from the retroperitoneal region and the left apical lung mass showed the presence of fibro-inflammatory process (retroperitoneal fibrosis) and nonspecific chronic inflammation, respectively. Methylprednisolone 1 mg/kg/day (64 mg/day) and methotrexate 10 mg/week were started for retroperitoneal fibrosis. Approximately two weeks later, the patient applied to the emergency service complaining of vomiting and malaise and detected to have neutrophilic leukocytosis, anemia, hyponatremia, hyperkalemia, and prerenal azotemia (Table 1). The patient’s arterial blood pressure was 70/40 mm/Hg; blood glucose level was 67 mg/dL. He had taken steroid treatment regularly before the admission; he did not have the complaints of trauma, fever, diarrhea, cough, or sputum. Maintenance intravenous fluid therapy was administered. Methylprednisolone dose was adjusted as 40 mg/day. There were no factors which may cause hyperkalemia. Elevated potassium levels remained despite the conducted hyperkalemia treatment. Considering adrenal crisis in spite of the ongoing steroid treatment, methylprednisolone treatment was re-arranged to 80 mg/day by intravenous way. Adrenocorticotrophic hormone (ACTH) and cortisol levels were found as 281 pg/mL (N = 7.2–63.3) and 0.657 μg/L, respectively. Accordingly, the diagnosis of primary adrenal insufficiency was established; fludrocortisone treatment was initiated at a dose of 0.05 mg and increased up to 0.1 mg/day. Meanwhile, adrenal gland biopsy showed caseous granulomatous inflammation (Fig. 2). While the biopsy sample was found to be negative for M. tuberculosis by polymerase chain reaction (TBC-PCR) assay, M. tuberculosis was found to be positive in broncho alveolar lavage (BAL) fluids sampling by TBC-PCR. Based on the diagnosis of pulmonary and extrapulmonary TBC and TBC-induced retroperitoneal fibrosis, first-line anti-tuberculosis treatment composed of four agents [ethambutol + isoniazid + streptomycin (as rifampicin caused eruption) + pyrazinamide] was initiated, methylprednisolone treatment was decreased, and fludrocortisone treatment was continued 0.1 mg/day. During the follow-up period, his symptoms regressed; values of sodium, potassium, blood urea nitrogen, and creatinine returned to normal levels. Table 1 summarizes all laboratory results during the follow-up period.
Discussion
We report a case that developed addisonian crisis receiving supraphysiological doses of steroid for the treatment of retroperitoneal fibrosis. Clinical evaluation showed us that the patient had both pulmonary and adrenal gland TBC and TBC-induced retroperitoneal fibrosis.
Retroperitoneal fibrosis is commonly idiopathic, but may be due to infections, drugs, and malignancies. It has been reported that M. tuberculosis may not only give rise to chronic periaortitis or retroperitoneal fibrosis [2, 3] but may also lead to retroperitoneal mass simulating retroperitoneal fibrosis with ureteric obstruction [4]. In our case, the presence of retroperitoneal fibrosis was confirmed by biopsy, and TBC was excluded by histological and microbiologic evaluations. The patient was initially diagnosed as having idiopathic retroperitoneal fibrosis without evaluation of the cause of adrenal mass, but his symptoms did not resolve after the initiation of steroid and progressed to adrenal insufficiency. Adrenal biopsy showed the presence of caseous granulomatous inflammation suggesting TBC. These results showed us that a meticulous investigation for secondary causes of retroperitoneal fibrosis was very crucial before reaching a conclusive diagnosis of idiopathic retroperitoneal fibrosis.
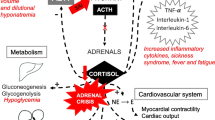
In an acute adrenal crisis, the cause is generally an adrenal hemorrhage or infarct, invasion by malignant diseases, or an abrupt interruption of blood flow to adrenal glands. But some infectious agents such as TBC, as identified in our case, HIV, cytomegalovirus (CMV), and other serious disseminated bacterial, viral or fungal infections should also be kept in mind [5]. TBC is a worldwide infectious disease which may cause death. Adrenal gland is the most frequently involved endocrine organ, but hypothalamus, pituitary gland, and thyroid might also be involved [6]. When TBC, through hematogenous spread, ultimately destroys 90% of the adrenal glands, Addison’s disease appears. Disease onset is about 10 years after the active TBC [7]. Almost one-third of chronic TBC patients may also be admitted due to adrenal crisis [8]. In the event of an TBC-induced Addison’s disease, large adrenal glands indicate a recent or active infection, whereas small, calcified glands stand for an inactive infection [9]. In our case, abdominal CT showed adrenal glands of usual size without any calcification. We noted, however, increased nodular thickness in adrenal glands bilaterally. For this reason, our patient might be accepted as an active case of TBC. In addition, adrenal gland biopsy showed a whole-layer destruction of the adrenal gland.
A question to be answered is, why an addisonian crisis took place despite a supraphysiological dose of steroid? As is known, daily endogenous cortisol production, which was formerly believed to be around 25–30 mg, was demonstrated to be at the level of 8–15 mg/day through stable isotope studies [10]. This amount is approximately equal to 20–30 mg of hydrocortisone [11]. In patients with adrenal insufficiency, daily need of steroid is 20–30 mg/day of hydrocortisone based on the variances in oral absorption. In febrile disease, injury and other similar stress conditions, however, two- to threefold increase is recommended in daily dose in order to prevent the patients from adrenal crisis [11]. In our patient, the daily dose of 48 mg methylprednisolone was expected to meet the stress-related demand. Nevertheless, it did not happen and the patient presented with adrenal crisis.
In the literature, we have encountered seven cases that were reported to have adrenal crisis while on high-dose steroid treatment [12–16]. Among them, the reason for corticosteroid treatment was vasculitis in 2, sarcoidosis in 1, asthma in 1, systemic lupus erythematosus (SLE) in 1, chronic hepatitis in 1, and pneumocystis jiroveci infection in the last 1 case. The possible causes of adrenal crisis and clinical features of the patients are given in Table 2.
Depending on our review, all of them were long-term receivers of steroid treatment except for a case. It is well established that long-term use of steroids suppresses HPA axis, thus reduces endogenous cortisol production and eliminates the stress response. When it comes to short-term use of steroids, on the other hand, in a case reported by Razzaq et al. [16], the patient was using steroid for 13 days and adrenal insufficiency was suggested to arise from adrenalitis induced by the intercurrent CMV infection. Similarly, our case was receiving high-dose steroid treatment for 14 days only and this might have caused TBC activation, thus leading to the necrosis of adrenal glands and ultimately a serious adrenal crisis.
TBC activation by steroid use is a well-recognized event. There is no, however, clear-cut data about duration and dosage of steroid use that causes immune suppression and activation of TBC. Still, recommendation on this subject states that steroid use for more than 1 month at a daily dose of 15 mg prednisolone or equivalent poses the risk of TBC [17]. Occurrence of adrenal insufficiency in patients on supraphysiological doses of steroids is not expected by most of the clinicians. When major pathophysiological mechanism of adrenal crisis, mineralocorticoid deficiency, is taken into account, prednisolone or methylprednisolone administered at supraphysiological doses fails to compensate for this mineralocorticoid effect, unless given in very high doses. Essentially having anti-inflammatory effects, prednisone and methylprednisolone have very little sodium-retaining activity. While sodium-retaining potency of fludrocortisone is 125 times more than that of cortisol, methylprednisolone has a potency of 0.5-fold [18]. In another words, 1 mg of methylprednisolone exerts a mineralocorticoid effect equal to that of approximately 0.004 mg of fludrocortisone. Methylprednisolone dose used by our patient was 40 mg and thus might be assumed to have an effect of that of 0.16 mg fludrocortisone. Although this dose lies within the range of 0.05–0.2 mg of fludrocortisone, which is the average daily dose used for mineralocorticoid replacement in primary adrenal insufficiency, obviously, it could not protect our patient from adrenal crisis. A possible explanation for this might be failure of methylprednisolone to have a full effect due to variances in oral absorption or steroid catabolism. In addition to the above-mentioned hypothesis, it might not be possible to achieve the desired response even with high-dose steroids due to the alterations in steroid receptors in some patients with favorable genetic background due to elevated cytokine synthesis in the event of inflammation [19]. In this way, steroid resistance might have developed in our patient through this mechanism despite the high-dose steroid, and thus, described clinical picture might have appeared.
In conclusion, TBC should be meticulously investigated as a possible cause of retroperitoneal fibrosis in countries where TBC is common. Although adrenal insufficiency and particularly adrenal crisis is very rarely expected while on high-dose steroid treatment, it must be considered in the presence of persistent hyperkalemia and hyponatremia, and detailed histopathologic and microbiological investigations should be performed, especially keeping infectious causes (CMV, TBC) in mind.
References
Puar TH, Stikkelbroeck NM, Smans LC, Zelissen PM, Hermus AR (2016) Adrenal crisis: still a deadly event in the 21(st) century. Am J Med 129(339):e1–e9
Molloy CB, Filer C, Ismail A (2005) Mycobacterium tuberculosis as a cause of chronic periaortitis. Rheumatology (Oxford) 44:696–697
Greco P, Vaglio A, Corradi D, Cobelli R, Zompatori M, Buzio C (2005) Tuberculosis as a trigger of retroperitoneal fibrosis. Clin Infect Dis 41:72–75
Bentley PG, Higgs DR (1976) Peritoneal tuberculosis with ureteric obstruction, mimicking retroperitoneal fibrosisBr. J Urol 48:170
Barthel A, Willenberg HS, Gruber M, Bornstein SR (2016) Adrenal insufficiency. In: Jameson JL, De Groot LJ, de Kretser DM et al (eds) Endocrinology: adult and pediatric, 7th edn, chap 102. Elsevier Saunders, Philadelphia, PA, pp 1763–1774
Kelestimur F (2004) The endocrinology of adrenal tuberculosis: the effects of tuberculosis on the hypothalamo-pituitary-adrenal axis and adrenocortical function. J Endocrinol Invest 27:380–386
Huebener KH, Treugut H (1984) Adrenal cortex dysfunction: CT findings. Radiology 150:195–199
Agarwal G, Bhatia E, Pandey R, Jain SK (2001) Clinical profile and prognosis of Addison’s disease in India. Natl Med J India 14:23–25
McMurry JF Jr, Long D, McClure R, Kotchen TA (1984) Addison’s disease with adrenal enlargement on computed tomographic scanning: report on two cases of tuberculosis and review of the literature. Am J Med 77:365–368
Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC et al (1991) Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab 72:39–45
Kyriazopoulou V (2007) Glucocorticoid replacement therapy in patients with Addison’s disease. Expert Opin Pharmacother 8:725–729
Jacobs TP, Whitlock RT, Edsall J, Holub DA (1988) Addisonian crisis while taking high-dose glucocorticoids: an unusual presentation of primary adrenal failure in two patients with underlying inflammatory diseases. JAMA 260(14):2082–2084
Marston RA (1992) Primary adrenocortical failure masked by exogenous steroid administration. Clin Endocrinol (Oxf) 36:519–520
Madoff DH, Dizon AM, Burd JK (1995) A case of mistaken identity: occult primary adrenal insufficiency in a patient taking exogenous glucocorticosteroids. The Endocrinologist 5:380–385
Cronin CC, Callaghan N, Kearney PJ, Murnaghan DJ, Shanahan F (1997) Addison disease in patients treated with glucocorticoid therapy. Arch Intern Med 157:456–458
Razzaq F, Dunbar EM, Bonington A (2002) The development of cytomegalovirus-induced adrenal failure in a patient with AIDS while receiving corticosteroid therapy. HIV Med 3:212–214
Diagnostic Standards and Classification of Tuberculosis in Adults and Children (2000) Am J Respir Crit Care Med 161(4):1376–1395
Schimmer B, Funder JW (2011) ACTH, adrenal steroids, and pharmacology of the adrenal cortex. In: Brunton L, Chabner B, Knollmann B (eds) Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12 edn. McGraw-Hill Companie, New York, NY, pp 1209–1235
Jiang Z, Zhu L (2016) Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease. Pulm Pharmacol Ther 37:1–8
Authors’ contribution
Study concept and design: DÜC and GBC; analysis and interpretation of data: DÜC and DA; drafting of the manuscript: GBC and DÜC; critical revision of the manuscript for important intellectual content: DÜC, CK and GBC; study supervision: DÜC and CK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Üsküdar Cansu, D., Cansu, G.B., Arik, D. et al. Adrenal crisis while on high-dose steroid treatment: what rheumatologist should consider?. Rheumatol Int 37, 657–662 (2017). https://doi.org/10.1007/s00296-016-3591-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3591-3