Abstract
The most dreaded complication of familial Mediterranean fever (FMF) is amyloidosis; controversy exists as to what acute phase reactant (APR) should be monitored in these patients. To analyze the best acute phase reactant for FMF follow-up to help guide physicians to decide on what APR parameter to use, we also attempted to define the best APR in predicting the complications of FMF, specifically the development of amyloidosis. Systematic review based on a sensitive search to capture studies that: (1) included FMF patients; (2) measured serum amyloid A (SAA), CRP (C-reactive protein), proteinuria, or ESR (erythrocyte sedimentation rate); (3) amyloidosis were the outcome measure; (4) sensitivity, specificity, predictive value, and other performance parameters could be calculated; and (5) had a longitudinal design. Of 1905 captured items, 26 were selected for detailed review, of which only two finally met the criteria, and the quality was only moderate; the articles did not analyzed the performance by means of sensitivity and specificity to predict, or even detect, amyloidosis, and thus had to be calculated based on text. The 26 screened studies were very heterogeneous in designs, parameters measured, and results, despite being set from research questions similar to ours. They were mainly descriptive, and it was very difficult to interpret the true performance of the tests. The correlation between the various APR is low. The evidence supporting the monitoring of FMF with any APR over the others is limited. Well designed longitudinal studies with a mixture of outcomes should be undertaken. Until them, recommending an APR over other would be based on expert opinion and indirect evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Familial Mediterranean fever (FMF) is a chronic inflammatory disease characterized by short self-limiting febrile attacks associated with signs of serositis (peritonitis, pleuritis, arthritis) and other more rare symptoms of organ involvement [1]. Between attacks patients are usually symptom-free.
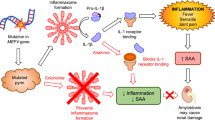
Due to the persistent inflammatory reaction, the most severe complication in FMF is the occurrence of AA-amyloidosis. It results from deposition of soluble serum amyloid A protein (SAA) in the extracellular space in an abnormal insoluble fibrillar form and can lead to functional organ loss. Before introduction of colchicine as standard therapy in FMF, this complication occurred in up to 60 % of patients [1]. Current data from different registries still reveal amyloidosis prevalence of 6.3 and 12.9 % within the group of FMF patients [2–5]. Whether this rather high rate is due to (partial) non-compliance, delayed diagnosis, and introduction of colchicine or insufficiently low prescribed colchicine dosages is unknown. The effective prevention of amyloid deposition by continuous colchicine treatment was initially shown in a large cohort, in which no analysis of the acute phase response was performed [6]. Current treatment recommendation uses solely clinical symptoms as criteria for treatment adaption [7, 8]. The control of subclinical inflammation is a major goal in FMF treatment.
In inflammatory diseases, biomarkers can be applied to monitor disease activity and treatment intensity. Ideally, for every disease entity, data on threshold values for the single biomarker indicating the long-term risk of the development of disease-associated complications should exist.
Since FMF is caused by dysregulation of inflammasome activity with a consecutive increase in IL-1β processing and secretion [9], the degree of inflammation would ideally be measured by serum levels of this cytokine. But this approach is hampered by the fact that IL-1β is virtually undetectable in human body fluids [10]. A variety of other cytokines have been analyzed in patients with FMF. Several of these proteins (e.g., interleukin (IL)-6, IL-8, IL-10, IL-12, IL-17, IL-18, TNF-α, INF-g and VEGFR-1) were increased during the acute attack and the attack-free interval underlining the problem of persisting subclinical inflammation in these patients [11]. However, the correlation of these markers with disease activity is weak, results are not consistent, and their availability in routine practice is limited; therefore, these biomarkers are not routinely applied. The S100 proteins represent another group of biomarkers, which sensitively detect inflammation in patients with attacks and during the attack-free intervals [12].
Until a more practical and less theoretical approach is agreed, in clinical practice, acute phase reactants (APRs)—i.e., C-reactive protein (CRP), SAA, and erythrocyte sedimentation rate (ESR)—are used to monitor FMF. CRP, SAA, and ESR are significantly increased during attacks [13–15]. But also in between attacks, a substantial degree of subclinical inflammation can be detected (e.g., increased CRP 34 % and increased ESR in 52 % of asymptomatic FMF patients [13]). However, in times of financial constraint, some clinics do not allow to measure three APR, and in addition, there is controversy as to which one of these APRs may be predicting best the risk of amyloidosis. The aim of the present study was to analyze the best APR for FMF follow-up to help guiding physicians to decide on what acute phase response parameter to use. We also attempted to define the best APR in predicting the complications of FMF, specifically the development of amyloidosis.
Methods
A systematic review was undertaken with the objective of identifying all studies published up until July 2014 providing information about the performance of APR, namely CRP, ESR, and SAA, also studying the predictive accuracy for amyloidosis in patients with FMF.
Search strategy
We first transformed the research question using the PICO approach and developed the search strategy accordingly. We searched MEDLINE (1950—December 2014), Embase (1980—December 2014), and The Cochrane Library (2014) by using comprehensive free text and MeSH synonyms for FMF, CRP, ESR, and SAA (the electronic search strategy is available in as supplementary material). We searched only published articles and placed no restrictions on time or language of publication. We supplemented searches by checking references cited in the included studies.
Study selection and data extraction
Criteria for inclusion were the following: (i) FMF patients of any age; (ii) measured CRP, ESR, or SAA; (iii) included amyloidosis as the outcome variable; and (iv) were longitudinal observational or diagnostic studies. One author (BE) assessed the electronic search results. He first screened by title and them by abstract in 10-min sessions aided by EndNote®. When an article title seemed relevant, the abstract was reviewed for eligibility. If there was any doubt, the full text of the article was retrieved and appraised for possible inclusion. The second author (ED) cross-checked the selection. Disagreements were resolved by discussion between the authors and with a third author (SO). A reason for exclusion was recorded in all cases if the article was not eligible or excluded. One author (BE) extracted the data from included articles in forms previously pilot-tested for feasibility and comprehensiveness, and differences were discussed. Data included the general characteristics of each study, study objectives, APR studied, and outcome definition and measure, among others.
Quality assessment and data synthesis
One of the authors (SO) checked the studies for risk of biases. She graded each article into low–moderate–high based on (1) whether the design was truly longitudinal and the duration of the study explicit, (2) the outcome measure was measured in an unbiased way in all patients, and (3) it was possible to calculate a measure of performance/prediction. Whenever possible a measure of performance was anticipated to be calculated based on the data obtained from individual studies. Otherwise, the individual results would be presented.
Results
The electronic search strategy yielded 1516 articles, seven of which were selected after the screening by title and abstract for detailed review. Only two studies [14, 16] met our inclusion criteria (Fig. 1).
Details of the two included studies may be found in Table 1 (Evidence table).
The aim of Duzova’s et al. [14] study was: (1) to compare the sensitivity of A-SAA and other acute phase proteins in determining subclinical inflammation in patients with FMF; (2) to define clinical, laboratory features modifying A-SAA level; and (3) to evaluate the effect of an increase in the colchicine dose on the A-SAA level. They found that homozygous and compound heterozygous patients had higher SAA levels than the heterozygous patients [129 (8–1500) vs. 29 mg/l (6–216), respectively, (P < 0.005)]. SAA was shown to be the best marker of subclinical inflammation in FMF. Although in Duzova’s study, SAA was shown to be the best marker of subclinical inflammation in FMF patients when compared to the other APRs such as CRP, erythrocyte sedimentation rate (ESR), fibrinogen and ferritin, APRs with the design and duration of the study failed to provide any prediction for the association with secondary amyloidosis.
In the study by Yalcinkaya et al. [16], the objectives were to determine the levels of APRs (including SAA, CRP and ESR) in FMF patients and to investigate a hypothetical role of levels of APRs for the development of amyloidosis. This was the study with the closest question to ours, despite the authors did not calculate a performance or a predictive value. The median levels of all APRs increased in the patients with FMF during attacks and a significant decrease was observed after the attack was over. However, the level of SAA was above reference range in all FMF patients during the attack-free period. The correlation between SAA and CRP during the attack-free period was only r = 0.557. Patients with amyloidosis had elevated levels of the three APRs but were not statistically different than those without amyloidosis. Of note, the largest SAA levels were not present in patients with demonstrated amyloidosis.
Discussion
With the present study, we aimed to evaluate the evidence to support the use of an APR over others in the monitoring of FMF patients by a systematic approach. Unfortunately, we were unable to find any study with which we could calculate a differential performance.
The typical clinical course of FMF is that of exacerbations and remissions, and the increased APR seen during these attacks usually returns to normal in attack-free periods [17]. However, in about 30 % of patients with FMF, the laboratory markers of inflammation, including SAA, do not return to normal levels [18]. Although it is unclear how exactly and why secondary amyloidosis develops during the course of FMF, there is enough clinical data that ongoing clinical and subclinical inflammation in any kind of disease, including chronic infectious diseases may lead to the development of amyloidosis.
Many of the screened studies actually compared the average levels of the APR in different groups. Lachmann et al., for instance, aimed to prospectively monitor inflammatory activity over a prolonged period in a cohort of Turkish patients with FMF, their healthy relatives, and healthy controls by looking CRP and SAA levels. They observed that both SAA and CRP were massively elevated during all reported clinical attacks of FMF in all patients, with median values of 693 (range 140–1330) mg/l and 115 (range 26–296) mg/l, respectively. SAA and CRP were also both elevated compared with the healthy control group even when these patients were free of FMF symptoms [median SAA 6.0 (range 0.7–1230) mg/l; median CRP 4.0 (range 2.7–262) mg/l]. Even when the patients were asymptomatic, only 29 % of SAA measurements in the FMF patients were less than 3 mg/l, i.e., within the normal range; 65 % were less than 10 mg/l and 13 % of SAA values exceeded 50 mg/l.
During the detailed review, a discrepancy in relation to the correlation between the levels of APRs was noted, from low–moderate as in the Yalcinkaya study to high study by Berkun et al. [18]. In addition, circularity in the interpretation of the results was noted: The definition of subclinical inflammation was the increase in the tests that were actually studied.
Korkmaz et al. [13] compared CRP and ESR between the attack and attack-free periods. They observed that CRP was the only APR that was increased in all FMF attacks, followed by ESR in 88 % of the attacks. In other studies, the levels of CRP or ESR were not even statistically different from controls [19] or from carriers without symptoms [15, 20].
This review is clearly limited by the quantity and quality of published manuscripts. Being such a relevant question in FMF, calls the attention that no study actually analyzed the performance by means of sensitivity and specificity to predict, or even detect, proteinuria or amyloidosis. Each study was designed completely ad hoc, thus, yielding a high heterogeneity. Many were described as longitudinal; however, the measures were actually performed cross-sectionally. A best design for this type of question would have been a long cohort with repeated measures of APRs within and between attacks and systematically searching in all patients for amyloidosis every certain time with an objective method. Until such study is performed, we will rely on indirect information to decide on what APR to follow-up.
References
Sohar E, Gafni J, Pras M, Heller H (1967) Familial Mediterranean fever. A survey of 470 cases and review of the literature. Am J Med 43(2):227–253
Akar S, Yuksel F, Tunca M, Soysal O, Solmaz D, Gerdan V et al (2012) Familial Mediterranean fever: risk factors, causes of death, and prognosis in the colchicine era. Medicine 91(3):131–136
Touitou I, Sarkisian T, Medlej-Hashim M, Tunca M, Livneh A, Cattan D et al (2007) Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum 56(5):1706–1712
Kasifoglu T, Bilge SY, Sari I, Solmaz D, Senel S, Emmungil H et al (2014) Amyloidosis and its related factors in Turkish patients with familial Mediterranean fever: a multicentre study. Rheumatology 53(4):741–745
Tunca M, Akar S, Onen F, Ozdogan H, Kasapcopur O, Yalcinkaya F et al (2005) Familial Mediterranean fever (FMF) in Turkey: results of a nationwide multicenter study. Medicine 84(1):1–11
Zemer D, Pras M, Sohar E, Modan M, Cabili S, Gafni J (1986) Colchicine in the prevention and treatment of the amyloidosis of familial Mediterranean fever. N Engl J Med 314(16):1001–1005
Hentgen V, Grateau G, Kone-Paut I, Livneh A, Padeh S, Rozenbaum M et al (2013) Evidence-based recommendations for the practical management of familial Mediterranean fever. Semin Arthritis Rheum 43(3):387–391
Kallinich T, Haffner D, Niehues T, Huss K, Lainka E, Neudorf U et al (2007) Colchicine use in children and adolescents with familial Mediterranean fever: literature review and consensus statement. Pediatrics 119(2):e474–e483
Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, Feigenbaum L et al (2011) Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity 34(5):755–768
Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S et al (2009) In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med 206(5):1029–1036
Ben-Zvi I, Livneh A (2011) Chronic inflammation in FMF: markers, risk factors, outcomes and therapy. Nat Rev Rheumatol 7(2):105–112
Kallinich T, Wittkowski H, Keitzer R, Roth J, Foell D (2010) Neutrophil-derived S100A12 as novel biomarker of inflammation in familial Mediterranean fever. Ann Rheum Dis 69(4):677–682
Korkmaz C, Ozdogan H, Kasapcopur O, Yazici H (2002) Acute phase response in familial Mediterranean fever. Ann Rheum Dis 61(1):79–81
Duzova A, Bakkaloglu A, Besbas N, Topaloglu R, Ozen S, Ozaltin F et al (2003) Role of A-SAA in monitoring subclinical inflammation and in colchicine dosage in familial Mediterranean fever. Clin Exp Rheum 21(4):509–514
Lachmann HJ, Sengul B, Yavuzsen TU, Booth DR, Booth SE, Bybee A et al (2006) Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology (Oxford) 45(6):746–750
Yalcinkaya F, Cakar N, Acar B, Tutar E, Guriz H, Elhan AH et al (2007) The value of the levels of acute phase reactants for the prediction of familial Mediterranean fever associated amyloidosis: a case control study. Rheumatol İnt 27(6):517–522
Ben-Chetrit E, Levy M (1998) Familial Mediterranean fever. Lancet 351(9103):659–664
Berkun Y, Padeh S, Reichman B, Zaks N, Rabinovich E, Lidar M et al (2007) A single testing of serum amyloid a levels as a tool for diagnosis and treatment dilemmas in familial Mediterranean fever. Semin Arthritis Rheum 37(3):182–188
Yildirim K, Uzkeser H, Keles M, Karatay S, Kiziltunc A, Kaya MD et al (2012) Relationship between serum interleukin-1beta levels and acute phase response proteins in patients with familial Mediterranean fever. Biochemia Medica. 22(1):109–113
Tunca M (1999) Acute phase response and evolution of familial Mediterranean fever. Lancet 353:1415
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This systematic review was funded by Eular (European League Against Rheumatism) as part of an initiative to develop recommendations on the management of FMF. Burak Erer has received a research fellow honorarium from EULAR, Erkan Demirkaya has received a research fellow honorarium from EULAR, and Seza Ozen has received research grant from EULAR.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Erer, B., Demirkaya, E., Ozen, S. et al. What is the best acute phase reactant for familial Mediterranean fever follow-up and its role in the prediction of complications? A systematic review. Rheumatol Int 36, 483–487 (2016). https://doi.org/10.1007/s00296-015-3413-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-015-3413-z