Abstract
An ideal inducible promoter presents inducibility with an inducer and no basal transcription without inducer. Previous studies have shown that PLRA3 in Pichia pastoris is a strong rhamnose-inducible promoter for driving the industrial production of recombinant proteins. However, another important profile of PLRA3, the basal transcription, was not investigated yet. In this study, the basal transcription of PLRA3 was assessed according to the profiles of two test strains grown in media lacking rhamnose: (1) the production of secretory β-galactosidase in P. pastoris GS115/PLRA3-LacB, in which lacB expression was regulated by PLRA3, and (2) growth in P. pastoris GS115/PLRA3-MazF, in which the expression of mazF, which encodes an intracellular toxic protein, was controlled by PLRA3. Analyses revealed low β-galactosidase production and non-obviously inhibited growth of the test strains, which suggests that there was a low basal transcription level of PLRA3 when rhamnose was absent. Thus, PLRA3 was an excellent candidate for genetic manipulation in P. pastoris due to its strict regulation, a strong and a low transcriptional activity with and without rhamnose, respectively. Subsequently, two systems were developed based on PLRA3 in P. pastoris: (1) an efficient markerless gene deletion system for single or multiple genes and (2) a high efficient piggyBac transposase-mediated mutation system for investigating the functions of unknown genes, as well as for the screening of expected mutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The precise control of the expression levels of desired genes is essential for basic biological research and biotechnological applications. Inducible promoters allow for the transcription of target genes to be switched on or off in the presence or absence of inducers (Meisner and Goldberg 2016). Therefore, inducible promoters are widely used for theoretical studies, as well as for the industrial production of target proteins. A variety of inducible promoters, including the methanol-inducible AOX1 promoter from Pichia pastoris (Unver et al. 2018; Zhao et al. 2018), rhamnose-inducible rhaBAD promoter from E. coli (Meisner and Goldberg 2016), galactose-inducible GAL promoter from S. cerevisiae (Ahn et al. 2013), and nitrate-inducible nitA promoter from R. rhodochrous (Herai et al. 2004), have been used for the industrial production of desired proteins. Additionally, a variety of inducible promoters can be applied to facilitate different types of molecular genetic manipulation: for example, the control of specific gene expression levels in metabolic engineering for improving the production of target products (Da Silva and Srikrishnan 2012; Trassaert et al. 2017), the development of markerless deletion systems (Yu et al. 2008; Luo et al. 2016), investigations of the connections between health and disease associated with gut microbiota (Lim et al. 2017), and the improved tetracycline-regulatable systems for conditional expression of genes in C. albicans and C. tropicalis (Bijlani et al. 2018).
Typically, an ideal promoter should be completely repressed in the absence of an inducer, as well as tunable to different transcription levels using various concentrations of an inducer (Rodriguez-Garcia et al. 2005). However, many inducible promoters exhibit obvious levels of background expression due to leaky expression and, as a result, target proteins, especially growth-inhibited or toxic proteins, are synthesized under non-inducible conditions and can result in host growth defects or even cell death (Giacalone et al. 2006). Thus, it is important to use tightly regulated promoters with low levels of leaky expression that enable host strains to grow normally until induction to produce target proteins, particularly when toxic proteins are produced (Saida et al. 2006).
A previous study from our research group showed that PLRA3 is a strong promoter that efficiently drives the production of desired proteins in P. pastoris using rhamnose as the inducer (Liu et al. 2016; Yan et al. 2018). However, due to leaky expression, the precise nature of the basal transcription of PLRA3 has yet to be fully elucidated. Thus, the present study evaluated the basal transcription profiles of PLRA3 in the absence of rhamnose induction. The results indicated that PLRA3 exhibited low basal transcription levels and has the potential for molecular genetic manipulation in P. pastoris. Subsequently, based on PLRA3, a markerless gene deletion system and a mutation system were developed in P. pastoris. The two systems were highly efficient and may be excellent tools for molecular genetic manipulation in P. pastoris.
Materials and methods
Strains and media
The present study used E. coli Trans1-T1 (TransGen; Beijing, China) as the gene cloning host, P. pastoris GS115 (Invitrogen; Carlsbad, CA, USA) as the expression host, and the pEasy-Blunt Simple Cloning Vector (TransGen). The plasmids (pGH01, pGHLRA3, and pGHGAP/gfp) and lacB-expressing strains (P. pastoris GS115/PLRA3-LacB, P. pastoris GS115/PAOX1-LacB, and P. pastoris GS115/PGAP-LacB) used in the present study have been described previously (Liu et al. 2016).
The MD, MR, and MM media contained 300 mM potassium phosphate, 1.34% YNB, 0.00004% biotin, and either 2% dextrose for MD, 2% rhamnose for MR or 1% (v/v) methanol, which was added every day, for MM.
The MDH, MRH, and MMH media contained 300 mM potassium phosphate, 1.34% YNB, 0.00004% biotin, 0.004% histidine, and either 2% dextrose for MDH, 2% rhamnose for MRH, or 1% (v/v) methanol, which were added every day, for MMH.
The YPR, YPD and YPM media contained 1% yeast extract and 2% peptone, and either 2% rhamnose for YPR, 2% dextrose for YPD, or 1% (v/v) methanol, which were added every day, for YPM.
The MDH medium was supplemented with leucine to a final concentration of 0.004% for preparation of the MDHL medium. The MDH, MRH and YPD media were supplemented with Zeocin to a final concentration of 100 µg/ml for preparation of the MDHZ, MRHZ and YPDZ media, respectively. To prepare the solid media, agar was supplemented into the above-mentioned media to a final concentration of 2% (w/v).
The primers used in this study are listed in Supplementary Table S1.
Determination of lacB expression and β-galactosidase activity
The single colonies of different recombinant Pichia strains (P. pastoris GS115/PLRA3-LacB, P. pastoris GS115/PAOX1-LacB, and P. pastoris GS115/PGAP-LacB with P. pastoris GS115 as a control strain) was inoculated into the YPD medium and cultured at 28 °C for 36 h while shaking (200 rpm). Next, the cultures were inoculated at 0.1% (v/v) into fresh MDH, MMH, YPD, and YPM media, respectively, and then cultured at 28 °C while shaking. The cultures were sampled at 12-h intervals to determine β-galactosidase activity in the fermentation supernatants using the following procedure.
First, 800 µl of 0.25% (w/v) ortho-nitrophenyl-β-galactoside (oNPG) in phosphate–citrate buffer (50 mM, pH 5.2) was pre-incubated at 60 °C for 5 min. Then, 200 µl of enzyme solution was added and incubated at 60 °C for 15 min. Next, 1 ml of 10% trichloroacetic acid and 2 ml of 1 M Na2CO3 were sequentially added into the mixture to stop this reaction for the chromogenic reaction, and absorbance was measured at 420 nm. One unit of β-galactosidase was defined as the amount of enzyme that released 1 µmol of o-nitrophenol per minute under standard conditions (pH 5.2, 60 °C, 15 min).
Construction and growth profiles of P. pastoris harboring PLRA3-MazF
Genomic DNA from P. pastoris was isolated using the TIANamp Yeast DNA Kit (Tiangen), and the left and right homologous DNA fragments of the sorbitol dehydrogenase gene (sdh) were cloned from the genomic DNA by two pairs of primers, sdh-F1/sdh-R1 and sdh-F2/sdh-R2, respectively. The two DNA fragments were fused into a single DNA fragment named “SDH” via overlap-extension polymerase chain reaction (PCR) with the primers sdh-F1 and sdh-R2. The SDH DNA fragment was ligated into the pEasy-Blunt Simple Cloning Vector to generate plasmid pPICS01, which could be digested by SnaB I due to the introduction of an additional restriction enzyme site for the insertion of a DNA fragment between the left and right homologous recombinant DNA fragments.
The toxic endoribonuclease MazF belonged to the members of Type II toxin–antitoxin systems from E. coli (Berghoff and Wagner 2017), and was used to assess the basal transcription level of PLRA3. The nucleotide sequence of the coding region of mazF (https://www.ncbi.nlm.nih.gov/gene/947252) was cloned from E. coli JM109. Next, mazF was ligated into plasmid pGHLRA3 via two restriction enzyme sites, EcoR I and Not I, which generated the mazF expression cassette in which mazF expression was controlled by PLRA3 as the promoter and AOX1TT as the terminator. Simultaneously, the zeocin expression cassette was amplified from plasmid pPICZA by PCR with a pair of primers, Zeocin-F and Zeocin-R. Subsequently, a tandem expression cassette (mazF–zeocin) containing the mazF expression cassette and the zeocin expression cassette was constructed via overlap-extension PCR and inserted into plasmid pPICS01 at the restriction enzyme site of SnaB I to generate plasmid pPICSmazF–zeo. After linearization with Swa I, the plasmid was introduced into P. pastoris GS115 via electroporation and the tandem expression cassette mazF–zeocin was integrated into chromosomal DNA at the sdh locus. The positive transformants, which were designated as P. pastoris GS115/PLRA3-MazF, were screened onto YPD with Zeocin (100 µg/ml) and then identified using PCR.
Pichia pastoris GS115/PLRA3-MazF, along with P. pastoris GS115 and P. pastoris GS115/PLRA3-LacB as control strains, was independently cultured in YPD at 28 °C for 48 h while shaking (200 rpm). Optical density at 600 nm (OD600) was measured in the cultures and adjusted to the same value using sterile water. Next, the cultures were inoculated at 0.1% (v/v) using fresh MDH and MRH media, respectively, and cultured at 28 °C while shaking. The cultures were sampled at 12-h intervals to determine OD600.
Markerless gene deletion plasmid pHISABCZ–mazF
For the markerless deletion of his4, a 1.2-kb left homologous sequence (A) and a 0.6-kb right homologous sequence (C) were amplified from chromosomal DNA via PCR with the following pairs of primers: his4A-F/his4A-R and his4C-F/his4C-R, respectively; there was a 29-bp overlap between his4A-R and his4C-F. Then, two restriction enzyme sites, Pme I and SnaB I, were introduced and DNA fragments A and C were fused into a single DNA fragment designated as HIS4AC, which was ligated into the pEasy-Blunt Simple Cloning Vector to generate plasmid pHISAC01. Additionally, a 0.6-kb DNA fragment (B) upstream of the 3′ end of DNA fragment A was amplified from chromosomal DNA via PCR and inserted into plasmid pHISAC01 at restriction site SnaB I to develop plasmid pHISABC01. Finally, the mazF–zeocin expression cassette was inserted into pHISABC01 at restriction site Pme I to generate the markerless deletion plasmid pHISABCZ–mazF.
Construction and identification of markerless gene deletion strains
After linearization with Swa I, plasmid pHISABCZ–mazF was introduced into P. pastoris GS115 and integrated into chromosomal DNA at the his4 locus. The positive transformants, designated as P. pastoris GS115/his4Z–mazF, were screened onto YPDZ and then identified using PCR.
Pichia pastoris GS115/his4Z–mazF was inoculated into YPD and incubated at 28 °C for 36 h while shaking. The cultures were then inoculated into the YPD and YPR media at 0.1% (v/v) each and grown at 28 °C while shaking. The OD600 values of the cultures were measured at 12-h intervals. Simultaneously, the cultures were each diluted with sterile water and plated onto solid YPD medium until single colonies occurred. Approximately 100 single colonies were transferred onto each of the YPD and YPDZ media, and the number of big colonies on the YPD medium and number of small colonies on the YPDZ medium were counted. PCR was performed using chromosomal DNA from the cells of small colonies as templates, and then the PCR products were sequenced to assay whether the target gene was deleted.
Construction of plasmid p53TRZ/PLRA3-PB
A DNA fragment containing the inverted terminal repeats of piggyBac (5TR/3TR) recognized by piggyBac transposase (GenBank: EF587698.1) and multiple cloning sites (MCS) was synthesized (Table S2). The DNA fragment was inserted into the pEasy-Blunt Simple Cloning Vector to generate the plasmid p53TR. Then, the zeocin expression cassette was cloned from plasmid pPICZA and inserted into plasmid p53TR at restriction enzyme site Pme I to develop plasmid p53TRZ.
The sequence of piggyBac encoding PB was synthesized by GenScript Biotech Corp in Nanjing, China. Then, piggyBac was inserted into plasmid pGHLRA3 via EcoR I and Not I to generate the resultant plasmid pGHLRA3/PLRA3-PB, in which piggyBac expression was under the control of PLRA3. Plasmid pGHLRA3/PLRA3-PB was digested by Asc I and Pac I and the DNA fragment containing piggyBac was ligated into plasmid p53TRZ after the digestion of Asc I and Pac I to generate the plasmid p53TRZ/PLRA3-PB.
Development of the leucine-deficient strain P. pastoris GS115/leu2T
The expression cassette of leu2 was amplified from the genomic DNA of P. pastoris cells by PCR with the following two pairs of primers: leu2-F1/leu2-R1and leu2-F2/leu2-R2. Using the above two PCR products and plasmid p53TRZ/PLRA3-PB as the templates and leu2-F1 and leu2-R2 as the primers for overlap-extension PCR, the PCR products were introduced into P. pastoris GS115 and then integrated into the leu2 locus via homologous recombination to develop the strain P. pastoris GS115/leu2T. This resultant strain, P. pastoris GS115/leu2T, was deficient in leu2 due to the insertion of an exogenous DNA fragment into the coding sequence (CDS) of leu2 and could not survive in leucine-deficient medium.
Mutation library of P. pastoris GS115
Pichia pastoris GS115/leu2T was grown into YPR or MRH media and produced PB following rhamnose induction at PLRA3. Once PB was expressed, the exogenous DNA fragment would move from the leu2 locus with four nucleotides (TTAA) remaining. These four nucleotides and two other nucleotides (GC) introduced into PCR primers in turn introduced two additional codons into the CDS of leu2. The strain designated as P. pastoris GS115/leu2m would then present with leucine-autotrophic growth. Subsequently, exogenous DNA would lose or insert into other TTAA sites, thereby mutating P. pastoris GS115; the mutants could be screened onto MDHZ or MRHZ media.
gfp expression and assays of green fluorescence intensity
Green fluorescent protein (GFP) gene gfp was obtained from plasmid pGHGAP/gfp via restriction enzymes EcoR I and Not I, and then was inserted into plasmid pPIC3.5 digested by EcoR I and Not I to develop plasmid pPIC3.5/gfp. Plasmid pPIC3.5/gfp after linearization with Bgl II was, respectively, introduced into P. pastoris GS115 and P. pastoris GS115/leu2T to develop strains P. pastoris GS115/PAOX1-GFP and P. pastoris GS115/ leu2T/PAOX1-GFP in which expression of gfp was regulated by PAOX1. Strain P. pastoris GS115/PGAP-GFP in which gfp expression was controlled by the strong constitute promoter PGAP was used a positive control.
The above three strains, P. pastoris GS115/PAOX1-GFP, P. pastoris GS115/leu2T/PAOX1-GFP, and P. pastoris GS115/PLRA3-GFP, were inoculated into the YPD medium and cultured at 28 °C for 36 h while shaking (200 rpm). Next, the cultures were inoculated at 0.1% (v/v) into fresh MRHZ media, respectively, and then cultured at 28 °C while shaking to OD600 reaching to ~ 2.0. Green fluorescence intensities from ~ 1,000,000 cells from each culture were monitored by cell analyzer (BD LSRFortessa).
Results
LacB expression profiles of P. pastoris GS115/PLRA3-LacB under non-inducible conditions
Although PLRA3 can be activated to efficiently drive the expression of target genes via induction with rhamnose (Liu et al. 2016; Yang et al. 2018), its basal transcription level has yet to be fully elucidated. Thus, to investigate the basal transcription level of PLRA3, the P. pastoris GS115/PLRA3-LacB strain, in which lacB expression was controlled by PLRA3, was developed. β-Galactosidase encoded by lacB can be secreted into the fermentation supernatants of cultures and, therefore, β-galactosidase activities can be used as an index of the transcription levels of PLRA3. Thus, the basal transcription level of PLRA3 was preliminarily assayed according to β-galactosidase activity in the fermentation supernatants of P. pastoris GS115/PLRA3-LacB grown under non-inducible conditions. Simultaneously, the P. pastoris GS115/PAOX1-LacB and P. pastoris GS115/PGAP-LacB strains, in which lacB expression levels were controlled by PAOX1 and PGAP, respectively, were used as the controls.
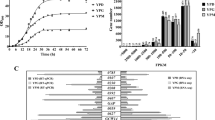
The three strains, P. pastoris GS115/PLRA3-LacB, P. pastoris GS115/PAOX1-LacB, and P. pastoris GS115/PGAP-LacB, were grown in non-inducible glucose-containing YPD and MD media and β-galactosidase activity in the fermentation supernatants was determined at various intervals (Fig. 1a, b). Very little β-galactosidase activity was detected in the fermentation supernatants of P. pastoris GS115/PLRA3-LacB and P. pastoris GS115/PAOX1-LacB. When estimated according to the specific activity of β-galactosidase (575 U/mg), the maximal amounts of β-galactosidase produced for P. pastoris GS115/PLRA3-LacB and P. pastoris GS115/PAOX1-LacB were 0.1 µg/ml and 0.017 µg/ml, respectively, in the YPD medium and 0.17 µg/ml and 0.012 µg/ml, respectively, in the MD medium. In the YPR and MR media containing inducible rhamnose, β-galactosidase production in the fermentation supernatants of P. pastoris GS115/PLRA3-LacB reached approximately 34.8 µg/ml and 17.4 µg/ml, respectively. Thus, PLAR3 exhibited low basal transcription levels under non-inducible conditions.
Basal transcription assay of PLRA3 according to β-galactosidase activities in the fermentation supernatants in P. pastoris GS115/PLRA3-LacB grown in YPD (a), MD (b), YPM (c), MM (d). Methanol-inducible promoter PAOX1 and constitute strong promoter PGAP were used as the controls. P. pastoris GS115/PLRA3-LacB, P. pastoris GS115/PAOX1-LacB and P. pastoris GS115/PGAP-LacB were the strains in which expression of lacB was under control of PLRA3, PAOX1 and PGAP, respectively. Each test was performed in triplicates, and the results are presented as means ± SD of three replicates
Methanol is the most commonly used inducer for the production of heterologous proteins in P. pastoris; therefore, the basal transcription profiles of PLRA3 in methanol-containing YPM and MM media were also investigated. The maximal β-galactosidase production levels in the fermentation supernatants of P. pastoris GS115/PLRA3-LacB cultured in YPM and MM media were approximately 0.17 µg/ml and 0.06 µg/ml, respectively (Fig. 1c, d). The control strains P. pastoris GS115/PAOX1-LacB and P. pastoris GS115/PGAP-LacB produced massive amounts of β-galactosidase in the fermentation supernatants of the cultures (Fig. 1c, d). Taken together, these results suggest that PLRA3 had a low leaky transcription profile when methanol was used as the main carbon. Furthermore, PLRA3 was tightly regulated by rhamnose and exhibited relatively low basal transcription levels under non-inducible conditions even though it was less tightly regulated than the well-known methanol-inducible promoter PAOX1.
Growth profiles of P. pastoris GS115/PLRA3-MazF
A secretory protein, β-galactosidase, was used as the reporter to indicate the basal transcription of PLRA3. Secretory proteins might not be fully secreted into the fermentation supernatants of cultures and non-secretory compartments are resident in cells; as a result, β-galactosidase activities in fermentation supernatants might not accurately reflect the basal transcription levels of PLRA3. To account for this possibility, the non-secretory protein MazF was used as an additional reporter for assay of the basal transcription levels of PLRA3. MazF is encoded by mazF, which is a sequence-specific endoribonuclease that can inhibit protein synthesis and lead to cell death. Theoretically, the growth profiles of P. pastoris GS115/PLRA3-mazF, in which mazF expression is regulated by PLRA3 would be closely related to the production of MazF. Due to the massive production of MazF that arises from rhamnose induction at PLRA3, P. pastoris GS115/PLRA3-mazF could not survive in the YPR and MRH media containing rhamnose as a carbon source. When inoculated into YPD and MDH media containing glucose as the carbon source, P. pastoris GS115/PLRA3-MazF grew normally if PLRA3 was tightly regulated by rhamnose, and died if PLRA3 exhibited some degree of basal transcription.
Several experiments were performed to determine if the above assumptions were correct. P. pastoris GS115/PLRA3-MazF and two control strains, P. pastoris GS115 and P. pastoris GS115/PLRA3-LacB, grew well on the YPD and MDH media (Fig. 2a, c), which indicated that the growth of P. pastoris GS115/PLRA3-MazF was not inhibited by low or trace amounts of MazF due to the low basal transcription of PLRA3. When inoculated into rhamnose-containing YPR and MRH media, P. pastoris GS115/PLRA3-MazF did not survive due to the massive production of MazF following rhamnose induction. The control strains P. pastoris GS115 and P. pastoris GS115/PLRA3-lacB grew normally on all media (Fig. 2b, d).
Growth profiles of P. pastoris GS115/PLRA3-MazF grown on/in YPD (a), YPR (b), MDH (c), MRH (d), liquid MDH (e), liquid MRH (f). P. pastoris GS115 and P. pastoris GS115/PLRA3-LacB were used as the control strains. a, b and c in A, B, C, and D represented the strains P. pastoris GS115, P. pastoris GS115/PLRA3-LacB, and P. pastoris GS115/PLRA3-MazF, respectively. Each test was performed in triplicates, and the results are presented as means ± SD of three replicates
The growth profiles were further confirmed by determining cell growth in liquid media. In MDH, P. pastoris GS115/PLRA3-MazF was able to grow, but at a relatively slower rate than P. pastoris GS115 and P. pastoris GS115/PLRA3-LacB (Fig. 2e); this indicated that the low production of MazF due to the basal transcription of PLRA3 weakly inhibited the growth of P. pastoris GS115//PLRA3-MazF. On the other hand, in MRH, P. pastoris GS115/PLRA3-mazF was completely inhibited by the severe toxicity of massive MazF production arising from rhamnose induction at PLRA3 (Fig. 2f). Taken together, these results further indicated that PLRA3 exhibited low basal transcription levels in the absence of rhamnose.
Markerless gene deletion system based on PLRA3
Due to the advantages of PLRA3, which include an almost complete attenuation of transcription in the absence of rhamnose and the maintenance of high transcription activity in the presence of rhamnose, it can be a useful tool for genetic manipulation. Thus, a markerless gene deletion system based on PLRA3 was developed in the present study (Fig. 3); six steps were involved in the deletion of a single gene. First, homologous fragment A and homologous fragment C were fused via overlap-PCR and two restriction enzyme sites, Pme I and SnaB I, were introduced. Second, the DNA fragment B, and the expression cassette mazF–zeocin, were inserted into the SnaB I and Pme I restriction enzyme sites, respectively. Third, homologous recombination occurred between the DNA fragment and the chromosomal DNA via fragment A and fragment C after introduction of the resultant DNA fragment into P. pastoris GS115 cells. Fourth, the positive recombinant strain, which was designated as P. pastoris GS115-MZ, was isolated with YPDZ and then verified by PCR. Fifth, a second recombination between the two repeats of fragment B led to deletion of the expression cassette mazF–zeocin. Cells without the expression cassette mazF–zeocin grew normally and were enriched in rhamnose-containing YPR medium.
Markerless gene deletion of his4
To evaluate the usefulness and efficiency of this gene deletion system, a his4 deletion procedure was performed. First, a markerless deletion plasmid, pHISABCZ–mazF, was constructed for his4 and the expression cassette mazF–zeocin was integrated into chromosomal DNA at the his4 locus after its introduction into P. pastoris GS115. The positive transformant, which was designated as P. pastoris GS115/his4Z–mazF, was then screened onto YPDZ medium. A single colony of P. pastoris GS115/his4Z–mazF was inoculated into a YPD medium and grown at 28 °C for 48 h while shaking. The cultures were then inoculated into YPD and YPR media at 0.1% (v/v). The OD600 values of cultures in the YPD and YPR media were determined simultaneously.
When P. pastoris GS115/his4Z–mazF was grown in YPD, the OD600 of the cultures increased rapidly to 9.0 at 36 h (Fig. 4a). Only trace amounts of MazF were produced due to the low basal transcription of PLRA3 in YPD and the growth of P. pastoris GS115/his4Z–mazF was slightly inhibited. On the contrary, the OD600 of the cultures slowly increased when P. pastoris GS115/his4Z–mazF was grown in YPR and reached 0.213 at 36 h (Fig. 4a). This might have been due to the massive production of MazF following the induction of rhamnose in YPR, which severely inhibited the growth of P. pastoris GS115/his4Z–mazF. Only the cells that abolished the expression cassette mazF–zeocin by homologous recombination of the two repeats of fragment B could survive and grow in YPR. The OD600 of cultures in YPD and YPR at the final incubation of 84 h were similar to previous values.
Markerless deletion of his4 in P. pastoris GS115. a Growth profiles of P. pastoris GS115/his4Z–MazF grown in YPD and YPR. b Growth profiles of sub-cultures of P. pastoris GS115/his4Z–MazF grown in YPD on YPD (a) and YPDZ (b); growth profiles of sub-cultures of P. pastoris GS115/his4Z–mazF grown in YPR on YPD (c) and YPDZ (d). c Strain verification via PCR. PCR products for wild-type strain, recombinant strain P. pastoris GS115-MZ, and his4-deleted strain were ~ 4.38 kb, ~ 4.46 kb, and ~ 1.7 kb, respectively. Lanes 1–6, 7–12, strains from big and small colonies on YPDZ, respectively; lane 13, wild-type strain P. pastoris GS115; lane M, DNA marker. d Nucleotide assay in his4-deleted strain by DNA sequencing
Theoretically, almost all cells from the cultures of P. pastoris GS115/his4Z–mazF grown in YPD and YPR should be Zeocin-resistant and Zeocin-sensitive, respectively. As expected, all of the cells collected from the sub-cultures of P. pastoris GS115/his4Z–mazF grown in YPD formed big colonies on YPD and YPDZ (Fig. 4ba, b), which indicates that homologous recombination did not occur between the two repeats of fragment B; thus, the expression cassette mazF–zeocin was not deleted. On the other hand, all of the cells collected from the sub-cultures of P. pastoris GS115/his4Z–mazF inoculated into YPR formed big colonies on YPD (Fig. 4bc) and small colonies on YPDZ (Fig. 4bd). These small colonies on YPDZ might have formed from mazF–zeocin-deleted cells and were designated as P. pastoris GS115/hisKO.
To verify these results, DNA fragments containing his4 were amplified using chromosomal DNA from cells in six big colonies and six small colonies that were randomly selected from YPDZ. The PCR products were of the expected size: 4.38 kb for the wild-type strain, 4.46 kb for the recombinant strain of P. pastoris GS115/his4Z–mazF, and 1.7 kb for the P. pastoris GS115/hisKO strain (Fig. 4c). The PCR products that responded to the his4-deleted strains were sequenced (Fig. 4d), which revealed that his4 and the expression cassette mazF–zeocin were deleted with four nucleotides (GTAG) remaining as the deletion scar. Three nucleotides (GTA) comprised the right part of the recognition sequence 5′-TACGTA-3′ for SnaB I, while the fourth nucleotide (G) was introduced after the recognition sequence of SnaB I in the primers (his4A-R and his4C-F). In fact, the fourth nucleotide (G) was not necessary and could not be introduced into the primers; i.e., this system exhibited high efficiency in realizing markerless gene deletion as well as scarless gene deletion, as only three nucleotides remained.
To further verify the validity of this system, a leu2 knockout was carried out. The construction of a deletion plasmid pLEU2ABCZ–mazF harboring the expression cassette mazF–zeocin was similar to that of pHISABCZ–mazF. The expression cassette mazF–zeocin was introduced into P. pastoris GS115/hisKO and then integrated at the leu2 locus. The transformants screened onto YPDZ were primarily formed by a single step of double-crossover recombination via the left and right homologous arms of leu2. A total of 400–800 transformants per microgram of plasmid occurred and the frequency of positive transformants that could not grow on MDH was relatively high, reaching 84% (42/50; Fig. 5a). The selective markerless strains obtained a frequency of nearly 100% after the positive transformants were grown in YPR, which was verified using PCR. The expected sizes of the PCR products were as follows: ~ 3.0 kb for the wild-type strain, ~ 3.8 kb for transformants harboring the expression cassette mazF–zeocin, and ~ 1.6 kb for the leu2-deleted strain (Fig. 5b).
Markerless deletion of leu2 in P. pastoris GS115/hisKO. a Growth profiles of sub-cultures of transformants grown on YPDZ and MDH. Positive transformants could grew on YPDZ instead of MDH due to leu2 disruption. b Strain verification by PCR. The PCR products were ~ 3.0 kb for the wild-type strain, ~ 3.8 kb for transformants harboring the expression cassette mazF–zeocin, and ~ 1.6 kb for the leu2-deleted strain
Mutation strategy based on PLRA3 and PB
Transposon insertional mutagenesis has been widely applied to investigate gene function and screen for expected mutants (Barquist et al. 2013). Recently, it was reported that the piggyBac transposon under control of the repressible MET3 promoter is effective in P. pastoris (Zhu et al. 2018). Compared to repressible promoters, inducible promoters are advantageous in that they promote the precise expression of a target gene. Thus, in the present study, PLRA3 was applied to regulate piggyBac expression for the construction of a mutation library of P. pastoris (Fig. 6a).
Mutation system based on PLRA3 regulating PB expression. a Schematic diagram of mutation system in P. pastoris. b Growth profiles of different strains on media MDHL and MDH. GS115, P. pastoris GS115; GS115/leu2T, P. pastoris GS115/leu2T; GS115/leu2m, P. pastoris GS115/leu2m. c Nucleotide assay of leu2 locus in P. pastoris GS115/leu2m by DNA sequencing
For the mutant library construction, two nucleotides (G and C), an exogenous DNA fragment containing the PB-recognizing sequence (PB/5TR and PB/3TR), a selective marker (zeocin), and piggyBac (the expression of which was under the control of PLRA3) were introduced into the CDS of leu2 via overlap-extension PCR to build the DNA fragment leu2T. The leu2T fragment was then introduced into P. pastoris GS115 and integrated into the leu2 locus via homologous recombination. Due to the leu2 disruption, P. pastoris GS115 cells harboring leu2T, which were designated as P. pastoris GS115/leu2T, could survive on a leucine-supplemented MDHL medium instead of an MDH medium. When P. pastoris GS115/leu2T was grown in rhamnose-containing media, such as MRH and YPR, piggyBac was intensively expressed. The exogenous DNA fragment in P. pastoris GS115/leu2T could be excised from the CDS of leu2 with four nucleotides (TTAA) remaining. As a result, six nucleotides (GTTAAC) were introduced into the CDS of leu2 to generate the modified version of leu2, which was termed leu2m. The leu2m gene could encode functional proteins involved in leucine biosynthesis and the P. pastoris GS115/leu2m strain could survive in the MDH medium. As expected, the P. pastoris GS115/leu2T strain could not grow on MDH medium but did grow on MDHL medium, whereas the P. pastoris GS115 and P. pastoris GS115/leu2m strains grew well on both media (Fig. 6b). DNA sequencing analyses further verified that the exogenous DNA fragment in P. pastoris GS115/leu2T was precisely excised from the leu2 locus with two artificially introduced nucleotides in primers (G and C) and four nucleotides (TTAA) remaining (Fig. 6c).
When the P. pastoris GS115/leu2T strain was cultured in a YPR or MRH medium, the exogenous DNA fragment could be excised from the leu2 locus with two resulting possibilities for the exogenous DNA fragment: reinsertion into a new genomic locus of TTAA or loss during/after cell division. The mutation occurred only when the exogenous DNA fragment was reinserted into another locus of TTAA and the mutants could be screened onto MDH medium with Zeocin (100 µg/ml). To determine the frequency of excision and reinsertion of the exogenous DNA fragment from the leu2 locus when P. pastoris GS115/leu2T was cultured in MRH, the colony-forming units (CFU) on the YPD, MDH, and MDHZ were assessed. The colonies on YPD were formed from all living cells, the colonies on MDH were formed from all cells in which the exogenous DNA fragment was excised from leu2, and the colonies on MDHZ were formed from cells in which the exogenous DNA fragment was excised from leu2 and then reinserted into another locus of TTAA. Based on the CFU number (Fig. 7), the excision frequency of the exogenous DNA fragment from the leu2 locus was 61.3% and the reinsertion frequency of the exogenous DNA fragment was 12.01%. These results indicate that this system was highly efficient for producing mutations in Pichia and may be an excellent tool to establish a mutant library.
Screening of rhamnose repression-resistant mutants
As a carbon source, rhamnose is preferred over methanol in P. pastoris, and methanol utilization is repressed in the presence of rhamnose. PAOX1 was not activated in rhamnose-containing media, such as YPR and MR, and P. pastoris GS115/PAOX1-GFP did not express GFP using rhamnose as a sole carbon source. To investigate mechanism of rhamnose repression, it was essential to isolate rhamnose repression-resistant mutants. To isolate these mutants, P. pastoris GS115/leu2T/PAOX1-GFP was thereby developed from P. pastoris GS115/PAOX1-GFP. When P. pastoris GS115/leu2T/PAOX1-GFP was grown in MRHZ medium, mutants occurred due to excision and subsequent insertion into other TTAA locus of the exogenous DNA fragment. The mutants expressed GFP which was under control of PAOX1 were considered as rhamnose repression-resistant mutants. Analyzing genomes of these mutants would provided some insights into rhamnose repression and into designing P. pastoris GS115 with rhamnose or other carbon sources instead of flammable and hazardous methanol as the inducers to activate PAOX1.
~ 1,000,000 cells from each culture of P. pastoris GS115/PAOX1-GFP grown in MRH, P. pastoris GS115/leu2T/PAOX1-GFP grown in MRHZ, and P. pastoris GS115/PGAP-GFP grown in MRH were analyzed. P. pastoris GS115/PAOX1-GFP was used as the negative control, and the gate without green fluorescence signal was drawn as P1 gate. Similarly, P. pastoris GS115/PGAP-GFP was used as the positive control, the area with stronger green fluorescence signal as P2 gate. Only two cells from P. pastoris GS115/PAOX1-GFP cultures fell into P1 gate, and no cells fell into P2 gate (Fig. 8a). As for P. pastoris GS115/PGAP-GFP, 92.2% and 30.9% of cells were in P1 and P2 gates, respectively (Fig. 8b). When P. pastoris GS115/leu2T/PAOX1-GFP was grown in selective medium MRHZ, only mutants could survive. Thus, ~ 1,000,000 cells from P. pastoris GS115/leu2T/PAOX1-GFP cultures were the mutant cells. It was shown that 0.055% and 0.009% of these mutant cells occurred in P1 and P2 gates, respectively (Fig. 8c). To avoid false positive samples, cells in P2 gate rather than P1 were considered as the positive mutants, the rhamnose repression-resistant mutants. These mutants were sorted, and some would be verified by genome sequencing to identify the mutation sites. The results further indicated that the developed mutation strategies were highly efficient to screen expected mutants and to investigate functions of specific genes.
Discussion
Inducible promoters are useful tools for controlling the expression of target genes; moreover, tightly regulated promoters are critical to achieve a massive yield of recombinant proteins and stability in engineered strains (McCutcheon et al. 2018; Rosano and Ceccarelli 2014). However, many inducible promoters are disadvantageous due to high basal expression levels; as a result, a variety of strategies have been adopted to reduce basal transcription in some promoters. For example, a T7 lysozyme or a lacO operator downstream of T7 promoter can be introduced in E. coli to reduce the basal transcription of some promoters (Gopal and Kumar 2013; McCutcheon et al. 2018).
The rhamnose-inducible promoter from E. coli is an ideal inducible promoter for the production of recombinant proteins due to its strong transcription as well as for theoretical studies due to its low basal expression. PLRA3 from P. pastoris also has a great deal of potential for massive production of recombinant proteins (Liu et al. 2016; Yan et al. 2018); thus, the present study conducted two experiments to determine its basal transcription level. The results indicated that PLRA3 maintained a low basal transcription level under non-inducible conditions based on two indexes: (1) low β-galactosidase production in the fermentation supernatants of P. pastoris GS115/PLRA3-LacB grown in non-inducible media and (2) the slightly slower growth of P. pastoris GS115/PLRA3-MazF grown in non-inducible media compared to wild-type P. pastoris GS115. However, it was also noted that PLRA3 was less tightly regulated than the well-known methanol-inducible promoter PAOX1. Thus, other strategies, such as the introduction of a glucose repression-related repressor-binding nucleotide sequence into PLRA3, were implemented to reduce leaky expression in another experiment.
Previously, a scarless gene deletion system was developed in Hansenula polymorpha using the split-marker method (Song et al. 2015). In this method, transformants with Zeocin resistance occurred via three-crossover homologous recombination, with two being due to homologous recombination between the left/right homologous arms and chromosomal DNA, and the third to the two split markers. Thus, transformation efficiency was low compared to classical transformation using an intact disruption cassette. Using the split-marker method, about 167 colonies of transformants per microgram of DNA occur and half of those are obtained from homologous recombination. In the present study, an intact disruption cassette with double-crossover homologous fragments was constructed in vitro via overlap-extension PCR and T4-ligase-mediated ligation, and then introduced into P. pastoris GS115. Transformants occurred following only one step of double-crossover homologous recombination, and about 400–800 colonies of transformants per microgram of DNA occurred, with over 80% of the transformants being positive. Compared to the split-marker method, the present method was advantageous in terms of transformation efficiency and a positive transformant ratio. However, one disadvantage to the present method was a small scar left in the chromosomal DNA via three or four nucleotides. Utilizing this system, a single strain with deletions of his4, leu2, and sdh plus gas1 was constructed by subsequent gene deletion, and this strain would be used as the host for expression of multiple secondary metabolite genes to produce expected metabolites in Pichia.
Zhu et al. (2018) recently used the MET3 promoter to regulate piggyBac expression and established piggyBac transposon-mediated mutagenesis in Pichia; the highest transposition frequency using the MET3 promoter to drive PB expression was 3%. In the present study, a Pichia mutation system based on the PLRA3-regulated expression of piggyBac was developed and the transposition frequency reached 12%, which was higher than that reported in Schizosaccharomyces pombe by Li et al. (2011). These findings indicate that this system could be used to isolate expected mutants and to identify the functions of unknown genes in Pichia.
The scarless gene deletion system in combination with the mutation system would be an excellent strategy to develop perfect Pichia hosts. Specific genes related to unfavorable profiles was first screened via the mutation system, and then were deleted using the markerless gene deletion system.
References
Ahn J, Park KM, Lee H, Son YJ, Choi ES (2013) GAL promoter-driven heterologous gene expression in Saccharomyces cerevisiae delta strain at anaerobic alcoholic fermentation. FEMS Yeast Res 13:140–142
Barquist L, Boinett CJ, Cain AK (2013) Approaches to querying bacterial genomes with transposon-insertion sequencing. RNA Biol 10:1161–1169
Berghoff BA, Wagner EGH (2017) RNA-based regulation in type I toxin–antitoxin systems and its implication for bacterial persistence. Curr Genet 63:1011–1016
Bijlani S, Nahar AS, Ganesan K (2018) Improved Tet-On and Tet-Off systems for tetracycline-regulated expression of genes in Candida. Curr Genet 64:303–316
Da-Silva NA, Srikrishnan S (2012) Introduction and expression of genes for metabolic engineering applications in Saccharomyces cerevisiae. FEMS Yeast Res 12:197–214
Giacalone MJ, Gentile AM, Lovitt BT, Berkley NL, Gunderson CW, Surber MW (2006) Toxic protein expression in Escherichia coli using a rhamnose-based tightly regulated and tunable promoter system. Biotechniques 40:355–364
Gopal GJ, Kumar A (2013) Strategies for the production of recombinant protein in Escherichia coli. Protein J 32:419–425
Herai S, Hashimoto Y, Higashibata H, Maseda H, Ikeda H, Omura S, Kobayashi M (2004) Hyper-inducible expression system for streptomycetes. Proc Natl Acad Sci 101:14031–14035
Li J, Zhang JM, Li X, Suo F, Zhang MJ, Hou WR, Han JH, Du LL (2011) A piggyBac transposon-based mutagenesis system for the fission yeast Schizosaccharomyces pombe. Nucleic Acids Res 39:e40
Lim B, Zimmermann M, Barry NA, Goodman AL (2017) Engineered regulatory systems modulate gene expression of human commensals in the gut. Cell 169:547–558
Liu B, Zhang YW, Zhang X, Yan CL, Zhang YH, Xu XX, Zhang W (2016) Discovery of a rhamnose utilization pathway and rhamnose-inducible promoters in Pichia pastoris. Sci Rep 6:27352
Luo X, Yang YW, Ling W, Zhuang H, Li Q, Shang GD (2016) Pseudomonas putida KT2440 markerless gene deletion using a combination of lambda Red recombineering and Cre/loxP site-specific recombination. Fems Microbiol Lett 363:fnw014
McCutcheon SR, Chiu KL, Lewis DD, Tan C (2018) CRISPR-Cas expands dynamic range of gene expression from T7RNAP promoters. Biotech J 13:1700167
Meisner J, Goldberg JB (2016) The Escherichia coli rhaSR-PrhaBAD Inducible promoter system allows tightly controlled gene expression over a wide range in Pseudomonas aeruginosa. Appl Environ Microbiol 82:6715–6727
Rodriguez-Garcia A, Combes P, Perez-Redondo R, Smith MCA, Smith MCM (2005) Natural and synthetic tetracycline-inducible promoters for use in the antibiotic-producing bacteria Streptomyces. Nucleic Acids Res 33:e87
Rosano GL, Ceccarelli EA (2014) Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol 5:172
Saida F, Uzan M, Odaert B, Bontems F (2006) Expression of highly toxic genes in E. coli: Special strategies and genetic tools. Curr Protein Pept Sci 7:47–56
Song P, Liu S, Guo XN, Bai X, He XP, Zhang BR (2015) Scarless gene deletion in methylotrophic Hansenula polymorpha by using mazF as counter-selectable marker. Anal Biochem 468:66–74
Trassaert M, Vandermies M, Carly F, Denies O, Thomas S, Fickers P, Nicaud JM (2017) New inducible promoter for gene expression and synthetic biology in Yarrowia lipolytica. Microb Cell Fact 16:141
Unver Y, Yildiz M, Kilic D, Taskin M, Firat A, Askin H (2018) Efficient expression of recombinant human telomerase inhibitor 1 (hPinX1) in Pichia pastoris. Prep Biochem Biotechnol 29:1–6
Yan CL, Xu XX, Zhang X, Zhang YW, Zhang YH, Zhang ZF, Zhang W, Liu B (2018) Decreased rhamnose metabolic flux improved production of target proteins and cell flocculation in Pichia pastoris. Front Microbiol 9:1771
Yang J, Cai H, Liu J, Zeng M, Chen J, Cheng Q, Zhang L (2018) Controlling AOX1 promoter strength in Pichia pastoris by manipulating poly (dA:dT) tracts. Sci Rep 8:1401
Yu BJ, Kang KH, Lee JH, Sung BH, Kim MS, Kim SC (2008) Rapid and efficient construction of markerless deletions in the Escherichia coli genome. Nucleic Acids Res 36:e84
Zhao T, Li Z, Guo Z, Wang A, Liu Z, Zhao Q, Li Y, McKenzie EA, Diao A (2018) Functional recombinant human legumain protein expression in Pichia pastoris to enable screening for legumain small molecule inhibitors. Protein Expr Purif 150:12–16
Zhu J, Zhu Q, Gong R, Xu Q, Cai M, Jiang T, Zhou X, Zhou M, Zhang Y (2018) PiggyBac transposon-mediated mutagenesis and application in yeast Komagataella phaffii. Biotechnol Lett 40:1365–1376
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number: 31671802) and Fundamental Research Funds for Central Non-profit Scientific Institution (Grant nos.: 1610392018006, Y2018LM02).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by M. Kupiec.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiao, J., Wang, S., Liang, M. et al. Basal transcription profiles of the rhamnose-inducible promoter PLRA3 and the development of efficient PLRA3-based systems for markerless gene deletion and a mutant library in Pichia pastoris. Curr Genet 65, 785–798 (2019). https://doi.org/10.1007/s00294-019-00934-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-019-00934-6