Abstract
Prions are infectious misfolded proteins that assemble into oligomers and large aggregates, and are associated with neurodegeneration. It is believed that the oligomers contribute to cytotoxicity, although genetic and environmental factors have also been shown to have additional roles. The study of the yeast prion [PSI +] has provided valuable insights into how prions form and why they are toxic. Our recent work suggests that SDS-resistant oligomers arise and remodel early during the prion formation process, and lysates containing these newly formed oligomers are infectious. Previous work shows that toxicity is associated with prion formation and this toxicity is exacerbated by deletion of the VPS5 gene. Here, we show that newly made oligomer formation and infectivity of vps5∆ lysates are similar to wild-type strains. However using green fluorescent protein fusions, we observe that the assembly of fluorescent cytoplasmic aggregates during prion formation is different in vps5∆ strains. Instead of large immobile aggregates, vps5∆ strains have an additional population of small mobile foci. We speculate that changes in the cellular milieu in vps5∆ strains may reduce the cell’s ability to efficiently recruit and sequester newly formed prion particles into central deposition sites, resulting in toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prions are misfolded aggregated proteins that are infectious. Several proteins have been shown to form prions in yeast, including Sup35p, Ure2p, Rnq1p, Swi1p, Cyc8p, and Mot3p (reviewed in Liebman and Chernoff 2012). In each case, the monomeric protein misfolds into an alternate stable conformation that in turn is able to convert normal copies of the protein to a misfolded form that is prone to aggregation. The process by which these proteins initially misfold and aggregate to form infectious particles is still unclear. Work in yeast has provided important insights into how prions form and when they become infectious.
Spontaneous prion formation in yeast is quite rare. Analysis of the Sup35 prion, or [PSI +], found that the spontaneous rate of formation is less than 1 in 1 million cells per generation (Lund and Cox 1981; Allen et al. 2005; Lancaster et al. 2010). Despite this low level, several approaches can increase the rate of de novo formation. An expansion of an oligopeptide repeat within the N-terminal prion forming region of Sup35 increases prion formation (Liu and Lindquist 1999). These extra repeats have been suggested to more readily direct the assembly of Sup35 proteins into ordered complexes. Prion formation has also been shown to be increased by exposure to environmental stress (Tyedmers et al. 2008). It is thought that the accumulation of misfolded proteins, including misfolded Sup35p, under these stress conditions may initiate prion formation. However, the most dramatic increase in de novo prion formation occurs by the overexpression of the Sup35 protein in a process called prion induction (Chernoff et al. 1993; Derkatch et al. 1996). This increase requires the presence of a second prion, such as the prion form of the Rnq1 protein, [PIN +] (otherwise known as [RNQ +]), or overexpressed glutamine and asparagine-rich proteins (Derkatch et al. 1997, 2001; Osherovich and Weissman 2001). Using this prion induction method, Derkatch et al. (1996) observed that the frequency of [PSI +] colonies after SUP35 overexpression in [psi −][PIN +] cultures was higher than 30%. The elevated Sup35 protein concentration likely increases the probability of protein misfolding (Chernoff et al. 1993; Derkatch et al. 1996), and the presence of a second prion allows for heterologous cross seeding for the de novo formation of [PSI +] (Derkatch et al. 2001; Osherovich and Weissman 2001; Arslan et al. 2015; Keefer et al. 2017).
Newly formed prion aggregates
The laboratory of Susan Liebman was the first to visualize prion induction in vivo (Zhou et al. 2001). The prion domain of Sup35p, which consists of the N-terminal and middle domain of the protein, can be fused to Green Fluorescent Protein (Sup35PrD-GFP). In cells lacking the [PSI +] prion, these fusion proteins are evenly distributed throughout the cytoplasm resulting in diffuse fluorescence. However, overexpression of this construct can induce [PSI +] formation in [PIN +] cells (Derkatch et al. 2001; Zhou et al. 2001). Observations of overnight cultures overexpressing the fusion protein show that a small percentage of cells formed large intracellular ring, line, and dot-like aggregates (Zhou et al. 2001). Since then, several studies have used periodic “snapshots” to infer how these aggregates are made. Small fluorescent foci initially appear, with some located near the vacuole. Later snapshot observations suggest that these small foci are replaced with the ring, line, and dot-like aggregates (Arslan et al. 2015), which are retained in the mother cell during cell division (Mathur et al. 2010). Isolation of cells that contain these newly formed aggregates can give rise to a proportion of progeny that are [PSI +], whereas sibling cells that lack fluorescent aggregates always give rise to progeny that lack the prion (Ganusova et al. 2006).
Since much of what we know about Sup35PrD-GFP ring, line, and dot-like aggregate formation during prion induction is due to temporal extrapolation, we recently employed 4D live cell imaging in order to continuously capture the initial formation of the Sup35PrD-GFP aggregate (Sharma et al. 2017). We found that cells displaying diffuse cytoplasmic fluorescence developed one or several small foci (which we called “early foci”) that quickly assembled into larger aggregates. While this assembly could result in rings, lines or dot-like structures, the frequency in which early foci formed each structure was similar.
The formation of SDS-resistant oligomers and infectivity of newly formed particles
It was originally observed that during prion induction, Sup35p forms large SDS-resistant oligomers that migrate differently than Sup35p oligomers associated with the propagating [PSI +] prion (Salnikova et al. 2005). In vitro studies showed that lysates containing these newly made oligomers were able to convert monomeric Sup35p to an aggregated form, suggesting that these newly formed oligomers can seed aggregation (Salnikova et al. 2005). However, the ability of these lysates to convert [psi −] cells to [PSI +] in vivo, thereby showing that these newly made prion oligomers are infectious, was unknown.
To begin to understand oligomer formation and infectivity, we looked at how the size of SDS-resistant oligomers changes during prion formation, and how these changes are correlated with the ability to convert [psi −] cultures to [PSI +] (Sharma et al. 2017). Using semi-denaturing detergent agarose gel electrophoresis (SDD-AGE; Kryndushkin et al. 2003), we resolved SDS-resistant Sup35PrD-GFP oligomers at different time points of the induction process. At early time points of induction, when cultures have diffuse Sup35PrD-GFP fluorescence, Sup35PrD-GFP forms a single band that is larger than the monomeric protein. At later time points, when approximately 20% of the cells exhibited ring, line, or dot-like aggregates, larger molecular weight smears are detected suggesting that Sup35PrD-GFP undergoes assembly into oligomers of diverse sizes. Endogenous Sup35p also forms SDS-resistant oligomers during induction that are different sizes than established [PSI +] oligomers (Sharma et al. 2017). These data suggest that between the time of prion formation and prion propagation, Sup35p oligomers must be remodeled or changed.
Next, we asked whether lysates from induced cultures could convert [psi −] cells to [PSI +]. The “protein-only” hypothesis proposed that misfolded prion proteins are infectious. In yeast, proof for this hypothesis has been demonstrated by several studies that show recombinant prionogenic proteins, either incubated to form fibrils or seeded with lysates from yeast cells containing prions, can convert non-prion containing cells to into the prion state (King and Diaz-Avalos 2004; Tanaka et al. 2004; Patel and Liebman 2007; Du et al. 2010). Even with strong proof for the protein-only hypothesis, little was known about when newly formed prion particles gain their infectivity.
To uncover when induced cultures become infectious, we obtained lysates from different time points of prion induction. We found that the transfection of fresh lysates from cells that overexpressed Sup35PrD-GFP for 16–24 h, and thus contained ring, line, or dot-like aggregates, were able to transform [psi −] cells into [PSI +] colonies. Conversely, cultures lacking cells with aggregates, such as uninduced cells or Sup35PrD-GFP overexpressed in the [pin −] background, could not convert [psi −] cells to the prion state (Sharma et al. 2017). Our data suggest that during the induction process, lysates contain infectious material.
An unanswered question is how newly made prion particles gain their infectivity. In vitro formed Sup35 fibers seeded with [PSI +] lysates can convert [psi −] to [PSI +] cells. Yet sonication of [PSI +] lysates prior to seeding significantly enhanced infectivity (King and Diaz-Avalos 2004). It is thought that this shearing by sonication mimics how prions are propagated in vivo. The Hsp104p chaperone has been shown to be required for [PSI +] propagation (Chernoff et al. 1995) and in conjunction with several other chaperones appears to shear and fragment larger prion aggregates into smaller seeds, or propagons, that can be inherited by progeny (reviewed in Liebman and Chernoff 2012; Cox and Tuite 2017). Inactivation of Hsp104p by low levels of guanidine-HCl blocks [PSI +] shearing, which limits the production of these smaller propagons (Eaglestone et al. 2000; Wegrzyn et al. 2001; Ness et al. 2002; Byrne et al. 2009). Hsp104p may also play an important role for ensuring the de novo formation of [PSI +]. During prion induction, Hsp104p has been shown to colocalize with newly formed Sup35PrD-GFP rings structures (Arslan et al. 2015). It is possible that Hsp104p immediately initiates the shearing of newly formed aggregates during de novo induction, which generates heritable, infectious propagons. Further infectivity studies will be required to understand whether Hsp104p plays a role in the infectivity of newly formed prion particles.
Prion induction-associated toxicity and newly made prion aggregates
The presence of [PSI +] alone does not have any adverse effect on cell growth, but increasing Sup35p expression in [PSI +] cells reduces viability (Derkatch et al. 1996). This [PSI +] associated toxicity has been shown to be relieved by the expression of the C-terminal translational termination domain of Sup35p or the Sup45 protein, suggesting that toxicity is due to the sequestration of these essential proteins into the prion aggregate (Vishveshwara et al. 2009). Other studies have shown that prion-associated toxicity can also be relieved by the overexpression of chaperones or by indirectly altering chaperone distribution within the cell (Douglas et al. 2008; Keefer and True 2016). Therefore, the sequestration of essential proteins into aggregates and changes in the protein quality control machinery could influence [PSI +] associated toxicity.
Toxicity has also been shown to be associated with prion induction. Cells containing newly formed aggregates are less viable than those with diffuse fluorescence (Ganusova et al. 2006). Overexpression of the C-terminal region of Sup35p was shown to suppress this prion induction-associated toxicity (Vishveshwara et al. 2009), suggesting that sequestration of the essential Sup35 protein into the newly formed aggregates results in its loss of function. It was also shown that the deletion of non-essential genes that code for proteins associated with cytoskeleton organization and biogenesis, response to stress, and cell budding decrease prion induction-associated toxicity (Tyedmers et al. 2008). Therefore, similar to prion-associated toxicity, prion induction-associated toxicity may be due to sequestration of essential proteins as well as several other factors.
Examining genetic mutants that enhance prion induction-associated toxicity could provide important clues to the causes of cell death. We previously characterized a genetic mutant that has increased prion induction-associated toxicity. Cells lacking the VPS5 open reading frame (YOR069w) form fewer ring, line, and dot-like structures compared to wild-type cells. Of the few vps5∆ cells containing these aggregates, we found that these cells were also significantly less viable than wild-type cells containing aggregates (Manogaran et al. 2011). It is possible that the inability to form ring and dot aggregates in vps5∆ cells may be due to toxicity.
VPS5 codes for a sorting nexin 1 homolog, a member of the retromer complex that mediates vesicle transport by ensuring the recycling of late endosome cargo to the Golgi (Nothwehr and Hindes 1997; Seaman et al. 1998). The retromer complex has also been implicated in having dual roles in cargo recycling and indirectly affecting Ypt7-dependent vacuole tethering and fusion (Liu et al. 2012). Strains lacking the VPS5 open reading frame exhibit vacuolar protein-sorting defects (Horazdovsky et al. 1997) and decreased autophagy in response to certain stress conditions (Dengjel et al. 2012). Interestingly, deletion of VPS5 also results in the loss of a second open reading frame on the opposite DNA strand (YOR068c; Fig. 1a). This other open reading frame, VAM10, appears to be required for the Sec18p independent priming stages of vacuole fusion (Kato and Wickner 2003).
vps5Δ strains have reduced aggregate formation frequency, yet show no change in SDS-resistant oligomers. a The VPS5 open reading frame (YOR069w) and VAM10 open reading frame (YOR068c) are located on chromosome 15 in the yeast genome. Site-directed mutagenesis was performed to generate plasmids that contain a mutation in the initiator methionine of either VPS5 or VAM10. Two nucleotide substitutions replaced the initiation methionine with an arginine in the VPS5 open reading frame, while leaving the VAM10 open reading frame untouched. In a second plasmid, a single nucleotide substitution at the beginning of the VAM10 open reading frame leads to a mutation that changes methionine for isoleucine, while maintaining the same wild-type amino acid (serine) in the VPS5 sequence encoded by the opposite strand. All plasmids were sequenced in both directions to confirm the engineered mutation and the opposite open reading frame sequence. b Plasmids containing wild-type versions of both genes (rescue), or mutated versions that maintain wild-type versions of only one gene (VAM10 or VPS5) were transformed into vps5Δ [PIN +] 74D-694 strains (Manogaran et al. 2011) along with a plasmid containing a copper inducible Sup35PrD-GFP allele. Sup35PrD-GFP was overexpressed for 24 h in wild-type, vps5Δ, or vps5Δ strains with the indicated plasmid. The number of cells containing ring, line, or dot-like aggregates was counted from a population of at least 300 cells from three independent transformants. Standard deviation is shown. Statistically significant differences from wild-type or vps5Δ strains were determined by unpaired two-tailed t test *p < 0.005. c Sup35PrD-GFP was overexpressed in wild-type and vps5Δ strains for 24 h. Cultures were lysed and immediately subjected to SDD-AGE immunoblots using anti-GFP antibody (left) to detect Sup35PrD-GFP and anti-Sup35C antibody to detect full length Sup35p (BE4; right) according to Sharma et al. (2017). [PSI +] lysates are run for the detection of established [PSI +] oligomers
To begin, we asked which gene (VPS5 or VAM10) was responsible for the reduced number of cells containing Sup35PrD-GFP ring, line, or dot-like aggregates during prion induction. Single-rescue plasmids that maintain the wild-type polypeptide sequence for one gene while eliminating the initiation methionine codon of the other gene on the opposite strand were introduced into mutants lacking both VPS5 and VAM10 open reading frames. We will refer to this deletion strain as vps5∆ below. We found that the introduction of wild-type versions of both genes was able to rescue the low level of Sup35PrD-GFP ring, line, and dot-like aggregates in vps5∆ strains (Fig. 1b). However, introduction of either individual wild-type gene showed the same low ring, line, and dot-like aggregate formation frequency as strains lacking both open reading frames. Our data suggest that Sup35PrD-GFP aggregation seems to require both genes and may involve a common pathway. Since both genes appear to play a role in vacuolar fusion, it is possible that impairment of vacuole fusion may underlie this change in aggregation state.
We next determined whether there were other differences between vps5∆ and wild-type strains that could explain the observed prion induction-associated toxicity. We found that overexpression of Sup35PrD-GFP in vps5∆ and wild-type strains produced Sup35PrD-GFP and endogenous Sup35 oligomers of similar sizes (Fig. 1c). Transfection of lysates from these induced strains was able to convert [psi −] recipient strains into [PSI +] (Table 1). However, conversion caused by vps5∆ lysates was approximately half of the conversion caused by parallel wild-type lysates (Table 1). Since ring, line, and dot-like aggregate formation in vps5Δ strains is half that of wild-type (Fig. 1b), the reduction in conversion is possibly correlated with less available infectious protein rather than cell death caused by a toxic particle.
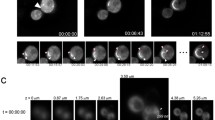
Next, we explored whether there were any differences in aggregate formation. We previously found that early foci can assemble into large ring, line and dot-like aggregates by four different pathways in wild-type cells (Sharma et al. 2017). We were able to follow the progression from early foci to large ring, line, and dot-like aggregates in 33 individual vps5Δ cells. Unlike wild-type cells, it appeared that the probability of vps5Δ cells to form aggregates by the four pathways was not equally likely (Fig. 2a). We also observed that the physical appearance of aggregates was quite different. In wild-type strains, diffuse fluorescence within the cytoplasm is initially observed upon Sup35PrD-GFP overexpression. The formation of early foci and the subsequent assembly into larger aggregates is correlated with a dramatic reduction in the diffuse cytoplasmic fluorescence, suggesting that the majority of soluble Sup35PrD-GFP is recruited into the large aggregates. While a similar reduction in diffuse fluorescence is observed in vps5Δ during the formation of large aggregates, many of the cells had an additional population of small anomalous aggregates not observed in wild-type (Fig. 2b, c). Approximately, 35% of these vps5Δ cells with ring and dot aggregates also had these anomalous aggregates (data not shown), many of which were mobile even after several hours of video recording.
Sup35PrD-GFP forms additional small anomalous aggregates during prion induction. a Sup35PrD-GFP was overexpressed in vps5Δ cells for 18 h, and then imaged using 8-well glass slides for an additional 6–12 h by 4D microscopy. Because of the reduced aggregate formation in vps5Δ, we selectively captured fields of cells in which the initial stages of early foci formation could be captured. Of the 149 cells imaged, we were able to view aggregate formation in 33 cells (17 cells in G1, and 16 cells in G2/M phase). We followed the formation of early foci into larger aggregates and categorized them into four distinct pathways previously characterized for wild-type cells by Sharma et al. (2017). Statistical analysis using Chi square goodness of fit tests indicates that while wild-type cells have an equal probability for each pathway, vps5Δ cells do not (p < 0.05). b Diagrammatic representation of the four pathways in vps5Δ strains. While the pathways were similar between wild-type and vps5Δ strains, we noticed small anomalous aggregates, many of which were mobile, associated with pathways I, II, and IV in vps5Δ strains. c Representative images of wild-type (WT) and vps5Δ strains are shown. Arrows indicate small anomalous aggregates. All images were subjected to 3D deconvolution using Autoquant deconvolution algorithms (Media Cybernetics) and are shown as maximum projection images
While it is unclear whether there is a direct correlation between the presence of these small anomalous aggregates and the higher toxicity in vps5Δ strains, it is possible that these aggregates could be a contributing factor. One could speculate that the changes in vacuolar fusion or autophagy caused by the loss of the VPS5 open reading frame may allow for the buildup of these anomalous aggregates, which could be detrimental to cell viability. Vacuolar defects in vps5Δ strains could also disrupt the cell’s ability to direct newly made prion particles to central protein holding places in the cell, such as the vacuole-associated insoluble protein deposit, IPOD. Since prion proteins have been shown to accumulate at IPOD (Kaganovich et al. 2008; Mathur et al. 2010; Saarikangas and Barral 2016), the sequestration and retention of these anomalous aggregates to a central location like IPOD in vps5Δ strains could be compromised.
These studies have begun to uncover the cellular nuances that direct prion formation and toxicity. Emerging evidence from mammalian systems has suggested that oligomeric species contribute to toxicity in human amyloid-based neurodegenerative diseases (Huang et al. 2013; Kayed and Lasagna-Reeves 2013; Gerson et al. 2016; Salahuddin et al. 2016). However, genetic and environmental factors have also been shown to exacerbate neurodegenerative progression of these diseases (Bertram and Tanzi 2005; Campdelacreu 2014; Agrawal et al. 2017). Therefore, these data suggest that the cellular context may direct the degree of toxicity caused by oligomers. Work with yeast prions allows for a quick and tractable system to uncover which cellular factors influence toxicity associated with oligomers. Studies focused on [PSI +] induction-associated toxicity will likely tease apart how both the newly made oligomers and cellular pathways contribute to cellular toxicity.
References
Agrawal S, Berggren KL, Marks E, Fox JH (2017) Impact of high iron intake on cognition and neurodegeneration in humans and in animal models: a systematic review. Nutr Rev 75:456–470. doi:10.1093/nutrit/nux015
Allen KD, Wegrzyn RD, Chernova TA, Muller S, Newnam GP, Winslett PA, Wittich KB, Wilkinson KD, Chernoff YO (2005) Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+]. Genetics 169:1227–1242
Arslan F, Hong JY, Kanneganti V, Park SK, Liebman SW (2015) Heterologous aggregates promote de novo prion appearance via more than one mechanism. PLoS Genet 11:e1004814
Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Investig 115:1449–1457
Byrne LJ, Cole DJ, Cox BS, Ridout MS, Morgan BJ, Tuite MF (2009) The number and transmission of [PSI] prion seeds (Propagons) in the yeast Saccharomyces cerevisiae. PLoS One 4:e4670
Campdelacreu J (2014) Parkinson disease and Alzheimer disease: environmental risk factors. Neurologia 29:541–549
Chernoff YO, Derkach IL, Inge-Vechtomov SG (1993) Multicopy SUP35 gene induces de-novo appearance of psi-like factors in the yeast Saccharomyces cerevisiae. Curr Genet 24:268–270
Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW (1995) Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268:880–884
Cox B, Tuite M (2017) The life of [PSI]. Curr Genet. doi:10.1007/s00294-017-0714-7
Dengjel J, Hoyer-Hansen M, Nielsen MO, Eisenberg T, Harder LM, Schandorff S, Farkas T, Kirkegaard T, Becker AC, Schroeder S et al (2012) Identification of autophagosome-associated proteins and regulators by quantitative proteomic analysis and genetic screens. Mol Cell Proteomics 11:M111-014035. doi:10.1074/mcp.M111.014035
Derkatch IL, Chernoff YO, Kushnirov VV, Inge-Vechtomov SG, Liebman SW (1996) Genesis and variability of [PSI] prion factors in Saccharomyces cerevisiae. Genetics 144:1375–1386
Derkatch IL, Bradley ME, Zhou P, Chernoff YO, Liebman SW (1997) Genetic and environmental factors affecting the de novo appearance of the [PSI+] prion in Saccharomyces cerevisiae. Genetics 147:507–519
Derkatch IL, Bradley ME, Hong JY, Liebman SW (2001) Prions affect the appearance of other prions: the story of [PIN(+)]. Cell 106:171–182
Douglas PM, Treusch S, Ren HY, Halfmann R, Duennwald ML, Lindquist S, Cyr DM (2008) Chaperone-dependent amyloid assembly protects cells from prion toxicity. Proc Natl Acad Sci USA 105:7206–7211
Du Z, Crow ET, Kang HS, Li L (2010) Distinct subregions of Swi1 manifest striking differences in prion transmission and SWI/SNF function. Mol Cell Biol 30:4644–4655
Eaglestone SS, Ruddock LW, Cox BS, Tuite MF (2000) Guanidine hydrochloride blocks a critical step in the propagation of the prion-like determinant [PSI(+)] of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 97:240–244
Ganusova EE, Ozolins LN, Bhagat S, Newnam GP, Wegrzyn RD, Sherman MY, Chernoff YO (2006) Modulation of prion formation, aggregation, and toxicity by the actin cytoskeleton in yeast. Mol Cell Biol 26:617–629
Gerson JE, Mudher A, Kayed R (2016) Potential mechanisms and implications for the formation of tau oligomeric strains. Crit Rev Biochem Mol Biol 51:482–496
Horazdovsky BF, Davies BA, Seaman MN, McLaughlin SA, Yoon S, Emr SD (1997) A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell 8:1529–1541
Huang P, Lian F, Wen Y, Guo C, Lin D (2013) Prion protein oligomer and its neurotoxicity. Acta Biochim Biophys Sin (Shanghai) 45:442–451
Kaganovich D, Kopito R, Frydman J (2008) Misfolded proteins partition between two distinct quality control compartments. Nature 454:1088–1095
Kato M, Wickner W (2003) Vam10p defines a Sec18p-independent step of priming that allows yeast vacuole tethering. Proc Natl Acad Sci USA 100:6398–6403
Kayed R, Lasagna-Reeves CA (2013) Molecular mechanisms of amyloid oligomers toxicity. J Alzheimers Dis 33(Suppl 1):S67–S78
Keefer KM, True HL (2016) Prion-associated toxicity is rescued by elimination of cotranslational chaperones. PLoS Genet 12:e1006431
Keefer KM, Stein KC, True HL (2017) Heterologous prion-forming proteins interact to cross-seed aggregation in Saccharomyces cerevisiae. Sci Rep 7:5853
King CY, Diaz-Avalos R (2004) Protein-only transmission of three yeast prion strains. Nature 428:319–323
Kryndushkin DS, Alexandrov IM, Ter-Avanesyan MD, Kushnirov VV (2003) Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J Biol Chem 278:49636–49643
Lancaster AK, Bardill JP, True HL, Masel J (2010) The spontaneous appearance rate of the yeast prion [PSI+] and its implications for the evolution of the evolvability properties of the [PSI+] system. Genetics 184:393–400
Liebman SW, Chernoff YO (2012) Prions in yeast. Genetics 191:1041–1072
Liu JJ, Lindquist S (1999) Oligopeptide-repeat expansions modulate ‘protein-only’ inheritance in yeast. Nature 400:573–576
Liu TT, Gomez TS, Sackey BK, Billadeau DD, Burd CG (2012) Rab GTPase regulation of retromer-mediated cargo export during endosome maturation. Mol Biol Cell 23:2505–2515
Lund PM, Cox BS (1981) Reversion analysis of [psi−] mutations in Saccharomyces cerevisiae. Genet Res 37:173–182
Manogaran AL, Hong JY, Hufana J, Tyedmers J, Lindquist S, Liebman SW (2011) Prion formation and polyglutamine aggregation are controlled by two classes of genes. PLoS Genet 7:e1001386
Mathur V, Taneja V, Sun Y, Liebman SW (2010) Analyzing the birth and propagation of two distinct prions, [PSI+] and [Het-s](y), in yeast. Mol Biol Cell 21:1449–1461
Ness F, Ferreira P, Cox BS, Tuite MF (2002) Guanidine hydrochloride inhibits the generation of prion “seeds” but not prion protein aggregation in yeast. Mol Cell Biol 22:5593–5605
Nothwehr SF, Hindes AE (1997) The yeast VPS5/GRD2 gene encodes a sorting nexin-1-like protein required for localizing membrane proteins to the late Golgi. J Cell Sci 110(Pt 9):1063–1072
Osherovich LZ, Weissman JS (2001) Multiple Gln/Asn-rich prion domains confer susceptibility to induction of the yeast [PSI(+)] prion. Cell 106:183–194
Patel BK, Liebman SW (2007) “Prion-proof” for [PIN+]: infection with in vitro-made amyloid aggregates of Rnq1p-(132-405) induces [PIN+]. J Mol Biol 365:773–782
Saarikangas J, Barral Y (2016) Protein aggregation as a mechanism of adaptive cellular responses. Curr Genet 62:711–724
Salahuddin P, Fatima MT, Abdelhameed AS, Nusrat S, Khan RH (2016) Structure of amyloid oligomers and their mechanisms of toxicities: targeting amyloid oligomers using novel therapeutic approaches. Eur J Med Chem 114:41–58
Salnikova AB, Kryndushkin DS, Smirnov VN, Kushnirov VV, Ter-Avanesyan MD (2005) Nonsense suppression in yeast cells overproducing Sup35 (eRF3) is caused by its non-heritable amyloids. J Biol Chem 280:8808–8812
Seaman MN, McCaffery JM, Emr SD (1998) A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 142:665–681
Sharma J, Wisniewski BT, Paulson E, Obaoye JO, Merrill SJ, Manogaran AL (2017) De novo [PSI +] prion formation involves multiple pathways to form infectious oligomers. Sci Rep 7:76
Tanaka M, Chien P, Naber N, Cooke R, Weissman JS (2004) Conformational variations in an infectious protein determine prion strain differences. Nature 428:323–328
Tyedmers J, Madariaga ML, Lindquist S (2008) Prion switching in response to environmental stress. PLoS Biol 6:e294
Vishveshwara N, Bradley ME, Liebman SW (2009) Sequestration of essential proteins causes prion associated toxicity in yeast. Mol Microbiol 73:1101–1114
Wegrzyn RD, Bapat K, Newnam GP, Zink AD, Chernoff YO (2001) Mechanism of prion loss after Hsp104 inactivation in yeast. Mol Cell Biol 21:4656–4669
Zhou P, Derkatch IL, Liebman SW (2001) The relationship between visible intracellular aggregates that appear after overexpression of Sup35 and the yeast prion-like elements [PSI(+)] and [PIN(+)]. Mol Microbiol 39:37–46
Acknowledgements
We thank Susan Liebman for the gifts of plasmids and strains used in this study. The Sup35C (BE4) antibody was a gift from Viravan Prapapanich and Susan Liebman. We would also like to thank Jane Dorweiler, Doug Lyke, and Emily Davis for critical reading of the manuscript. This work was supported by a grant from the National Institutes of Health (GM109336) to A. L. M., and B. T. W. and E. R. L. were supported by the Marquette University Honors Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Wisniewski, B.T., Sharma, J., Legan, E.R. et al. Toxicity and infectivity: insights from de novo prion formation. Curr Genet 64, 117–123 (2018). https://doi.org/10.1007/s00294-017-0736-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-017-0736-1