Abstract
In Saccharomyces cerevisiae, the large majority of the genes coding for cytoplasmic ribosomal proteins (RPs) depend on the general regulatory factor Rap1 for their transcription, but a small cohort of them relies on Abf1 regulatory activity. A recent study showed that unlike Rap1, whose association with RP gene promoters is not affected by environmental changes causing RP gene repression/reactivation, Abf1 association with both RP gene and ribosome biogenesis (Ribi) gene promoters dynamically responds to changes in growth conditions. This observation changes the paradigm of general regulatory factors as relatively static DNA-binding proteins constitutively bound to highly active promoters, and point to Abf1, which binds hundreds of non-RPG promoters within the yeast genome, as a possible key regulatory switch in nutrient- and stress-dependent transcriptional modulation. Moreover, the frequent presence of Abf1 binding sites in the promoters of mitochondrial RP genes evokes the possibility that Abf1 might orchestrate still unexplored levels of co-regulation involving growth-related gene networks in yeast cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Saccharomyces cerevisiae genome is interspersed with binding sites for relatively abundant DNA-binding proteins collectively known as general regulatory factors (GRF) because of their involvement as global regulators in diverse chromosomal functions. GRFs include Rap1, Reb1, Tbf1 and Abf1 proteins, all containing Myb-related motifs capable of sequence-specific DNA binding (Azad and Tomar 2016; Brigati et al. 1993; Chasman et al. 1990; Ju et al. 1990; Shore 1994). GRF-binding sites are scattered throughout the genome, with a higher frequency within promoter regions, where the presence of bound GRFs influences promoter activity, but also within telomeric and subtelomeric regions, where GRFs contribute to telomere structure and regulation (Fourel et al. 1999; Grunstein 1997), and at replication origins whose function can be influenced by bound GRFs (Raychaudhuri et al. 1997). It is thought that a common role shared by these DNA-binding proteins at different chromosome locations might be the establishment and maintenance of well-defined chromatin structures (Ganapathi et al. 2011; Hartley and Madhani 2009).
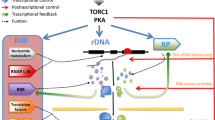
At promoter regions, GRFs may additionally act by recruiting the basal transcription machinery (Papai et al. 2010). Interestingly, promoter regions marked by GRFs generally belong to heavily transcribed genes, many of which are involved in growth-related functions such as ribosome biogenesis (Bosio et al. 2011). Indeed, it has long been known that in S. cerevisiae most of the 138 ribosomal protein (RP) genes contain Rap1-binding sites in their promoter regions, with the exception of a small subset of RP genes for which Rap1 is replaced by Abf1 (Lascaris et al. 1999). These two GRFs appear to be interchangeable in this case, as they both act as constitutive transcriptional activators (Mager and Planta 1990).
Given the high number of binding sites located across the genome and the ability of GRFs to influence nucleosome occupancy/chromatin structure, GRFs have the potential to act as master regulators of genome expression in response to nutritional cues or other environmental perturbations. Suggestively with respect to this possibility, the four GRFs mentioned above have all been reported to be phosphoproteins (Albuquerque et al. 2008; Francesconi and Eisenberg 1991; Morrow et al. 1990). In particular, the balance between Abf1 hyper- and hypo-phosphorylated forms was reported to vary in response to nutrient availability (Silve et al. 1992), and Abf1 was included among candidate proximal targets of TORC1 by a recent phosphoproteomic study (Oliveira et al. 2015). However, until recently no evidence was reported for regulated changes in GRF association with cognate promoter elements, nor for any other functional alteration in their activity at promoters, in response to environmental stimuli. This consolidated the notion of GRFs as permanent “placeholders” constitutively acting at promoter regions by counteracting nucleosome deposition and, thus, favoring regulatory transactions due to other, more tunable transcription factors (Bhattacharya and Warner 2008).
Recently, a study by our laboratory challenged this notion by showing that the association of Abf1 with at least some of its target promoters is responsive to nutritional status (Fermi et al. 2016). In particular, we found that inhibition of the nitrogen-sensitive TORC1 pathway by rapamycin, a perturbation known to cause quick repression of ribosomal protein (RP) gene transcription, unexpectedly entails a large increase in Abf1 association with the small cohort of RP genes whose promoter is demarcated by Abf1 instead of Rap1 binding sites. Even though the dependence of this phenomenon on changes of Abf1 phosphorylation state is unknown at present, this observation points to Abf1 as the first GRF whose association to DNA is modulated by nutritional status, and, at the same time, raises at least three orders of questions: (a) why should a repressive stimulus (TORC1 inactivation) entail an increased recruitment of an activator to transcriptionally repressed promoters? (b) what is the physiological reason (if any) for the existence in S. cerevisiae of two distinct subsets of RP gene promoters, one characterized by nutrient-unaffected Rap1 binding, the other by nutrient-dependent fluctuations in Abf1 binding? (c) considering other genes bound by Abf1 in their promoter regions, how does Abf1 behave at these promoters in response to nutritional stress, and which are the possible regulatory interconnections between the different categories of Abf1-demarcated genes?
As to the first question, we provided evidence that increased Abf1 association with RP gene promoters observed in response to TOR pathway inactivation might help the transcriptional rescue of these repressed promoters, once more favorable nutritional conditions are established (Fermi et al. 2016). But quick changes in promoter occupancy by Abf1, which were also observed, albeit to lower extents, at ribosome-unrelated promoters in response to TORC1 inhibition (Fermi et al. 2016), might also be related to extra-transcriptional roles of Abf1 (Reed et al. 1999; Yu et al. 2009).
The second and third questions are related to each other, because the different behaviors of Abf1 at its target promoters [which have already been noted in association with different chromatin organization propensities (Paul et al. 2015)] might deal with still unexplored levels of co-regulation between different groups of Abf1-demarcated genes. An intriguing possibility is that the Abf1-dependent subset of RP genes shares some specific regulatory features with the genes required for ribosome biogenesis belonging to the so-called Ribi regulon. According to such hypothesis, the promoters of these genes are enriched in Abf1-binding sites (Yarragudi et al. 2007; Bosio et al. 2016), and their association with Abf1 increases in response to TORC1 inactivation exactly as it does in the case of Abf1-dependent RP genes (Fermi et al. 2016).
In a systematic search for other S. cerevisiae gene regulons whose promoter regions tend to be enriched in Abf1 binding sites, we noticed that such cis-acting elements appear to be overrepresented also in the promoter regions of the nuclear genes coding for mitochondrial ribosomal proteins (MRPs). As shown in Table 1, among the 74 S. cerevisiae genes coding for MRPs (according to the Saccharomyces Genome Database), those displaying in the promoter region one or more Abf1-binding sites are 24, 13 of which have been shown to be bound by Abf1 in vivo and/or to be transcriptionally dependent on Abf1 (Chang et al. 2011; Schlecht et al. 2008; Yarragudi et al. 2007). Interestingly, 15 more mitochondrial ribosomal protein (MRP) gene promoters contained one or more Reb1-binding sites, and 3 more MRP promoters displayed one or more Rap1-binding sites (Chang et al. 2011). Therefore, the majority of MRP promoters are demarcated by GRFs, in particular by Abf1. In light of these observations, it is tempting to speculate that Abf1 might orchestrate a network of subtle regulatory interconnections between genes involved in the biogenesis and structure of both cytoplasmic and mitochondrial ribosomes. With this respect, it is worth noting that Abf1 has been suggested by a previous study to be involved in the intergenomic signaling pathway of mitochondrial–nuclear communication (Woo et al. 2009).
Given that there are several hundreds of Abf1 target promoters in the budding yeast genome, with many of them regulating growth-related genes, this transcription factor might represent one of the key regulators of genome expression reprogramming which allows for rapidly alternating growth arrest and growth burst phases in response to changing nutrient availability (Ho and Gasch 2015; Soontorngun 2016).
References
Albuquerque CP, Smolka MB, Payne SH, Bafna V, Eng J, Zhou H (2008) A multidimensional chromatography technology for in-depth phosphoproteome analysis. Mol Cell Proteom: MCP 7:1389–1396
Azad GK, Tomar RS (2016) The multifunctional transcription factor Rap1: a regulator of yeast physiology. Front Biosci 21:918–930
Bhattacharya A, Warner JR (2008) Tbf1 or not Tbf1? Mol Cell 29:537–538
Bosio MC, Fermi B, Spagnoli G, Levati E, Rubbi L, Ferrari R, Pellegrini M, Dieci G et al (2016) Abf1 and other general regulatory factors control ribosome biogenesis gene expression in budding yeast (Submitted)
Brigati C, Kurtz S, Balderes D, Vidali G, Shore D (1993) An essential yeast gene encoding a TTAGGG repeat-binding protein. Mol Cell Biol 13:1306–1314
Chang DT, Huang CY, Wu CY, Wu WS (2011) YPA: an integrated repository of promoter features in Saccharomyces cerevisiae. Nucl Acids Res 39:D647–D652
Chasman DI, Lue NF, Buchman AR, LaPointe JW, Lorch Y, Kornberg RD (1990) A yeast protein that influences the chromatin structure of UASG and functions as a powerful auxiliary gene activator. Genes Dev 4:503–514
Fermi B, Bosio MC, Dieci G (2016) Promoter architecture and transcriptional regulation of Abf1-dependent ribosomal protein genes in Saccharomyces cerevisiae. Nucl Acids Res. doi:10.1093/nar/gkw194
Fourel G, Revardel E, Koering CE, Gilson E (1999) Cohabitation of insulators and silencing elements in yeast subtelomeric regions. EMBO J 18:2522–2537
Francesconi SC, Eisenberg S (1991) The multifunctional protein OBF1 is phosphorylated at serine and threonine residues in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 88:4089–4093
Ganapathi M, Palumbo MJ, Ansari SA, He Q, Tsui K, Nislow C, Morse RH (2011) Extensive role of the general regulatory factors, Abf1 and Rap1, in determining genome-wide chromatin structure in budding yeast. Nucl Acids Res 39:2032–2044
Grunstein M (1997) Molecular model for telomeric heterochromatin in yeast. Curr Opin Cell Biol 9:383–387
Harbison CT, Gordon DB, Lee TI, Rinaldi NJ, Macisaac KD, Danford TW, Hannett NM, Tagne JB, Reynolds DB, Yoo J, Jennings EG, Zeitlinger J, Pokholok DK, Kellis M, Rolfe PA, Takusagawa KT, Lander ES, Gifford DK, Fraenkel E, Young RA (2004) Transcriptional regulatory code of a eukaryotic genome. Nature 431:99–104
Hartley PD, Madhani HD (2009) Mechanisms that specify promoter nucleosome location and identity. Cell 137:445–458
Ho YH, Gasch AP (2015) Exploiting the yeast stress activated signaling network to inform on stress biology and disease signaling. Curr Genet 61:503–511
Ju QD, Morrow BE, Warner JR (1990) REB1, a yeast DNA-binding protein with many targets, is essential for growth and bears some resemblance to the oncogene myb. Mol Cell Biol 10:5226–5234
Lascaris RF, Mager WH, Planta RJ (1999) DNA-binding requirements of the yeast protein Rap1p as selected in silico from ribosomal protein gene promoter sequences. Bioinformatics 15:267–277
Mager WH, Planta RJ (1990) Multifunctional DNA-binding proteins mediate concerted transcription activation of yeast ribosomal protein genes. Biochim Biophys Acta 1050:351–355
Morrow BE, Ju Q, Warner JR (1990) Purification and characterization of the yeast rDNA binding protein REB1. J Biol Chem 265:20778–20783
Oliveira AP, Ludwig C, Zampieri M, Weisser H, Aebersold R, Sauer U (2015) Dynamic phosphoproteomics reveals TORC1-dependent regulation of yeast nucleotide and amino acid biosynthesis. Sci Signal 8:rs4
Papai G, Tripathi MK, Ruhlmann C, Layer JH, Weil PA, Schultz P (2010) TFIIA and the transactivator Rap1 cooperate to commit TFIID for transcription initiation. Nature 465:956–960
Paul E, Tirosh I, Lai W, Buck MJ, Palumbo MJ, Morse RH (2015) Chromatin mediation of a transcriptional memory effect in yeast. G3 5:829–838
Raychaudhuri S, Byers R, Upton T, Eisenberg S (1997) Functional analysis of a replication origin from Saccharomyces cerevisiae: identification of a new replication enhancer. Nucl Acids Res 25:5057–5064
Reed SH, Akiyama M, Stillman B, Friedberg EC (1999) Yeast autonomously replicating sequence binding factor is involved in nucleotide excision repair. Genes Dev 13:3052–3058
Schlecht U, Erb I, Demougin P, Robine N, Borde V, van Nimwegen E, Nicolas A, Primig M (2008) Genome-wide expression profiling, in vivo DNA binding analysis, and probabilistic motif prediction reveal novel Abf1 target genes during fermentation, respiration, and sporulation in yeast. Mol Biol Cell 19:2193–2207
Shore D (1994) RAP1: a protean regulator in yeast. Trends Genet 10:408–412
Silve S, Rhode PR, Coll B, Campbell J, Poyton RO (1992) ABF1 is a phosphoprotein and plays a role in carbon source control of COX6 transcription in Saccharomyces cerevisiae. Mol Cell Biol 12:4197–4208
Soontorngun N (2016) Reprogramming of nonfermentative metabolism by stress responsive transcription factors in the yeast Saccharomyces cerevisiae. Curr Genet. doi:10.1007/s00294-016-0609-z
Woo DK, Phang TL, Trawick JD, Poyton RO (2009) Multiple pathways of mitochondrial-nuclear communication in yeast: intergenomic signaling involves ABF1 and affects a different set of genes than retrograde regulation. Biochim Biophys Acta 1789:135–145
Yarragudi A, Parfrey LW, Morse RH (2007) Genome-wide analysis of transcriptional dependence and probable target sites for Abf1 and Rap1 in Saccharomyces cerevisiae. Nucl Acids Res 35:193–202
Yu S, Smirnova JB, Friedberg EC, Stillman B, Akiyama M, Owen-Hughes T, Waters R, Reed SH (2009) ABF1-binding sites promote efficient global genome nucleotide excision repair. J Biol Chem 284:966–973
Acknowledgments
We thank Paola Frigeri (University of Parma) for help with MRP promoter analysis. This work was supported by a Grant from the Italian Ministry of Education, University and Research (MIUR, PRIN 2009 to G.D.) and Italian Association for Cancer Research (AIRC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Rights and permissions
About this article
Cite this article
Fermi, B., Bosio, M.C. & Dieci, G. Multiple roles of the general regulatory factor Abf1 in yeast ribosome biogenesis. Curr Genet 63, 65–68 (2017). https://doi.org/10.1007/s00294-016-0621-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-016-0621-3