Abstract
The study was performed to examine 11 isolates of Trichoderma for their bio-control potentials against Sclerotium rolfsii Sacc. causing stem rot in groundnut. The antagonists Trichoderma were subjected to sequence related amplified polymorphism (SRAP) based molecular diversity analysis and compared with their hardness to S. rolfsii with respect to secretary antifungal and antioxidant profile. T. virens NBAII Tvs 12 evident highest (87.91 %) growth inhibition of test pathogen followed by T. koningii MTCC 796 (67.03 %) at 7 days after inoculation (DAI). Microscopic study confirmed biocontrol mechanism as mycoparasitism for Tvs 12 and antibiosis for MTCC 796. The growth inhibition of test pathogen was significantly negatively correlated with sclerotia formation and lipid peroxidation during antagonism due to release of secretary bioactive antioxidants by antagonists to terminate oxidative burst generated by S. rolfsii and causing inhibition of sclerotium formation. The GC–MS profile identified antifungal and antioxidant constituents hexadecane, 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester, 1-hexadecanesulfonyl chloride, and octadecane in potent antagonists Tvs 12; and nonacosane and octadecane in MTCC 796 along with two novel compounds 1-pentadecene and 1-heneicosyl formate for biocontrol activity. Molecular diversity of Trichoderma isolates associated with antagonistic activity was assessed by SRAP markers. The 115 primer combinations generate total 1328 amplified products of which, 1095 are shared polymorphic and 199 are unique polymorphic. The 15 SRAP combinations produced 18 bands to diagnose best antagonist Tvs 12 and 13 SRAP combinations generated 19 unique bands for identification of MTCC 796. The mycoparasitic antagonist Tvs 12 would be the best antagonist and released unique antifungal and antioxidant constituents to combat pathogen infection. The SRAP based genetic diversity indicates Tvs12 strain clustered with T. viride NBAII Tv23 and shared only 52 % similarity with other isolates of Trichoderma. The SRAP similarities explained substantial diversity (19–68 %) across Trichoderma isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The stem rot disease incited by S. rolfsii is a serious soil borne disease in groundnut (Arachis Hypogaea L.). A S. rolfsii infection may cause an average 25 % reduction in groundnut yield (Mayee and Datar 1988). Various management strategies have been adopted to prevent S. rolfsii infections in groundnut crop. Many seed dressing fungicides are reported to be effective against stem rot of groundnut but very little is known about successful exploitation of bio-control agents on disease management. Biological control of stem rot caused by S. rolfsii using Trichoderma is a cheap and better way to sustain the crop production.
Trichoderma is one of the most important filamentous fungi common in soil and root ecosystems and used as an effective biocontrol agents for soil borne fungal plant pathogens and some species are also known for their abilities to enhance systemic resistance to plant diseases (Gajera et al. 2015). The Trichoderma antagonists release a variety of compounds that induce localized or systemic resistance responses, and this explains lack of pathogenicity to plants. The plant root microorganism associations cause substantial changes to the plant proteome and metabolism. Trichoderma control plant diseases through various mechanisms such as antibiosis, competition, suppression, mycoparasitism, induced resistance, hypovirulence and predation (Mayee and Datar 1988; Tseng et al. 2008).
Screening and characterization of Trichoderma from different geographical locations provide diversity which can be exploited further for the control of plant diseases. There are various well-established molecular markers that had been widely used for diversity analysis in Trichoderma and other fungi including isozyme variation (Royse and May 1982), DNA sequences in internal transcribed spacer region (ITS1) (Sokol et al. 1999), restriction fragment length polymorphism (RFLP) (Abang et al. 2002), amplified fragment length polymorphism (AFLP) (O’Neill et al. 1997) and random amplified polymorphic DNA (RAPD) (Agrawal and Kotasthane 2009; Graham et al. 1994). However, inter simple sequence repeats (ISSR) and simple sequence repeats (SSR) markers are reliable, but are rarely reported for Trichoderma characterization. The marker technology sequence related amplified polymorphism (SRAP) is consistent and repeatable than RAPDs, and are less time-consuming compared to AFLPs, can be used for studying molecular diversity in Trichoderma.
Li and Quiros (2001) developed a SRAP for the amplification of open reading frames (ORFs). It is based on two-primer amplification. The primers are 17 or 18 nucleotides long and consist of the following elements. Core sequences, which are 13–14 bases long, where the first 10 or 11 bases starting at the 5′ end, are sequences of no specific constitution (“filler” sequences), followed by the sequence CCGG in the forward primer and AATT in the reverse primer. The core is followed by three selective nucleotides at the 3′ end. The filler sequences of the forward and reverse primers must be different from each other and can be 10 or 11 bases long. SRAP combines simplicity, reliability, moderate throughput ratio and facile sequencing of selected bands. Further, it targets coding sequences in the genome and results in a moderate number of co-dominant markers.
All Trichoderma strains could not work equally against specific soil borne disease as Trichoderma antagonists have different mechanisms of pathogen recognition. In the backdrop of this scenario, the aim of the present study was (1) to evaluate the antagonistic activity of Trichoderma isolates as a biocontroller against pathogen Sclerotium rolfsii, causing stem rot of groundnut (2) to observe mechanism of antagonist for growth inhibition of S. rolfsii using microscopy, (3) to identify antifungal and antioxidant bioactive constituents present in best antagonist Trichoderma for biocontrol activity, and (4) to assess molecular diversity analysis of antagonist Trichoderma using SRAP markers and identification of unique strain specific markers to diagnose best antagonists.
Materials and methods
Sources and maintenance of microbes
Culture of 11 isolates of Trichoderma including one local isolates [(1) T. harzianum NBAII Th1; (2) T. harzianum NRRL 13879; (3) T. harzianum NRRL 20565; (4) T.harzianum Local; (5) T. viride NBAII Tv23; (6) T. viride NRRL 6418; (7) T. virens NBAII Tvs12; (8) T. hamantum NBAII Tha 1; (9) T. koningii MTCC 796; (10) T. pseudokoningii MTCC 2048; (11) T. species NRRL 5242] were procured either from Indian Type Culture Collection (ITCC, indicating accession number with NBAII), New Delhi; Microbial Type Culture Collection (MTCC), Chandigarh; or Agricultural Research Service Culture Collection (NRRL), Illinois, USA. The Trichoderma strains are available at their respective culture collection centre for future uses. One local isolate of T. harzianum was collected from culture collection of Department of Plant Pathology, Junagadh Agricultural University, Junagadh. The phytopathogen S. rolfsii were isolated from infected groundnut root by hyphal tip method (Sinclair and Dhingra 1985) and maintained on PDA by storing it under refrigeration (4 °C). Phytopathogen isolated from infected groundnut root was sent for deposition and identification at Indian type culture collection (ITCC), Division of plant pathology, IARI, New Delhi and they identified the pathogen as Sclerotium rolfsii (identity number 9107.13).
In vitro antagonism of Trichoderma against S. rolfsii and microscopic characterization during antagonism
The Trichoderma isolates were evaluated for their antagonistic activity against the S. rolfsii pathogen by dual culture assay on PDA plate (Dennis and Webster 1971). The time of inoculation of pathogen was decided based on its growth rate with respect to antagonist. The radial growth of the pathogen was measured at 7 DAI from control and antagonists plate and percent inhibition was calculated using the formula given by Vincent (1927). Total sclerotia formed from S. rolfsii during antagonism along with control plate at 7 DAI were counted. The experiment was conducted in three independent replications; and mean values of index of antagonism and sclerotial count of test pathogen were pointed up in graphical representation depicting standard deviation (bar) between replications.
Determination of growth rates, mechanism of inhibition and mycoparasitism were observed on PDA media using light microscope. Thin layer of PDA was prepared on microscopic slide under aseptic condition. The antagonist and pathogen were inoculated on same slide at opposite pole and slides were incubated at 28 °C for 7 days. The antagonistic activity was observed under binocular microscope attached with image analyzer (Nicon). Phase contrast images of antagonism were taken using 10× and 100× lenses.
Lipid peroxidation of S. rolfsii and total lipid estimation
Lipid peroxidation in the periphery of the mycelial colony of S. rolfsii both in the presence and absence of the antagonist at 7 DAI was measured as the amount of thiobarbituric acid reactive substances (TBARS) determined by the thiobarbituric acid (TBA) reaction (Heath and Packer 1968). Lipid peroxides were measured in chloroform extracts of total lipids as malondialdehydes (MDA) formed from the decomposition of lipid hydroperoxides during the heating stage of the assay (Georgiou 1997). The mycelia of S. rolfsii (0.5 g) from inhibition zone of antagonists plate were harvested and suspended in 0.5 ml chloroform for lipid extraction. For total phospholipid determination, 0.1 ml of the chloroform suspension of extracted total lipids were vacuum-dried and phosphorus were estimated from the dry lipid residue using Bartlett method (Bartlett 1959). The known aliquot (0.4 ml) of the chloroform suspension of extracted total lipids from mycelial samples were vacuum dried and re-suspended in a 1 ml 0.1 M NaCl, 0.5 ml 25 % (v/v) HCl and 0.5 ml 1 % (w/v) TBA (in 0.05 M NaOH) for lipid peroxidationl. The mixture was incubated for 15 min in a 100 °C water-bath. The developed chromogen was extracted with 1.5 ml isobutanol and the absorbance was read at 532 nm with an UV–Visible spectrophotometer. The value for non-specific absorption at 600 nm was subtracted from the 532 nm reading. Lipid peroxides as MDA-TBA adduct were estimated as mM. MDA M−1 of total phospholipids using a molar extinction coefficient of 155 mM−1 cm−1 and represent in a graphical illustration with means of three determinations. The molar extinction coefficient is parameter defining how strongly a substance absorbs light at a given wavelength per molar concentration.
Bioactive constituents from antagonist Trichoderma by GC–MS
The S. rolfsii was grown in a 250 ml potato dextrose broth at 28 °C. It was then incubated while being shaken at 150 rpm in a 500 ml Erlenmeyer flask for 7 days. Mycelia were collected by centrifugation at 3000 rpm for 10 min at room temperature. The mycelia were washed twice using 50 ml sterile deionized water and then boiled for 20 min twice to obtain deactivated mycelia, which were then stored at −4 °C until use as carbon source (Tseng et al. 2008).
The best Trichoderma antagonists (T. virens NBAII Tvs12; T. koningii MTCC 796) and least (T. viride NRRL 6418) were grown on synthetic media (SM) containing 0.1 % glucose or 0.1 % inactive cell wall of pathogen S. rolfsii as carbon sources, 1.4 g (NH4)2 SO4, 0.2 gm KH2PO4, 6.9 g NaH2PO4.H2O, 0.3 gm MgSO4·7H2O for release of bioactive compounds and incubated for 7 days in shaker incubator at 28 °C (125 rpm). The content was centrifuged for 15 min at 8000 rpm and supernatant collected followed by filtration with Whatman-1 to remove fungal mycelia. The mycelia free culture filtrates were extracted with ethyl acetate at volume ratio of 1:1 by use of a separating funnel. The extract was passed through a pad of anhydrous sodium sulphate to remove excess water and thereafter evaporated to dryness using a rotary vacuum evaporator (Siddiquee et al. 2012).
The extracts were used for GC–MS profile (Keszler et al. 2000). The GC–MS analysis was carried out with fused silica capillary column (RT × 5 MS 30 m length, 0.25 µm diameter, 0.25 mm thickness), operating in electron impact mode at 70 eV, and helium (99.999 %) was used as carrier gas at a constant flow of 1 ml min−1 and an injection volume of 1 µl was employed (split ratio of 10:1), injector temperature 280 °C; ion-source temperature 230 °C. The column over temperature was programmed from 80 °C (isothermal for 7 min), with an increase of 6–290 °C/min, ending with a 35 min isothermal at 280 °C. Interpretation of mass spectra of GC–MS was done using the database of national institute standard and technology (NIST) having more than 2,82,000 patterns. The mass spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular formula, molecular weight and biological activity of the compounds of the test materials were ascertained.
PCR-SRAP analysis
Total genomic DNA was extracted using CTAB method from all 11 isolates of Trichoderma and pathogen S. rolfsii (Narayanasamy and Saravana 2009). The purity of DNA was checked in 0.8 % agarose gel electrophoresis and found single intact band. DNA was quantified using agarose gel electrophoresis. The quantity of genomic DNA isolated from 11 Trichoderma strains and S. rolfsii was measured in piccodrop (Picodrop PET01) and 20 ng/µl DNA concentration was maintained for PCR-SRAP amplification. The SRAP was used for molecular characterization of Trichoderma and pathogen S. rolfsii for studying genetic diversity and similarity across 11 Trichoderma strains which differed in their antagonistic activity. Molecular characterization of Trichoderma using PCR-SRAP was done as method given by Li and Quiros (2001). The amplification of 256 combinations of 16 forward and 16 reverse SRAP primers (Table 1) were screened using one Trichoderma strain resulted to 141 primer combinations gave positive amplification. Therefore, these 115 primer combinations used for further diversity analysis across 11 Trichoderma isolates and test pathogen.
The PCR reaction mixture (15 µl) contained 1.5 µl 10× Taq Buffer, 0.3 µl 1U/µl Taq polymerase, 1.2 µl 10 mM/µl dNTP mix, 1.2 µl Primer, 1.2 µl Genomic DNA and 9.6 µl Nuclease free water. The reaction mixtures were heated to 96 °C for 4 min, then subjected to 5 cycles of 1 min at 94 °C; 1.15 min at 35 °C and 2 min at 72 °C, then subjected to 30 cycles of 1 min at 94 °C; 1.15 min at 50 °C and 2 min at 72 °C and a final extension for 10 min at 72 °C (Li and Quiros 2001). The amplification products were separated in 2 % (w/v) agarose gel in 1× TBE buffer and visualized by staining with 0.1 % ethidum bromide. The PCR-SRAP repeated twice and consistence bands were considered for analysis. The bands were recorded as presence (1) and absence (0) across 11 Trichoderma strains and pathogen S. rolfsii in binary matrix and utilized for polymorphic pattern and phylogenetic relationship.
Statistical analysis
Antgonistic (biocontrol) activities of 11 Trichoderma isolates against phytopathogen S. rolfsii (viz., growth inhibition of test pathogen, Sclerotium count and lipid peroxide products) were carried out in three independent experiments and data were analyzed for standard deviation between antagonists (Fisher and Yates 1948). GC–MS profile of beat and least antagonists were carried out in duplicate and mean values were presented. Variations among 11 Trichoderma strains and pathogen S. rolfsii across the SRAP primers used in the present study were evaluated from pairwise comparison for the proportion of shared bands amplified (Nei 1987). The similarity coefficients were calculated by using the Jaccard’s similarity coefficient and cluster analysis by UPGMA using NTSYSpc-2.02i software developed by Rohlf (1998). A polymorphic information index (PIC) for SRAP profiles was calculated as PIC = 1 − p 2 − q 2, where, p is band frequency and q is no band frequency (Ghislain et al. 1999). The PIC values were then used to calculate a SRAP primer index (SPI), which was generated by adding up the PIC values of all the markers amplified by the same primer (Anderson et al. 1993).
Results
Antagonist activity and microscopic characterization of Trichoderma inhibiting S. rolfsii
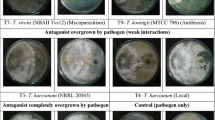
Growth inhibition of S. rolfsii during in vitro interaction with biocontrol agents Trichoderma was observed at 7 DAI (Fig. 1). The growth of test pathogen was significantly reduced at 7 DAI (Fig. 2). Percent growth inhibition of pathogen S. rolfsii was found significantly higher in T. virens NBAII Tvs12 (T7) followed by T. koningii MTCC 796 (T9), T. viride NBAII Tv23 (T5), T. harzianum NBAII Th1 (T1) and T. species NRRL 5242 (T11) at 7 DAI. The strain Tvs 12 was found to be best antagonist inhibiting 87.91 % growth inhibition of test pathogen followed by MTCC 796 with 67.03 % inhibition of test pathogen.
Percent growth inhibition, sclerotium count and lipid perixidation of S. Rolfsii during in vitro antagonism with Trichoderma strains at 7 DAI (bars indicate standard deviations between three experiments). T1 = T. harzianum (NBAII Th1) × S. rolfsii; T2 = T. harzianum (NRRL 13879) × S. rolfsii; T3 = T. harzianum (NRRL 20565) × S. rolfsii; T = T.harzianum (Local) × S. rolfsii; T5 = T. viride (NBAII Tv23) × S. rolfsii; T6 = T. viride (NRRL 6418) × S. rolfsii; T7 = T. virens (NBAII Tvs12) × S. rolfsii; T8 = T. hamantum (NBAII Tha 1) × S. rolfsii; T9 = T. koningii (MTCC 796) X S. rolfsii; T10 = T. pseudokoningii (MTCC 2048) × S. rolfsii; T11 = T. species (NRRL 5242) × S. rolfsii; T12 = S. rolfsii control (9107.13)
The intersection effect between antagonists and test pathogen were examined under microscope at 7 DAI (Fig. 1). The Tvs 12 (T7) isolates overgrew completely on the pathogen with mycoparasitism as observed in microscopic observations. The strong antagonist Tvs 12 (T7) grew over mycelia of test pathogen with surround coiling and hock like structure formation followed by disintegration and disruption of mycelia of pathogen. Thus, the T7 antagonist (i.e. interaction between Trichoderma strain T. virens—NBAII Tvs12 and S. rolfsii) has best antagonistic activity followed by T9 antagonist (i.e. interaction between Trichoderma strain T. koningii—MTCC 796 and S. rolfsii) as compared to the other biocontrol agents. The best antagonist T. virens—NBAII Tvs 12 strain inhibited the growth of pathogen by using mode of action mycoparasitism whereas other isolates including second best antagonist T. koningii—MTCC 796 exhibited strong antibiosis and formed about 2–4 mm zone of inhibition after 7 days and the same were confirmed in microscopic examinations.
Lipid peroxidation and sclerotium count of S. rolfsii during antagonism with Trichoderma isolates
The lipid peroxidation of total lipids of test pathogen S. rolfsii during antagonism was examined at 7 DAI and lipid peroxides as MDA-TBA adduct were found highest 25.3 mM MDA M−1 of total phospholipids in absence of antagonists Trichoderma (control plate) with formation of maximum number (85) of sclerotia. The interactions of S. rolfsii with best antagonists T. virens—NBAII Tvs12 and T. koningii—MTCC 796 diminished lipid peroxidation of test pathogen mycelia at inhibition zone without sclerotial formation compared to least antagonists acquaintances (Fig. 2). The growth inhibition of test pathogen was significantly negatively correlated (P 0.001) with number of sclerotia (r = −0.7236) and lipid peroxides (r = −0.8248) in antagonists plate. However, lipid peroxidation was positively correlated (r = 0.5490) with sclerotial formation. It indicates that lipid peroxidation is an essential phenomenon to elevate cellular oxidative processes for sclerital differentiation and promote growth of test pathogen S. rolfsii. However, best biocontrol agents secreted antioxidant bioactive constituents in the antagonists plate during antagonism which cease oxidative burst and stop the development of the test pathogen to the undifferentiated mycelial stage and thereby restrain growth of S. rolfsii.
Comparative evaluation of bioactive compounds from antagonist Trichoderma
The best (T. virens NBAII Tvs12 and T. koningii MTCC 796) and least (T. viride NRRL 6418) antagonists Trichoderma were grown in synthetic media containing inactive cell wall of pathogen as carbon sources to release bioactive constituents under influence of pathogen cell wall (Figs S1–S4). The GC–MS profile of SM containing glucose and cell wall of pathogen without inoculation of antagonists Trichoderma were also preformed as negative control and the compounds detected common in inoculated and without inoculation of Trichoderma were eliminated before comparison of best and least antagonists.
Total 30 bioactive compounds were identified in best antagonist strain NBAII Tvs12 (T7) when inoculated with SM containing glucose as normal growth. However, replacing the glucose with cell wall of pathogen in SM altered the metabolic constituents of antagonist and about 27 bioactive compounds were identified. The antagonist T7 evident maximum growth inhibition of test pathogen through mycoparasitism. The antagonist T. koningii MTCC 796 (T9) inhibited growth of pathogen differently with development of inhibition zone and secreted 19 bioactive compounds using cell wall of pathogen as carbon source. The comparative GC MS profile of bioactive compounds found in best and least inhibitory antagonists along with molecular formula, molecular weight and biological activity are depicted in Table 2.
The compounds contributing more than 2 % area as relative abundance were considered as major bioactive constituents. Major bioactive constituents in the best Tvs12 (T7) (SM + glucose) are alpha-bisabolol (8.29 %), 2-[4-methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1 carboxaldehyde (5.99 %), pentadecanoic acid (5.71 %) and 9-tricosene (5.41 %). The key compounds in T7 (SM + pathogen cell wall) were evident 1-heneicosyl formate (10.23 %), 1-hexadecanesulfonyl chloride (4.39 %), 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester (3.95 %) and 1-pentadecene (3.55 %). The major compounds in T9 (SM + Cell wall) were found to be 1-heneicosyl formate (8.15 %) and octadecane (1.69 %). Total 20 compounds were identified in least antagonists T. viride NRRL 6418 (T6) (SM + pathogen cell wall). The foremost compounds in the T6 were found to be octadecanoic acid (4.00 %), 13-tetradecen-1-ol acetate (2.97 %) and oleic acid (2.08 %).
Molecular diversity of Trichoderma isolates using SRAP markers
The genomic DNA of 11 Trichoderma and test pathogen S. rolfsii were utilized for molecular diversity analysis using SRAP marker. Total 115 SRAP primer combinations amplified to generate the 1328 bands across 11 isolates Trichoderma and pathogen S. rolfsii (Table 3). The SRAP-22 primer produced maximum number of 21 bands, while SRAP-15 produced minimum number of 1 band. Out of 1328, 1291 bands were polymorphic and 32 bands were monomorphic. Among the 1291 polymorphic bands, 1095 alleles were shared polymorphic within two or more isolates, while 199 bands were unique-polymorphic. The percent polymorphism obtained for SRAP primers were ranged from 61.50 to 100 % with an average value of 97.00 % per primer. The PIC values for SRAP marker ranged from 0.58 (SRAP-88) to 0.94 (SRAP-22 and SRAP-242) with an average value of 0.86 per primer and SPI differed from 2.33 (SRAP-88) to 19.76 (SRAP-22).
Based on PIC and SPI, SRAP-22 and SRAP-242 are most informative markers for polymorphism of genomic DNA of Trichoderma strains. Among 115 SRAP primers, 80 primers showed the amplification of specific unique bands. Maximum 10 unique bands were produced by SRAP-151. The SRAP 26 amplified specific unique bands of 232 bp to diagnose best antagonists T. virens NBAII Tvs12; and 1133 and 125 bp for T. koningii MTCC 796 (Fig. 3). Total 18 unique bands were produced by 15 SRAP primers to identify best antagonist strain Tvs12 However, MTCC 796 were identified by 19 unique bands produced from 13 SRAP primer pairs (Table 4).
SRAP profile of antagonist and test pathogen using different SRAP primers [M = DNA Marker (100 bp); 1, T. harzianum (NBAII Th1); 2, T. harzianum (NRRL 13879); 3, T. harzianum (NRRL 20565); 4, T.harzianum (Local); 5, T. viride (NBAII Tv23); 6, T. viride (NRRL 6418); 7, T. virens (NBAII Tvs12); 8, T. hamantum (NBAII Tha 1); 9, T. koningii (MTCC 796); 10, T. pseudokoningii (MTCC 2048); 11, T. species (NRRL 5242); 12, S. rolfsii (9107.13)]
The dendrogram was constructed using UPGMA based Jaccard’s similarity coefficient and found in the range from 19 to 68 % based on SRAP data across 11 Trichoderma strains and pathogen S. rolfsii (Fig. 4). The 11 isolates of Trichoderma and S. rolfsii were grouped into two main clusters: cluster A and cluster B and shared 26 % similarity. The cluster A comprised of two clusters, cluster A1and cluster A2. while Cluster B contains pathogen S. rolfsii. Cluster A1 was further subdivided into sub clusters, cluster A1(a) and cluster A1(b). Cluster A1(a) consisted of T. harzianum NBAII Th1, T. viride NBAII Tv23 and T. harzianum NBAII Tha1 while cluster A1(b) contained T. koningii MTCC 796, T. pseudokoningii MTCC 2048 and T.species NRRL 5242. Cluster A2 was further subdivided into sub clusters, cluster A2(a) and cluster A2(b). Cluster A1(a) consisted of T. harzianum NRRL 13879 and T. harzianum Local. Test pathogen out grouped from all trichoderma isolates and share minimum 19 % similarity. however best antagonist T. virens NBAII Tvs12 grouped with T. viride NBAII Tv23 and shared 52 % similarity with other isolates of Trichoderma.
Discussion
Different biocontrol agents can be used for the control of stem rot disease in groundnut including bacteria, fungi and actinomycetes. The most important biocontroller belongs to the genus Trichoderma. In vitro antagonism of 11 Trichoderma isolates with S. rolfsii indicated maximum (87.91 %) growth inhibition of test pathogen with T. virens NBAII Tvs 12 followed by T. koningii–MTCC 796 (67.03 %) at 7 DAI. The microscopic depiction of antagonist during interaction confirmed mycoparasitism as mode of action for potential Tvs 12 and antibiosis for MTCC 796 strain. The unique antifungal and antioxidant constitutes are released by dominant antagonists Tvs 12 and MTCC 796 to combat the test pathogen.
Similar to present study, Anand and Reddy (2009) studied biocontrol potential of 42 Trichoderma strains, isolated from rhizosphere soil samples from cultivated lands, against S. rolfsii. On day 5, maximum percentage reduction of the pathogen growth over control indicated T30 (43.22 %) and T6 (40.0 %) to be the best performers while T13 (−41.22 %) recorded the least. Pan and Bhagat (2007) used dual culture technique for screening isolates of Trichoderma against five most widely occurring soil-borne pathogenic fungi viz. R. solani, S. rolfsii, M. phaseolina, F. oxysporium and Pythium spp. Their study revealed that Trichoderma isolates not only differed in their reaction against different pathogens but antagonists themselves differed in the reaction against any single phytopathogen, required 3–7 days after incubation to attain S1 phase (the antagonist completely overgrown on the growth of pathogen). This may be due to variability in antagonistic isolates isolated from different ecological niches. The pathogens Pythium spp. and R. solani were highly affected by all Trichoderma isolates (T. harzianum, T. viride, G. virens), whereas S. rolfsii and M. phaseolina were comparatively less inhibited.
Joshi et al. (2008) evaluated the antagonistic potential of seventeen fungal isolates (15 Trichoderma harzianum and two Fusarium solani isolates) in vitro and in glasshouse against two important stages (sclerotia and mycelium) in the infection cycle of three plant pathogens viz., Rhizoctonia solani, Sclerotinia sclerotiorum and Sclerotium rolfsii. Out of the 17 isolates, only three isolates (S-1, S-6 and S-12) of T. harzianum, exhibited significantly higher sclerotial parasitism and hyphal inhibition activity against all the three pathogens.
The antagonism between Trichoderma strain and test pathogen were examined under microscope at 7 DAI. The best antagonist Tvs 12 (T7) showed mycoparasitism on the pathogen while T. koningii—MTCC 796 exhibited strong antibiosis and formed about 2–4 mm zone of inhibition after 7 days. Similar to present study, Yogendra and Singh (2002) studied the effect of Trichoderma based biocontrol agents, viz. T. viride and T. harzianum on the growth of Sclerotium rolfsii in vitro. T. harzianum exhibited strong mycoparasitism and covered 100 % colony growth on the pathogen, where as T. viride showed strong antibiosis and formed 2–3 mm zone of inhibition after 6 days of incubation in dual culture. T. harzianum and T. longibrachiatum inhibited the in vitro growth and produced coiling around mycelium of Sclerotium rolfsii resulting in lysis of hyphae.
Gajera et al. (2012) reported pathogen-specific mechanism of antagonists Trichoderma for biocontrol activity. T. koningii MTCC 796 was capable of overgrowing and degrading M. phaseolina mycelia, coiling around the hyphae with apressoria and hook-like structures. Formation of apressoria-like structures enabled the hyphae of Trichoderma spp. to firmly attach to the surface of its host mycelium. However, some antagonists (T. viride NBAII Tv 23, T. hamatum NBAII Tha 1) used different mechanisms against M. phaseolina just touched the hyphae without coiling. Whereas, T. pseudokoningii showed spore around pathogen not attached to hyphae. Present study agreed with the results suggesting all Trichoderma strains could not work equally against specific soil borne disease as various Trichoderma antagonists have different mechanisms of pathogen recognition.
Lipid peroxidation has been implicated in a large number of cellular processes including cell proliferation and differentiation in fungi. The filamentous fungi exhibit a marked increase in lipid peroxidation during differentiation. The S. rolfsii causes noticeable increase in lipid peroxidation during its differentiation state (Georgiou 1997). The lipid peroxidation is a indicator of high oxidative stress and lipofuscins were identified as lipid peroxidation products in all fungal representatives of sclerotial differentiation. The sclerotial biogenesis in S. rolfsii was accompanied by the accumulation of high levels of lipid peroxidation products and it is a well-established oxidative stress indicator. Present study evident that lipid peroxidation elevated in absence of antagonists Trichoderma with formation of maximum sclerotia by S. rolfsii and promote growth of test pathogen. The best antagonists Tvs12 and MTCC 796 strains diminished mycelial lipid peroxidation of test pathogen without formation of sclerotia compared to least antagonists. The growth inhibition of test pathogen was significantly negatively correlated with sclerotial formation and lipid peroxidation in antagonists plate due to release of secretary bioactive antioxidants to terminate oxidative burst generated by S. rolfsii and causing inhibition of sclerotium formation. The role of antioxidant beta-carotene in sclerotial metamorphosis is supported by the finding that when it is administered in the growth medium at concentrations that do not inhibit growth, it causes a concentration-dependent reduction of oxidative stress (lipid peroxidation) of S. rolfsii, S. minor, and S. sclerotiorum undifferentiated mycelia, and an equally proportional reduction of sclerotial differentiation (Georgiou et al. 2006).
The bioactive constituents of best strains Tvs12 and MTCC 796; and least T. viride NRRL 6418 antagonists were identified using GC–MS profile. The compounds identified in the study are saturated hydrocarbons (C7–C30), cyclohexane, cyclopentane, fatty acids, alcohols, esters, sulfur-containing compounds, simple pyrane and benzene derivatives. Similarly, Siddiquee et al. (2012) carried out separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC–MS using three different capillary columns. They were identified more than 278 volatile compounds which are in analogous nature to present study.
The antagonist Tvs12 evident maximum growth inhibition of test pathogen through mycoparasitism and identified 27 bioactive constituents while MTCC 796 evident 19 bioactive compounds using cell wall of pathogen as carbon source. The nonadecane, triacontane, 1-bromo and 3,7-diazabicyclo[3.3.1]nonane, 9,9-dimethyl were found unique in best Tvs12 (SM + glucose), which were reported for antimicrobial and cytotoxic effect (Akpuaka et al. 2013). The Tvs12 strain under the influence of pathogen cell wall evident to produce hexadecane which were reported for pesticide, nematicide, antioxidant, hypocholesterolemic, antiandrogenic activity, hemolytic and 5-alpha reductase inhibitor; octadecanoic acid for antifungal, antibacterial, hypocholesterolemic, antitumoral and antioxidant activities (Akpuaka et al. 2013, Govindappa et al. 2014; Roy et al. 2011) and 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester and 1-hexadecanesulfonyl chloride for antifungal, antibacterial, antiviral and antioxidant activities (Manorenjitha et al. 2013). The nonacosane was noticed in MTCC 796 (SM + pathogen cell wall) for antibacterial activity (Akpuaka et al. 2013). However, octadecane found in best MTCC 796 antagonists with higher relative abundance (1.69 %) followed by Tvs 12 (1.44 %) and least NRRL 6418 (1.30 %) when inoculated in SM containing pathogen cell wall and reported for hypocholesterolemic activity (Manorenjitha et al. 2013). The novel compounds 1-pentadecene (3.55 %) was evident only in best antagonists Tvs 12 strain while 1-heneicosyl formate was found in Tvs 12 (10.23 %) and MTCC 796 (8.15 %) strains inoculated with SM containing pathogen cell wall for biocontrol activity.
Present study evidenced that secretary antioxidants like hexadecane, 1,2-benzenedicarboxylic acid, mono (2-ethylhexyl) ester, 1-hexadecanesulfonyl chloride, pentadecanoic acid and octadecanoic acid released by antagonists ((Tvs 12 and MTCC 796) cause S. rolfsii to form very thick mycelia without sclerotia during interaction compared to control plate having sole test pathgen S. rolfsii. The results are supported with antioxidants N-acetylcysteine (Patsoukis and Georgiou 2008), ascorbic acid (Georgiou et al. 2003), hydroxyl radical scavengers (Georgiou et al. 2000), beta-carotene (Georgiou et al. 2001) when they are supplemented in the growth medium of S. rolfsii. The best antagonists (Tvs 12 and MTCC 796) secreted vast bioactive antioxidants which act as a damping them into the growth medium and causing inhibition of sclerotium formation by S. rolfsii. The high antioxidant concentrations inhibited sclerotial differentiation in any sclerotium producing phytopathogenic filamentous fungi including S. rolfsii, and thereby restrain the ability of S. rolfsii to proliferate via sclerotium formation. Meanwhile, these antioxidant at high concentrations could arrest growth and halt the development of the fungus to the undifferentiated mycelial stage, where mycelia are more vulnerable to degrade by antagonists (Georgiou et al. 2006; Papapostolou and Georgiou 2010). Therefore, antioxidants secretary antagonists act as an antifungal alternatives to traditional fungicides.
Bansode et al. (2011) reported SRAP markers as a useful tool for genetic diversity and phenetic relationship among 80 isolates of Trichoderma. Total 43 pairs of SRAP primers were used for diversity analysis of 83 Trichoderma isolates belonging to 4 different species. These 43 pairs of primers produced 595 amplified products among which 587 were polymorphic. These SRAP primers produced 97.98 % polymorphism. However, present study demonstrated better genetic diversity (0.19–0.68 Jaccard similarity coefficient) for Trichoderma and identified unique bands for best biocontrollers Tvs 12 and MTCC 796. Total 115 primer combinations generating 1328 amplified products. Budak et al. (2004) used SRAP markers for estimation of genetic diversity and phenetic relationship in natural and domesticated populations of turfgrass. Their initial screening showed SRAP is highly polymorphic and more informative when compared with AFLP, RAPD and SSR markers. They applied SRAP markers to obtain an overview of genetic diversity and phenetic relationships present among cool season (C3) and warm season (C4) turfgrass species and their relationship with other Gramineae species were tested. They found widespread genetic variation among C3 and C4 turfgrass species. However, present study suggested genetic variation and phylogenetic relationship between 11 Trichoderma isolates which were differentially potential for antagonistic biocontrol activity against phytopathogen S. rolfsii.
Further, the SRAP markers were reported for various application of molecular biology viz. construction of a detailed linkage map for Brassica oleracea (Okazaki et al. 2007; Gao et al. 2007), mapping QTL for cotton fibre quality (Lin et al. 2005), the genes for lateral branch traits in cucumber (Wang et al. 2005), constructing a linkage map of cotton (Zhongxu et al. 2003), examine comparative genetics of different species (Fernando et al. 2006), tagging of the gene for resistance to fusarium wilt in eggplant (Mutlu et al. 2008).
Trichoderma virens NBAII Tvs 12 is evidently best antagonist inhibiting 87.91 % of growth of test pathogen S. rolfsii followed by T. koningii—MTCC796 (67.03 % inhibition). The best antagonists Tvs 12 inhibited growth of pathogen through mycoparasitism and continued to grow over pathogen without formation of inhibition zone during antagonism. However, T. koningii—MTCC 796 suppressed the growth of pathogen with formation of inhibition zone (strong antibiosis) and did not overgrow on pathogen. The growth inhibition of test pathogen was negatively correlated with sclerotium formation and lipid peroxides products in antagonists plate. The GC–MS profile of best Tvs 12 antagonist identified 27 bioactive constituents while MTCC 796 evident 19 bioactive compounds using cell wall of pathogen as carbon source. The molecular diversity analysis using SRAP marker clearly distinguished antagonist Trichoderma and test pathogen with minimum 19 % similarity. However, within Trichoderma antagonists, the clustering pattern were found according to biocontrol activity of Trichoderma rather than species.
Conclusion
Among the 11 isolates of Trichoderma, mycoparasitic T. virens NBAII Tvs 12 was the best bioagent inhibited maximum growth of fungal pathogen S. rolfsii. The best antagonists Tvs 12 inhibited growth of pathogen through mycoparasitism and continued to grow over pathogen without formation of inhibition zone during antagonism. However, T. koningii—MTCC 796 suppressed the growth of pathogen with formation of inhibition zone (strong antibiosis) and did not overgrow on pathogen. The growth inhibition of the test pathogen was negatively correlated with sclerotium formation and lipid peroxides products in antagonists plate. The antagonists secretary antifungal and antioxidant bioactive constituents responsible for disintegration and disruption of pathogen cell wall were identified with GC–MS profile. The SRAP based molecular analysis indicates significant genetic diversity (19–68 %) present in the Trichoderma antagonists and the best antagonist Tvs 12 were distinguished with 18 unique fragments generated by 15 SRAP markers. The Tvs 12 strain might have a significant role in the control of stem rot disease by reducing the virulence of S. rolfsii in the groundnut rhizosphere.
References
Abang MM, Winter S, Green TKR, Hoffmann P, Mignpuna HD, Wolf GA (2002) Molecular identification of Colletotrichum gloeosporioides causing yam anthracnose in Nigeria. Plant Pathol 51:63–71
Agrawal T, Kotasthane AS (2009) Assessment of diversity in isolates of Trichoderma species from soils of Chhattisgarh region in Central India using RAPD markers. J Mycol Plant Pathol 139(3):484–489
Akpuaka A, Ekwenchi MM, Dashak DA, Dildar A (2013) Biological activities of characterized isolates of n-hexane extract of Azadirachta indica (Neem) leaves. Nat Sci 11(5):141–147
Anand S, Reddy J (2009) Biocontrol potential of Trichoderma spp. against plant pathogens. Int J Agric Sci 1(2):30–39
Anderson JA, Churchill GA, Sutrique JE, Tanksley SD, Sorrels ME (1993) Optimizing parental selection for genetic linkage maps. Genome 36:181–186
Bansode R, Bhat S, Krishnaraj PU, Nishani S (2011) Detection of antifungal activity and molecular diversity in Trichoderma isolates using SRAP markers. J Mycol Pl Pathol 41(1): 82–87
Bartlett RJ (1959) Phosphorus assay in column chromatography. J Biol Chem 234:466–471
Budak H, Shearman RC, Parmaksiz I, Gaussoin RE, Riordam TP, Dweikat I (2004) Molecular characterization of Buffalograss germplasm using sequence—related amplified polymorphism markers. Theor Appl Genet 108:328–334
Dennis CJ, Webster J (1971) Antagonism properties of species groups of Trichoderma, III. Hyphal Interaction. Trans Br Mycol Soc 57:363–369
Fernando WGD, Zhang JX, Dusabenyagasani M, Guo XW, Ahmed H (2006) Genetic diversity of Gibberella zeae isolates from Manitoba. Plant Dis 90:1337–1342
Fisher R, Yates N (1948) Statistical methods for research workers. In: Crew FAE, Ward Cutler D (eds) Biological monograph and manuals, 12th edn. Oliver and Boyd, Edinburg, London, pp 130–131
Gajera HP, Bambharolia RP, Patel SV, Khatrani TJ, Goalkiya BA (2012) Antagonism of Trichoderma spp. against Macrophomina phaseolina: evaluation of coiling and cell wall degrading enzymatic activities. J Plant Pathol Microb 3:149
Gajera HP, Savaliya DD, Patel SV, Goalkiya BA (2015) Trichoderma viride induces pathogenesis related defense response against rot pathogen infection in groundnut (Arachis hypogaea L.). Infect Genet Evol 34:314–325
Gao M, Li G, Yang B, Qiu D, Farnham M, Quiros C (2007) High- Density Brassica oleracea linkage map: identification of useful new linkages. Theor Appl Gene 115:277–287
Georgiou CD (1997) Lipid peroxidation in Sclerotium rolfsii: a new look into the mechanism of sclerotial biogenesis in fungi. Mycol Res 101:460–464
Georgiou CD, Tairis N, Sotiropoulou A (2000) Hydroxyl radical scavengers inhibit lateral-type sclerotial differentiation and growth in phytopathogenic fungi. Mycologia 92:825–834
Georgiou CD, Zervoudakis G, Tairis N, Kornaros M (2001) Beta-carotene production and its role in sclerotial differentiation of Sclerotium rolfsii. Fungal Genet Biol 34:11–20
Georgiou CD, Zervoudakis G, Petropoulou PK (2003) Ascorbic acid might play a role in sclerotial differentiation of Sclerotium rolfsii. Mycologia 95:308–316
Georgiou CD, Patsoukis Ν, Papapostolou Ι, Zervoudakis G (2006) Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integr Comp Biol 46:691–712
Ghislain M, Zhang D, Fajardo D, Huamann Z, Hijmans RH (1999) Marker assisted sampling of the cultivated Andean potato Solanum phureja collection using RAPD markers. Genet Resour Crop Evol 46:547–555
Govindappa M, Prathap S, Vinay V, Channabasava R (2014) Chemical composition of methanol extract of endophytic fungi, Alternaria Sp. of Tebebuia Argentea and their antimicrobial and antioxidant activity. Int J Biol Pharm Res 5:861–869
Graham GC, Mayers P, Henry RJ (1994) A simplified method for the preparation of fungal genomic DNA for PCR and RAPD analysis. Biotechniques 16:49–50
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 251:567–576
Joshi D, Hooda KS, Bhatt JC (2008) Biocontrol potential of fungal antagonists from western Himalayan region against sclerotial plant pathogens. Pesticide Res J 20(1):1–5
Keszler A, Forgacs E, Kotai L (2000) Components in the fermentation broth of trichoderma atroviride by solid-phase extraction and gas chromatography–mass spectrometry. J Chromatogr Sci 38:421–424
Li G, Quiros F (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet 103(2):455–461
Lin Z, He D, Zhang X, Nie Y, Guo X, Feng C, Stewart J (2005) Linkage map construction and mapping QTL for cotton fibre quality using SRAP, SSR and RAPD. Plant Breed 124:180–187
Manorenjitha MS, Norita AK, Norhisham S, Asmawi MZ (2013) GC–MS analysis of bioactive components of Ficus religiosa (Linn.) stem. Int J Pharm Bio Sci 4(2):99–103
Mayee CD, Datar VV (1988) Diseases of groundnut in the tropics. Review Trop Plant Pathol 5:169–198
Mutlu N, Boyaca FH, Gocmen M, Abak K (2008) Development of SRAP, SRAPRGA, RAPD and SCAR markers linked with a Fusarium wilt resistance gene in eggplant. Theor Appl Genet 117:1303–1312
Narayanasamy PD, Saravana M (2009) RAPD analysis of Trichoderma and it’s antagonistic affect with Pseudomonas fluorescens. J Plant Pathol Microb 20(4):223–233
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
O’Neill NR, Van Berkum P, Lin JJ, Kuc J, Ude GN, Kenworthy W, Saunders JA (1997) Application of amplified restriction fragment length polymorphism for genetic characterization of Colletotrichum pathogens of alfalfa. Phytopathology 87:745–750
Okazaki K, Sakamoto K, Kikuchi R, Saito A, Togashi E, Kuginuhi Y, Matsumoto S, Hiras M (2007) Mapping and characterization of FLC homologs and QTL analysis of flowering time in Brassica oleracea. Theor Appl Genet 114:595–608
Pan S, Bhagat S (2007) Effect of substrates physical factors on mass multiplication of Trichoderma harzianum in management of seedling blight of jute. Biol Control 21:127–136
Papapostolou I, Georgiou CD (2010) Superoxide radical induces sclerotial differentiation in filamentous phytopathogenic fungi: a superoxide dismutase mimetics study. Microbiology 156:960–966
Patsoukis Ν, Georgiou CD (2008) Thiol redox state and oxidative stress affect sclerotial differentiation of the phytopathogenic fungi Sclerotium rolfsii and Sclerotinia sclerotiorum. J Appl Microbiol 104:42–50
Rohlf FJ (1998) Numerical taxonomy and multivariate analysis system version 1.80 manual. Exeter Software, New York
Roy P, Amdekar S, Kumar A, Singh V (2011) Preliminary study of the antioxidant properties of flowers and roots of Pyrostegia venusta (Ker Gawl) Miers. BMC Complement Altern Med 11:69
Royse DJ, May B (1982) Use of isozyme variation to identify genotypic classes of Agaricus brunnescens. Mycologia 74:93–102
Siddiquee S, Cheong BE, Taslima K, Kausar H, Hasan M (2012) Separation and identification of volatile compounds from liquid cultures of Trichoderma harzianum by GC–MS using three different capillary columns. J Chromatogr Sci 50:358–367
Sinclair JB, Dhingra OD (1985) Basic plant pathology method. CRC Press, Inc., Corporate Blud, Boca Rotan, Florida
Sokol S, Kaldorf M, Bothe H (1999) Molecular characterization and taxonomic affinities of species of the white rot fungus Ganoderma. Mycologia 54:314–318
Tseng SH, Liu SY, Yang HH, Lo CT, Peng KC (2008) Proteomic study of biocontrol mechanisms of Trichoderma harzianum ETS 323 in response to Rhizoctonia solani. J Agric Food Chem 56:6914–6922
Vincent JM (1927) Distortion of fungal hyphae in the presence of certain inhibitor. Nature 2:159–850
Wang G, Junsong P, Xiaozun L, Maunle HF, Zhong W (2005) Construction of cucumber genetic linkage map with SRAP markers and location of the genes for lateral branch traits. Life Sci 148:213–220
Yogendra S, Singh Y (2002) Biological control of Sclerotium blight of Gmelina rborea. Indian For 128(1):41–44
Zhongxu L, Xianlong Z, Yichun N, Daohua H, Maoqing WU (2003) Construction of a genetic linkage map for cotton based on SRAP. Chin Sci Bull 48(19):2063–2067
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kupiec.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hirpara, D.G., Gajera, H.P., Bhimani, R.D. et al. The SRAP based molecular diversity related to antifungal and antioxidant bioactive constituents for biocontrol potentials of Trichoderma against Sclerotium rolfsii Scc.. Curr Genet 62, 619–641 (2016). https://doi.org/10.1007/s00294-016-0567-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-016-0567-5