Abstract
Osmoregulation encompasses active homeostatic processes that ensure proper cell volume, shape and turgor as well as an intercellular milieu optimal for the diverse biochemical processes. Recent studies demonstrate that yeast cells operate within a tight window of cellular water concentrations that still allows rapid diffusion of biomolecules while already moderate cell compression following hyper-osmotic stress leads to macromolecular crowding and a slow-down of cellular processes. Yeast cells accumulate glycerol as compatible osmolyte under hyper-osmotic stress to regain cell volume and turgor and release glycerol following a hypo-osmotic shock. The high osmolarity glycerol (HOG) response pathway controls glycerol accumulation at various levels, where each mechanism contributes to the temporal and quantitative pattern of volume recovery: inhibition of glycerol efflux, direct activation of the first enzyme in glycerol biosynthesis, stimulation of glycolytic flux as well as upregulation of expression of genes encoding enzymes in glycerol biosynthesis and an active glycerol uptake system. The HOG mitogen-activated protein kinase (MAPK) pathway communicates with the other yeast MAPK pathways to control cell morphogenesis. Cross-talk between the MAPK pathways has recently been used to re-wire osmostress-controlled expression of glycerol biosynthesis genes from Hog1 to Kss1-Fus3. The results of this study further illustrate the key importance of glycerol accumulation under osmostress and allow studying Hog1-dependent and independent processes as well as redundancy and robustness of the MAPK system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Probably all cells, even individual cells in multi-cellular organisms, such as human beings, are capable of sensing, responding and adapting to changes in the water activity of their environment. Decreased water activity, i.e. diminished availability of free water, in the surrounding medium causes water to follow its concentration gradient and diffuse out of cells. This leads to cell shrinking and cells experience hyper-osmotic stress (Hohmann 2002; Wood 2011). Two recent studies demonstrate that even relatively moderate volume loss causes a slow-down of cellular processes in yeast cells (Babazadeh et al. 2013; Miermont et al. 2013). It has been known for some time that the stronger the stress level (i.e. the higher the concentration of osmoticum) the longer it takes cell to respond (Van Wuytswinkel et al. 2000). For instance, the Hog1 protein kinase (see further) requires more time to accumulate in the nucleus at 0.8 M NaCl as compared to 0.4 M NaCl (Babazadeh et al. 2013). Also the nuclear accumulation of unrelated regulators as well as vesicular trafficking is delayed when cells are exposed to strong osmostress (Miermont et al. 2013). The cytosolic diffusion of Hog1 is dramatically diminished when cells are treated with 0.8 M NaCl, which causes an initial ca 40 % drop of the cell volume. Already 0.4 M NaCl, which causes an initial 20 % drop of the cell volume, affects Hog1 diffusion (Babazadeh et al. 2013). These observations suggest that the yeast cell operates within a narrow window of water concentrations that still allows diffusion processes to occur at a rate required for optimal functioning of the cellular biochemical and molecular processes. A rather moderate drop of the water concentration, however, appears to lead to macro-molecular crowding. As a consequence, free diffusion is progressively limited, resulting in a slow-down of the molecular processes (Mika and Poolman 2011; Wood 2011). Therefore, osmoregulation not only is critical for cell shape, turgor and morphogenesis but also for ensuring a milieu optimal for intracellular dynamics. Moreover, the observation that already moderate hyper-osmotic stress appears to cause macro-molecular crowding provides scope for potential mechanisms of intracellular osmosensing. The stimulus that cells perceive as osmostress is still poorly understood (Poolman et al. 2002; Wood 2011). Known osmosensors are commonly transmembrane proteins that may monitor membrane stretching/curving or altered interactions between the plasma membrane and the cell wall. However, fungi seem to possess also intracellular osmosensing histidine kinases (Meena et al. 2010). Perhaps the stimulus that those proteins sense may be related to macro-molecular crowding and/or restriction of diffusion.
Glycerol as compatible solute in yeast
The accumulation of compatible solutes to compensate for water loss is a universal strategy of cells (Yancey et al. 1982). Solutes are compatible because they are inert with respect to intracellular processes and either replace water or/and revert the water concentration gradient and drive water back into cells. The nature of solutes that is used by different organisms differs widely (Yancey et al. 1982). Polyols are commonly used in fungi and the yeast S. cerevisiae uses glycerol as compatible solute when grown on sugar-containing medium (Blomberg and Adler 1992; Hohmann 2002).
Glycerol plays diverse roles in the physiology of S. cerevisiae (for glycerol metabolic pathways see Fig. 1) (Hohmann 2002; Nevoigt and Stahl 1997). It can serve as a source for carbon and energy and is found in yeast’s natural environment mainly as a product of yeast and fungal metabolism. Glycerol is taken up via the H+-coupled active uptake system Stl1 (Ferreira et al. 2005) and converted by the Gut1 glycerol kinase and the Gut2 FAD-dependent glycerol dehydrogenase to the glycolytic and gluconeogenic intermediate dihydroxyacetonephosphate, DHAP (Nevoigt and Stahl 1997). Glycerol is also normally produced by yeast as a by-product of sugar metabolism. In a normal wine fermentation, for instance, glycerol is initially produced as a response to osmostress because of the high sugar concentration in the must. At later stages of the fermentation, however, when oxygen is absent and hence respiration cannot be used for the re-oxidation of NADH, part of that NADH is re-oxidised via glycerol production from DHAP (Scanes et al. 1998). This is necessary because part of the glycolytic intermediates are used for biosynthetic pathways and hence not sufficient pyruvate is available to re-oxidise NADH via ethanol production (Ansell et al. 1997). Glycerol production may also help to balance the cytosolic level of Pi at the switch from gluconeogenic to glycolytic metabolism (Thevelein and Hohmann 1995; van Heerden et al. 2014). Pi is a substrate for the glyceraldehyde kinase reaction in the lower part of glycolysis. For instance, a tps1Δ mutant, which suffers from accumulation of sugar phosphatase and depletion of Pi can be suppressed by overexpression of the first enzyme in glycerol biosynthesis (Thevelein and Hohmann 1995).
Yeast glycerol metabolism. Glycerol is produced from the glycolytic intermediate dihydroxyacetone phosphate DHAP (glycolysis only sketched) catalysed by NAD-dependent glycerol-3-phosphate dehydrogenase Gpd1 and Gpd2 and glycerol-3-phosphatase, Gpp1 and Gpp2. There is an alternative pathway via dihydroxyacetone, which, however, does not seem to play a role in glycerol production under osmostress. Glycerol is exported via Fps1. Glycerol is assimilated by yeast following active uptake via Stl1. Glycerol is phosphorylated by glycerol kinase, Gut1, and oxidised by FAD-dependent glycerol-3-phosphate dehydrogenase to DHAP, which enters glycolysis/gluconeogenesis. Glycerol production and utilisation probably do never occur simultaneously because the utilisation pathway is repressed by glucose
Glycerol is produced in two dedicated steps from DHAP by NAD-dependent glycerol-3-phosphate dehydrogenase and glycerol-3-phosphatase (Hohmann 2002; Nevoigt and Stahl 1997). Glycerol-3-phosphate dehydrogenase is encoded by two genes: GPD1, which encodes the osmostress-specific isoform as well as GPD2, which plays an important role in NADH redoxidation under anaerobic conditions. While deletion of GPD1 causes moderate sensitivity to hyper-osmotic stress and deletion of GPD2 causes slower growth in the absence of oxygen, only deletion of both genes causes strong osmo-sensitivity and complete growth arrest under anaerobicity (Ansell et al. 1997; Hohmann 2002). Hence, the two genes/enzymes have distinct but overlapping functions. Glycerol-3-phosphatase is also encoded by two isogenes, GPP1 and GPP2, which, however, seem to have largely redundant roles. The expression of GPD1 as well as GPP1 and GPP2 is stimulated by hyper-osmotic stress (Albertyn et al. 1994; Ansell et al. 1997; Hohmann 2002; Nevoigt and Stahl 1997; Norbeck et al. 1996).
To fine tune the intracellular glycerol content and to rapidly release glycerol following hypo-osmotic stress yeast employs the Fps1 glycerol channel, an aquaglyceroporin (Ahmadpour et al. 2014). Deletion of FPS1 renders yeast cells sensitive to hypo-osmotic shock as well as to anaerobicity, suggesting that it is the main or sole pathway for glycerol out of the cells. There is another aquaglyceroporin encoded by the yeast genome, Yfl054c. It appears, however, that it does not seem to serve a function redundant to that of Fps1 (Ahmadpour et al. 2014; Oliveira et al. 2003).
The high osmolarity glycerol HOG pathway
Glycerol accumulation is controlled mainly by the HOG pathway, although hog1Δ cells still accumulate ca 50 % of the amount of glycerol accumulated by wild type cells (Brewster and Gustin 2014; Chen and Thorner 2007; Hohmann 2002, 2009; Saito and Posas 2012; Sheikh-Hamad and Gustin 2004). As will be discussed later this may be due to other protein kinases taking over in the absence of Hog1. Mutations that inactivate the HOG pathway render yeast cells highly sensitive to hyperosmotic stress while mutations causing uncontrolled HOG pathway activity are lethal.
The HOG Mitogen-Activated Protein Kinase MAPK pathway is one of the best studied and characterised signalling pathways in eukaryotic cells. Approaches employing genetics, cell and molecular biology as well as biochemistry and systems biology have provided a wealth of detailed molecular and systems level information about this signalling system (Chen and Thorner 2007; Saito and Posas 2012).
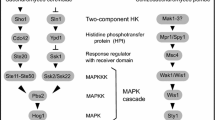
The HOG pathway has been subject of numerous reviews [for instance (Chen and Thorner 2007; Hohmann 2002; Saito and Posas 2012)]. Briefly, the pathway consist of two branches, the Sln1 branch and the Sho1 branch (Fig. 2). The Sln1 branch is controlled by a sensor histidine kinase, the eukaryotic version of bacterial two-component systems. Sln1 has four transmembrane domains and is located in the plasma membrane. Sln1 is a negative regulator of the Sln1 HOG pathway branch. Under normal conditions, the Sln1 histidine kinase is active, resulting in auto-phosphorylation and transfer of the phosphate group via the phosphotransfer protein Ypd1 to the response regulator protein Ssk1, which is kept in an inactive state by phosphorylation. Deletion of SLN1 or YPD1 causes constitutive Hog pathway activation, which is lethal to yeast cells. Following hyper-osmotic shock Sln1 is inactivated and Ypd1 and eventually Ssk1 become dephosphorylated. In the dephosphorylated form Ssk1 interacts with the MAPKKKs Ssk2 and Ssk22 and relieves their auto-inhibition. Ssk2 and Ssk22 auto-phosphorylate and activate themselves, which enables them to phosphorylate and activate the MAPKK Pbs2. Active Pbs2, in turn, phosphorylates Hog1 on two adjacent sites, leading to activation of Hog1 and its accumulation in the nucleus. While nuclear Hog1 is essential for stimulating gene expression, it also has numerous cytosolic targets. In fact, if Hog1 is prevented from transfer to the nucleus, cells are still capable of adapting to hyper-osmotic stress, highlighting the importance of cytosolic targets (Westfall et al. 2008). Nuclear Hog1 stimulates initiation of transcription by interacting with DNA-binding proteins, such as Hot1 and is also involved in subsequent steps of transcription (de Nadal et al. 2011). Recently, it has been shown that Hog1 also may control gene expression via transcription of lncRNAs (Nadal-Ribelles et al. 2014), as discussed in detail elsewhere in this issue of Current Genetics (Sole et al. 2014).
The yeast HOG pathway and its effects on glycerol accumulation. The HOG-pathway consists of two branches that converge of the MAPKK Pbs2 to phosphorylate and activate (see text for further details). Hog1 appears to control closing of the glycerol export channel Fps1, indirectly stimulate activity of Gpd1 in glycerol production as well as glycolytic flux via Pfk26 activation. Hog1 also mediates alleviated expression of the genes encoding enzymes in glycerol production, Gpd1 and Gpp2, as well as the active glycerol uptake system Stl1. Hog1 also seems down-regulate expression of the aquaporin Aqy2 and thereby restrict water loss and potentially cell adhesion and invasion (Furukawa et al. 2009). Dotted lines refer to an increase in the amount of protein, normal lines refer to regulatory effects mediated by phosphorylation/dephosphorylation
The Sho1 branch is named after the Sho1 protein, which is a plasma membrane-localised scaffold protein for interaction with different components of the pathway. The sensors in the Sho1 branch probably are the single membrane-spanning mucin-like proteins Hkr1 and Msb2. Stimulation of those sensors results, in a presently poorly characterised manner, in a series of protein interaction changes, which involve Msb2 and Hkr1, Sho1, the G-protein Cdc42 and the protein kinases Ste20 and Cla4. The association with Cdc42 enables Ste20-Cla4 to phosphorylate and activate the MAPKKK Ste11. In the process, also Pbs2, which in addition to its role as a MAPKK also serves as a scaffold protein, is recruited to the cell surface by interaction with Sho1. Ste11, in turn, is recruited to the membrane by its association partner Ste50 by interaction Cdc42, Sho1 and another membrane protein, Opy2. Activated Ste11 phosphorylates and activates Pbs2, which in turn phosphorylates and activates Hog1 (Saito and Posas 2012).
Several protein phosphatases appear to function as negative regulators of the HOG pathway by targeting Hog1: the PP2C Ptc1 as well as the phospho-tyrosine phosphatases Ptp2 and Ptp3. It appears that Ptc1 and Ptp3 are located in the cytosol, while Ptp2 is located primarily in the nucleus. Double mutants lacking both PTC1 and PTP2 are inviable because of constitutive HOG pathway activation even under non-stress conditions, demonstrating that the HOG pathway displays significant basal activity (Hohmann 2002; Saito and Posas 2012). Several additional pieces of evidence illustrate the high basal signalling activity, which appears to be solely contributed by the Sln1 branch and down-regulation of which depends on Hog1 in an unknown manner: a kinase-dead Hog1 is constitutively phosphorylated and an analogue-sensitive Hog1 variant becomes phosphorylated upon inhibition. The basal signalling activity appears to be important for fast and efficient responses (Macia et al. 2009).
The two branches of the HOG pathway appear to function independently. It is possible that the different sensing systems perceive different types of stimuli as a result of osmotic stress, such as membrane stretching or altered contact between the plasma membrane and the cell wall. The Sln1 branch is more sensitive to osmotic changes, i.e. it displays a lower threshold. The Sln1 branch alone can mediate full osmo-resistance while mutants with only the Sho1 branch are sensitive to high stress levels (Hohmann 2002; Saito and Posas 2012). In a wild type situation the Sho1 branch probably contributes little to the overall response, suggesting that it also has other roles. In fact, in filamentous fungi it appears that the Sho1 branch does not lead to Pbs2 activation and hence this branch appears to have a completely different role, for instance in sensing contact with surfaces (Furukawa et al. 2005). This makes also sense in yeast, because the Sho1 branch shares components with the pheromone response and invasive growth pathways. Invasive growth is obviously associated with surface contact. In fact, in a hog1Δ mutant the osmostress signal derived from the Sho1 branch is directed towards Fus3 and Kss1, the MAPKs of the pheromone and invasive growth pathways (Davenport et al. 1999). In a hog1Δ mutant, osmostress leads to Fus3-Kss1 mediated gene expression responses as well as development of cell progressions mimicking invasive growth (O’Rourke and Herskowitz 1998, 2004; Rep et al. 2000). It has been shown that deletion of KSS1 prevents this behaviour and partly suppresses the osmo-sensitivity of the hog1Δ mutant, indicating that osmostress signal diversion contributes to the osmo-sensitivity of the mutant [(Davenport et al. 1999) see further].
The HOG pathway controls glycerol accumulation at different levels
Glycerol accumulation is essential for yeast adaptation to hyper-osmotic stress since mutants that block the synthesis of glycerol or that cause leakage of glycerol out of the cell confer an osmo-sensitive phenotype (Hohmann 2002). Glycerol accumulation and the associated volume recovery also seems to confer the main feedback control mechanism on HOG pathway activation (Klipp et al. 2005). Stimulation of Hog1 phosphorylation following a hyper-osmotic shock is transient and the period of Hog1 phosphorylation correlates with the degree of osmostress (stronger stress—longer activation) and with the time it takes that volume recovery starts after the initial volume loss (Babazadeh et al. 2013). As predicted by simulation using a mathematical model, mutants with decreased ability to accumulate glycerol show prolonged HOG pathway activation and mutants with increased capacity to accumulate glycerol show shorter periods of HOG pathway activation (Klipp et al. 2005; Krantz et al. 2004). While HOG pathway intrinsic feedback control mechanisms appear to exist (Macia et al. 2009; Sato et al. 2003), pathway down-regulation following hyper-osmotic shock is clearly linked to glycerol accumulation and volume recovery. Since glycerol accumulation is controlled by Hog1 at different steps those mechanisms constitute per se feedback mechanisms on Hog1 activity (Hohmann 2009).
Recently we have investigated the temporal and quantitative contributions of the different mechanisms by which Hog1 controls glycerol accumulation (Petelenz-Kurdziel et al. 2013). We employed mathematical modelling and simulation supported by quantitative experimental data. This analysis provides a more detailed picture of the roles of different control mechanisms. The analysis also showed that glycerol accumulation is accompanied by a re-routing of metabolism at the expense of biomass formation.
Briefly (Fig. 2), it appears that closure of Fps1 to prevent glycerol efflux, activation of glycerol-3-phosphate dehydrogenase Gpd1 as well as stimulation of glycolysis by activation of phosphofructose-2-kinase provide means for starting glycerol accumulation rapidly by altering activity of existing proteins. Then gene expression changes lead to increased amounts of glycerol biosynthetic enzymes as well as increased capacity for glycerol uptake, especially in the absence of glucose as carbon source.
Control of Gpd1
In addition to increasing the capacity for glycerol production by raising the amount of the biosynthetic enzymes, the activity of Gpd1 is directly controlled by phosphorylation (Lee et al. 2012; Oliveira et al. 2012). The two TORC2-dependent protein kinase Ypk1 and Ypk2 phosphorylate Gpd1 on Ser24 (Lee et al. 2012). At the same time it appears that in fact four adjacent sites, Ser23, Ser24, Ser25 and Ser27, are phosphorylated in Gpd1 and that only mutating all four sites to Ala results in an enzyme that is two-fold more active than wild type (Oliveira et al. 2012). Following hyper-osmotic shock Ypk1 and 2 are apparently no longer stimulated by TORC2 and Gpd1 becomes dephosphorylated by an unknown protein phosphatase, leading to its activation (Lee et al. 2012). Increased transcription of GPD1 and activation of the enzyme both appear to contribute to glycerol production. It has previously been observed that glycerol production following osmostress is kicked on well before enzyme production increases (Klipp et al. 2005) hence activation of the existing enzyme pool may be one mechanism by which rapid glycerol production following osmoshock is achieved. The information of Gpd1 activation following osmoshock was not available at the time of our modelling effort and hence its temporal and quantitative contributions could not be further studied at that point.
Control of glycolytic flux
Key enzymes of glycolysis are controlled by allosteric mechanisms. Phosphofructokinase is targeted by a number of molecules that affect the enzyme activity and fructose-2.6-bisphosphate is the probably most potent activator of the enzyme. The cellular level of Fru-2.6-bP is about 1,000× lower than that of the glycolytic intermediate Fru-1.6-bP (Muller et al. 1997). Fru-2.6-bP is produced by a dedicated phosphofructo-2-kinase encoded by the genes PFK26 and PFK27 (Boles et al. 1996). Pfk26 appears to be the target of a number of different protein kinase such as protein kinase A, the Slt2 MAPK and Hog1 (Dihazi et al. 2001, 2003, 2004). Stimulation of Pfk26 by Hog1 has the potential to increase glycolytic flux. Modelling and simulation suggests that the main purpose for this activation may be stabilisation of downstream glycolytic flux rather than increased glycerol production (Petelenz-Kurdziel et al. 2013). In other words, the increased flux due to PFK activation may help compensating for the flux that is redirected towards glycerol.
Control of Fps1-mediated glycerol efflux
The glycerol efflux aquaglyceroporin is rapidly closed following hyper-osmotic shock in order for the glycerol produced to be kept inside the cell. Mutations of Fps1 where closure is impaired result in poor glycerol accumulation, excretion of glycerol to the growth medium and glycerol overproduction in an attempt by the cell to compensate for glycerol loss (Ahmadpour et al. 2014). Modelling and simulation as well as experimentally determined glycerol accumulation profiles suggest that Fps1 closure is one of the first events and especially important for the rapid onset of glycerol accumulation following osmoshock (Petelenz-Kurdziel et al. 2013).
Fps1 is an unusual aquaglyceroporin in that it possesses long (ca 230 and 250 amino acids, respectively) extension N- and C-terminal of the core domain with its six transmembrane domains. These extensions have been shown to be required for Fps1 closure since deletion of either domain renders Fps1 hyperactive (Ahmadpour et al. 2014). There are conflicting results about the involvement of Hog1 in Fps1 closure. Fps1 appears to be the entry route for two toxic substances, arsenite as well as acetic acid. Also those stress conditions stimulate the Sln1 branch of the HOG pathway in a manner that is not well understood. While it appears to be well documented that Hog1 controls Fps1 via phosphorylation of Thr231 following arsenite and acetic acid treatment (Mollapour and Piper 2007; Thorsen et al. 2006), Hog1 seems to be dispensable for Fps1 closure after hyperosmotic shock (Luyten et al. 1995). Still, mutants making Thr231 in the long N-terminal extension of Fps1 unphosphorylatable as well as certain mutants in the well conserved motif around this residue or ablation of the entire N-terminal extension all cause hyperactive Fps1, glycerol loss and osmo-sensitivity (Ahmadpour et al. 2014). One explanation for those conflicting observations could be that under osmostress another protein kinase partly or completely takes over the role of Hog1 when HOG1 is deleted.
Recent work (Lee et al. 2013) demonstrates that Hog1 binds to the conserved sequence in the N-terminal extension, which contains a MAPK docking site to phosphorylate Rgc2 (as well as probably also the redundant Rgc1). Rgc2 binds to the C-terminal extension of Fps1 within a plekstrin-like sequence around residue 615. Rgc2 appears to keep Fps1 open. Following phosphorylation Rgc2 dissociate from Fps1, which results in Fps1 closure (Lee et al. 2013). Probably the C-terminal domain, or both the C-terminal and N-terminal domains, fold back to close the transmembrane pore as was suggested by certain point mutations at the cytosolic mouth of the pore (Geijer et al. 2012; Karlgren et al. 2004). However, the details of the pore closing process are not understood and X-ray structural information is unfortunately not available.
In addition to Thr231, there is a potential conserved MAPK phosphorylation site in the C-terminal domain of Fps1 (S537), which seems to be important for arsenite efflux and hence may be involved in keeping Fps1 in an open state [(Hedfalk et al. 2004) and our unpublished data]. There is a diverse body of evidence that suggests that the Slt2 MAPK, which is stimulated by hypo-osmotic shock, cell wall stress and morphogenic changes (Levin 2011), plays a role in opening Fps1 (see also further) under arsenite stress (our unpublished data) but also during mating and perhaps during hypo-osmotic shock (Baltanas et al. 2013; Philips and Herskowitz 1997). Whether Slt2 phosphorylates Fps1 on S537 and what the functional role of such phosphorylation might be in light of the recent results from the Levin lab described above, remains to be elucidated.
Interestingly, it appears that Fps1 like aquaglyceroporins as characterised by the conserved functional domains in the termini, are restricted to yeasts closely related to S. cerevisiae and have not been found in genomes from other fungi (Pettersson et al. 2005). Although Fps1 plays a central role in yeast osmoregulation and albeit its regulation seems to be complex, involving different conserved regulators, it appears to be restricted to a small group of group of organisms. Perhaps Fps1 and its regulation evolved in the context of employing glycerol as osmolyte in an environment with rapidly changing osmotic pressure.
Stimulation of gene expression
Hog1 mediates stimulated expression (de Nadal et al. 2011) of more than 100 genes (O’Rourke et al. 2002). Among those are the genes GPD1 and GPP2, to a lesser extent also GPP1, as well as STL1. In all those cases it appears that Hog1 collaborates with the DNA-binding protein Hot1 (Alepuz et al. 2003; Rep et al. 2000). While expression of GPD1 and GPP2 displays residual stimulation by osmostress in the absence of Hog1 or Hot1, osmostress-induced expression of STL1 is completely dependent on Hog1 and Hot1 (Rep et al. 1999, 2000).
It appears that yeast can adapt to high osmolarity even if gene expression changes are prevented by tethering Hog1 to the cytosol (Westfall et al. 2008). However, increased capacity to produce glycerol may play a role at later stages of the adaptation, especially to sustain growth in adapted cells. Yeast cells growing in high osmolarity medium, when Hog1 activity is already back to basal levels, clearly show increased levels of Gpd1 activity, which may confer the level of glycerol production required for growth (Klipp et al. 2005). This said, it also appears that the level of intracellular glycerol drops at later stages of batch cultures, suggesting that glycerol accumulation is an osmoadaptation strategy specifically employed by cells actively growing in glucose medium (Klipp et al. 2005).
In standard glucose medium the stimulated expression of STL1 may not play much of a role in osmoadaptation (Ferreira and Lucas 2007; Ferreira et al. 2005), in particular since there is no glycerol available in the growth medium. However, under conditions when glycerol production is impaired for one or the other reason (experimentally in deletion mutants, or in the absence of a fermentable carbon source in more natural conditions), active glycerol uptake through Stl1 may very well contribute to glycerol accumulation (Petelenz-Kurdziel et al. 2013). Expression of STL1 is very strongly upregulated upon osmostress in glucose medium but expression also falls back quickly [for instance (Alepuz et al. 2003)]. Expression of STL1 is also controlled by glucose repression as well as glucose inactivation of the protein product (Ferreira et al. 2005), which further indicates that active glycerol uptake may play a more important role in the absence of a fermentable carbon source.
Three MAPKs cooperate to control morphogenic changes in yeast
The HOG pathway is part of a signalling network that contains four MAPK: Fus3 and Kss1, Hog1, as well as Slt2 (Chen and Thorner 2007). Four pathways are characterised based on specific stimuli and specific responses: the pheromone response pathway (stimulus: mating pheromone; response: cell cycle arrest, morphogenic changes, mating; MAPK: Fus3 and Kss1), the invasive growth pathway (stimulus: probably nutrient starvation, perhaps also surface attachment; response: morphogenic and budding pattern changes; MAPK: Kss1 and Fus3), the HOG pathway (stimulus: osmotic changes; response: cell cycle arrest, glycerol accumulation) as well as cell wall integrity pathway (stimulus: hypo-osmotic stress and other stress factors, cell wall damage; response: stimulation of cell wall repair mechanisms; MAPK: Slt2). While these pathways were defined by specific stimulus/response relations they also communicate and cross-talk and they share components (for instance the protein kinases Ste20-Cla4 and Ste11 are shared by the pheromone response, invasive growth and HOG pathway Sho1-branch; some of the same protein phosphatases seem to act on all MAPKs). Two recent studies provide novel insight how these signalling systems cooperate and cross-talk and how such cross-talk can be employed for re-routing signalling.
The Colman-Lerner lab adapted yeast cells to hyper-osmotic stress (Baltanas et al. 2013). Under such conditions, then, the HOG pathway is down-regulated and cells maintain an appropriate glycerol level to counteract the hyper-osmotic conditions. Such cells were then treated with pheromone. As expected, pheromone activated the pheromone response pathway leading to phosphorylation of Fus3 and subsequently also to phosphorylation of Slt2. This had been observed previously: the Fus3-mediated morphogenic changes cause cell wall stress and Slt2 activation is required for mating (Buehrer and Errede 1997; Rajavel et al. 1999). However, unexpectedly, also the HOG pathway was activated under these conditions. Activation of the HOG pathway in osmoadapted, pheromone-treated cells was dependent on Slt2 and Fps1 (Baltanas et al. 2013).
Those data suggest the following scenario. Activation of Fus3 causes cell morphogenic/cell wall stress signals that stimulate Slt2. Stimulation of Slt2 in turn activates Fps1-mediated glycerol efflux (which was measured experimentally). In fact, Fps1 had previously been reported to be required for successful cell fusion during mating, probably to release, perhaps locally, turgor pressure to allow cell wall degradation and plasma membrane fusion without cell bursting (Philips and Herskowitz 1997). In osmo-adapted cells, however, opening of Fps1 and release of glycerol causes hyper-osmotic stress, because the growth medium osmolarity is still at an increased level. Therefore, the cell counteracts and activated the HOG pathway (Baltanas et al. 2013).
Although in this scenario, no direct cross-talk between the three MAPK pathways involved occurs, there are at least two interesting conclusions that can be drawn from those observations. First, the sequential activation of three MAPK occurs because the response mediated by one MAPK generates the stimulus for the subsequent pathway in the system: Fus3-mediated responses lead to Slt2 stimulation and Slt2 action appears to lead to Hog1 stimulation. While the sequential activation of three MAPK was observed in a rather specific experimental setup (although completely possible in nature), the interplay between the MAPK systems almost certainly occurs continuously to control cell morphogenesis and osmotic stability. Second, Fps1 appears to integrate different signals and plays a central role in the control of osmotic stability and morphogenic homeostasis. It appears to be controlled, directly or indirectly, by at least two MAPKs and is, based on mutant phenotypes, involved in the response to hyper- and hypo-osmotic stress as well as cell function during mating. There is presently no evidence that the HOG- and cell wall integrity pathways directly communicate, rather it appears that they influence each other through the intracellular level (Garcia-Rodriguez et al. 2005) and here in particular via Fps1, which may be a regulatory target for both Hog1 and Slt2.
Re-routed osmoregulation enables studying osmoadaptation without Hog1
Deletion of only one of the many genes upregulated in a Hog1-dependent manner following hyperosmotic shock causes an osmo-sensitive phenotype, like that of HOG1 does: GPD1 for glycerol-3-phosphate dehydrogenase. Encouraged by the previous observations that overexpression of GPD1 caused a shorter period of Hog1 activation (Krantz et al. 2004) and that osmostress activates in the hog1Δ mutant via the Sho1 branch Fus3-Kss1 (Hall et al. 1996) we set out to re-route osmostress signalling and establish a Hog1-independent osmoadaptation system. A hog1Δ strain (Fig. 3) that expresses under the Fus3-Kss1 controlled FUS1 promoter an unphosphorylatable hyperactive version of Gpd1 as well as Gpp2 displayed glycerol accumulation and volume recovery profiles essentially as wild type, and could also grow under high osmolarity conditions (Babazadeh et al. 2014). Quite remarkably it appears that in this system Fps1 is closed even in the absence of Hog1 since expression of an N-terminal truncation of Fps1 caused osmo-sensitivity and deletion of FPS1 did not affect osmotolerance of the strain. Most probably another (MAP) kinase takes over the role of Hog1 in this scenario but it is neither Fus3 nor Kss1, since deletion of these kinase does not lead to osmo-sensitivity in a hog1Δ mutant that overexpressed hyperactive Gpd1 from a strong constitutive promoter. Clearly more research is needed to understand the mechanisms that control Fps1 in a better way.
In the re-routed Hog1-independent osmoadaptation system Ste11 activates Ste7 and Fus3/Kss1. GPP2 and GPD1*, which encodes the non-phosphorylatable, hyperactive Gpd1, are transcribed under control of the FUS1 promoter, which is controlled by Ste12 and Tec1. Fps1 still appears to be closed following hyperosmotic shock by an unknown mechanism
It has previously been reported by the Thorner lab that when Hog1-dependent transcription is prevented by tethering Hog1 to the cytosol, cells can adapt to moderate osmostress (Westfall et al. 2008). This observation, together with the properties of the re-routed osmoregulation system (Babazadeh et al. 2014) suggest that glycerol accumulation and volume recovery are critical in osmoadaptation and if those are possible they may overcome lack of many other Hog1-dependent effects, probably also because volume recovery diminishes the stimulus and hence signalling through the HOG system, which through its routing to Fus3 and Kss1 may cause deleterious effects. In the presence of Hog1, volume recovery allows deactivation of Hog1 and relieve of the cell cycle block mediated by active Hog1.
What is lacking?
In this mini-review I have focused on a couple of recent developments, with particular focus on the diverse roles of Hog1 in glycerol accumulation and volume recovery. Our understanding of the underlying molecular and systems level control mechanisms has increased further in recent years. Still we lack many details of the control of the Fps1 glycerol channel, which seems to play a central role in osmoregulation both under stress as well as during cell morphogenesis and which seems to integrate signals from diverse pathways. We also still lack a systems level understanding of the interaction of the diverse MAPK signalling pathways in controlling homeostasis and morphogenesis, especially under non-stress conditions. Under such conditions the activity changes may be rather subtle and short lived and hence escape population-level measurements. Single cell studies with sensitive probes and reporters as well as sophisticated tools to steer pathway activity in the absence of normal external stimuli may be needed to address those questions. Finally, the re-routed osmoregulation system offers opportunities to study osmoadaptation in yeast in the absence of the Hog1 kinase and may prove a useful tool to elucidate Hog1-dependent and independent mechanisms as well as redundancy and robustness of the MAPK system.
References
Ahmadpour D, Geijer C, Tamas MJ, Lindkvist-Petersson K, Hohmann S (2014) Yeast reveals unexpected roles and regulatory features of aquaporins and aquaglyceroporins. Biochim Biophys Acta 1840:1482–1491
Albertyn J, Hohmann S, Thevelein JM, Prior BA (1994) GPD1, which encodes glycerol-3-phosphate dehydrogenase is essential for growth under osmotic stress in Saccharomyces cerevisiae and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol 14:4135–4144
Alepuz PM, de Nadal E, Zapater M, Ammerer G, Posas F (2003) Osmostress-induced transcription by Hot1 depends on a Hog1-mediated recruitment of the RNA Pol II. EMBO J 22:2433–2442
Ansell R, Granath K, Hohmann S, Thevelein JM, Adler L (1997) The two isoenzymes for yeast NAD+-dependent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 have distinct roles in osmoadaptation and redox regulation. EMBO J 16:2179–2187
Babazadeh R, Adiels CB, Smedh M, Petelenz-Kurdziel E, Goksor M, Hohmann S (2013) Osmostress-induced cell volume loss delays yeast Hog1 signaling by limiting diffusion processes and by Hog1-specific effects. PLoS One 8:e80901
Babazadeh R, Furukawa T, Hohmann S, Furukawa K (2014) Rewiring yeast osmostress signalling through the MAPK network reveals essential and non-essential roles of Hog1 in osmoadaptation. Sci Rep 4:4697
Baltanas R, Bush A, Couto A, Durrieu L, Hohmann S, Colman-Lerner A (2013) Pheromone-induced morphogenesis improves osmoadaptation capacity by activating the HOG MAPK pathway. Sci Signal 6:ra26
Blomberg A, Adler L (1992) Physiology of osmotolerance in fungi. Adv Microb Physiol 33:145–212
Boles E, Gohlmann HW, Zimmermann FK (1996) Cloning of a second gene encoding 5-phosphofructo-2-kinase in yeast, and characterization of mutant strains without fructose-2,6-bisphosphate. Mol Microbiol 20:65–76
Brewster JL, Gustin MC (2014) Hog1: 20 years of discovery and impact. Sci Signal 7:re7
Buehrer BM, Errede B (1997) Coordination of the mating and cell integrity mitogen-activated protein kinase pathways in Saccharomyces cerevisiae. Mol Cell Biol 17:6517–6525
Chen RE, Thorner J (2007) Function and regulation in MAPK signaling pathways: lessons learned from the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1773:1311–1340
Davenport KD, Williams KE, Ullmann BD, Gustin MC (1999) Activation of the Saccharomyces cerevisiae filamentation/invasion pathway by osmotic stress in high-osmolarity glycogen pathway mutants. Genetics 153:1091–1103
de Nadal E, Ammerer G, Posas F (2011) Controlling gene expression in response to stress. Nat Rev Genet 12:833–845
Dihazi H, Kessler R, Eschrich K (2001) Phosphorylation and inactivation of yeast 6-phosphofructo-2-kinase contribute to the regulation of glycolysis under hypotonic stress. Biochemistry 40:14669–14678
Dihazi H, Kessler R, Eschrich K (2003) Glucose-induced stimulation of the Ras-cAMP pathway in yeast leads to multiple phosphorylations and activation of 6-phosphofructo-2-kinase. Biochemistry 42:6275–6282
Dihazi H, Kessler R, Eschrich K (2004) High osmolarity glycerol (HOG) pathway-induced phosphorylation and activation of 6-phosphofructo-2-kinase are essential for glycerol accumulation and yeast cell proliferation under hyperosmotic stress. J Biol Chem 279:23961–23968
Ferreira C, Lucas C (2007) Glucose repression over Saccharomyces cerevisiae glycerol/H+ symporter gene STL1 is overcome by high temperature. FEBS Lett 581:1923–1927
Ferreira C et al (2005) A member of the sugar transporter family, Stl1p is the glycerol/H+ symporter in Saccharomyces cerevisiae. Mol Biol Cell 16:2068–2076
Furukawa K, Hoshi Y, Maeda T, Nakajima T, Abe K (2005) Aspergillus nidulans HOG pathway is activated only by two-component signalling pathway in response to osmotic stress. Mol Microbiol 56:1246–1261
Furukawa K, Sidoux-Walter F, Hohmann S (2009) Expression of the yeast aquaporin Aqy2 affects cell surface properties under the control of osmoregulatory and morphogenic signalling pathways. Mol Microbiol 74:1272–1286
Garcia-Rodriguez LJ, Valle R, Duran A, Roncero C (2005) Cell integrity signaling activation in response to hyperosmotic shock in yeast. FEBS Lett 579:6186–6190
Geijer C et al (2012) Yeast aquaglyceroporins use the transmembrane core to restrict glycerol transport. J Biol Chem 287:23562–23570
Hall JP, Cherkasova V, Elion E, Gustin MC, Winter E (1996) The osmoregulatory pathway represses mating pathway activity in Saccharomyces cerevisiae: Isolation of a FUS3 mutant that is insensitive to the repression mechanism. Mol Cell Biol 16:6715–6723
Hedfalk K et al (2004) A regulatory domain in the C-terminal extension of the yeast glycerol channel Fps1p. J Biol Chem 279:14954–14960
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372
Hohmann S (2009) Control of high osmolarity signalling in the yeast Saccharomyces cerevisiae. FEBS Lett 583:4025–4029
Karlgren S, Filipsson C, Mullins JGL, Bill RM, Tamas MJ, Hohmann S (2004) Identification of residues controlling transport through the yeast aquaglyceroporin Fps1 using a genetic screen. Eur J Biochem 271:771–779
Klipp E, Nordlander B, Kruger R, Gennemark P, Hohmann S (2005) Integrative model of the response of yeast to osmotic shock. Nat Biotechnol 23:975–982
Krantz M, Nordlander B, Valadi H, Johansson M, Gustafsson L, Hohmann S (2004) Anaerobicity prepares Saccharomyces cerevisiae cells for faster adaptation to osmotic shock. Eukaryot Cell 3:1381–1390
Lee YJ, Jeschke GR, Roelants FM, Thorner J, Turk BE (2012) Reciprocal phosphorylation of yeast glycerol-3-phosphate dehydrogenases in adaptation to distinct types of stress. Mol Cell Biol 32:4705–4717
Lee J et al (2013) MAPK Hog1 closes the S. cerevisiae glycerol channel Fps1 by phosphorylating and displacing its positive regulators. Genes Dev 27:2590–2601
Levin DE (2011) Regulation of cell wall biogenesis in Saccharomyces cerevisiae: the cell wall integrity signaling pathway. Genetics 189:1145–1175
Luyten K, Albertyn J, Skibbe WF, Prior BA, Ramos J, Thevelein JM, Hohmann S (1995) Fps1, a yeast member of the MIP family of channel proteins, is a facilitator for glycerol uptake and efflux and is inactive under osmotic stress. EMBO J 14:1360–1371
Macia J, Regot S, Peeters T, Conde N, Sole R, Posas F (2009) Dynamic signaling in the Hog1 MAPK pathway relies on high basal signal transduction. Sci Signal 2:ra13
Meena N, Kaur H, Mondal AK (2010) Interactions among HAMP domain repeats act as an osmosensing molecular switch in group III hybrid histidine kinases from fungi. J Biol Chem 285:12121–12132
Miermont A, Waharte F, Hu S, McClean MN, Bottani S, Leon S, Hersen P (2013) Severe osmotic compression triggers a slowdown of intracellular signaling, which can be explained by molecular crowding. Proc Natl Acad Sci USA 110:5725–5730
Mika JT, Poolman B (2011) Macromolecule diffusion and confinement in prokaryotic cells. Curr Opin Biotechnol 22:117–126
Mollapour M, Piper PW (2007) Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27:6446–6456
Muller S, Zimmermann FK, Boles E (1997) Mutant studies of phosphofructo-2-kinases do not reveal an essential role of fructose-2,6-bisphosphate in the regulation of carbon fluxes in yeast cells. Microbiology 143:3055–3061
Nadal-Ribelles M, Sole C, Xu Z, Steinmetz LM, de Nadal E, Posas F (2014) Control of Cdc28 CDK1 by a stress-induced lncRNA. Mol Cell 53:549–561
Nevoigt E, Stahl U (1997) Osmoregulation and glycerol metabolism in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 21:231–241
Norbeck J, Påhlman AK, Akhtar N, Blomberg A, Adler L (1996) Purification and characterization of two isoenzymes of dl-glycerol-3-phosphatase from Saccharomyces cerevisiae. Identification of the corresponding GPP1 and GPP2 genes and evidence for osmotic regulation of Gpp2p expression by the osmosensing mitogen-activated protein kinase signal transduction pathway. J Biol Chem 271:13875–13881
Oliveira R, Lages F, Silva-Graca M, Lucas C (2003) Fps1p channel is the mediator of the major part of glycerol passive diffusion in Saccharomyces cerevisiae: artefacts and re-definitions. Biochim Biophys Acta 1613:57–71
Oliveira AP, Ludwig C, Picotti P, Kogadeeva M, Aebersold R, Sauer U (2012) Regulation of yeast central metabolism by enzyme phosphorylation. Mol Syst Biol 8:623
O’Rourke SM, Herskowitz I (1998) The Hog1 MAPK prevents cross talk between the HOG and pheromone response MAPK pathways in Saccharomyces cerevisiae. Genes Dev 12:2874–2886
O’Rourke SM, Herskowitz I (2004) Unique and redundant roles for HOG MAPK pathway components as revealed by whole-genome expression analysis. Mol Biol Cell 15:532–542
O’Rourke SM, Herskowitz I, O’Shea EK (2002) Yeast go the whole HOG for the hyperosmotic response. Trends Genet 18:405–412
Petelenz-Kurdziel E et al (2013) Quantitative analysis of glycerol accumulation, glycolysis and growth under hyper osmotic stress. PLoS Comput Biol 9:e1003084
Pettersson N, Filipsson C, Becit E, Brive L, Hohmann S (2005) Aquaporins in yeasts and filamentous fungi. Biol Cell 97:487–500
Philips J, Herskowitz I (1997) Osmotic balance regulates cell fusion during mating in Saccharomyces cerevisiae. J Cell Biol 138:961–974
Poolman B, Blount P, Folgering JHA, Friesen RHE, Moe PC, van der Heide T (2002) How do membrane proteins sense water stress? Mol Microbiol 44:889–902
Rajavel M, Philip B, Buehrer BM, Errede B, Levin DE (1999) Mid2 is a putative sensor for cell integrity signaling in Saccharomyces cerevisiae. Mol Cell Biol 19:3969–3976
Rep M, Reiser V, Gartner U, Thevelein JM, Hohmann S, Ammerer G, Ruis H (1999) Osmotic stress-induced gene expression in Saccharomyces cerevisiae requires Msn1p and the novel nuclear factor Hot1p. Mol Cell Biol 19:5474–5485
Rep M, Krantz M, Thevelein JM, Hohmann S (2000) The transcriptional response of Saccharomyces cerevisiae to osmotic shock—Hot1p and Msn2p/Msn4p are required for the induction of subsets of high osmolarity glycerol pathway-dependent genes. J Biol Chem 275:8290–8300
Saito H, Posas F (2012) Response to hyperosmotic stress. Genetics 192:289–318
Sato N, Kawahara H, Toh-e A, Maeda T (2003) Phosphorelay-regulated degradation of the yeast Ssk1p response regulator by the ubiquitin-proteasome system. Mol Cell Biol 23:6662–6671
Scanes KT, Hohmann S, Prior BA (1998) Glycerol production by the yeast Saccharomyces cerevisiae and its relevance to wine: a review. South Afr J Enol Viticul 9:17–24
Sheikh-Hamad D, Gustin MC (2004) MAP kinases and the adaptive response to hypertonicity: functional preservation from yeast to mammals. Am J Physiol Renal Physiol 287:F1102–F1110
Sole C, Nadal-Ribelles M, de Nadal E, Posas F (2014) A novel role for lncRNAs in cell cycle control during stress adaptation. Curr Genet. doi:10.1007/s00294-014-0453-y
Thevelein JM, Hohmann S (1995) Trehalose synthase, guard to the gate of glycolysis in yeast? Trends Biochem Sci 20:3–10
Thorsen M et al (2006) The MAPK Hog1p modulates Fps1p-dependent arsenite uptake and tolerance in yeast. Mol Biol Cell 17:4400–4410
van Heerden JH et al (2014) Lost in transition: start-up of glycolysis yields subpopulations of nongrowing cells. Science 343:1245114
Van Wuytswinkel O, Reiser V, Siderius M, Kelders MC, Ammerer G, Ruis H, Mager WH (2000) Response of Saccharomyces cerevisiae to severe osmotic stress: evidence for a novel activation mechanism of the HOG MAP kinase pathway. Mol Microbiol 37:382–397
Westfall PJ, Patterson JC, Chen RE, Thorner J (2008) Stress resistance and signal fidelity independent of nuclear MAPK function. Proc Natl Acad Sci USA 105:12212–12217
Wood JM (2011) Bacterial osmoregulation: a paradigm for the study of cellular homeostasis. Annu Rev Microbiol 65:215–238
Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress: evolution of osmolyte systems. Science 217:1214–1222
Acknowledgments
I thank D. E. N. Rangel for arranging the ISFUS meeting (International Symposium on Fungal Stress Responses) and the special issue in Current Genetics. Work in my laboratory has been supported by the Swedish Research Council as well as the European Commission. This review article was supported in part by a grant from São Paulo Research Foundation (FAPESP) of Brazil # 2014/01229-4.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D.E.N. Rangel.
This article is part of the Special Issue “Fungal Stress Responses”.
Rights and permissions
About this article
Cite this article
Hohmann, S. An integrated view on a eukaryotic osmoregulation system. Curr Genet 61, 373–382 (2015). https://doi.org/10.1007/s00294-015-0475-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-015-0475-0