Abstract
In Saccharomyces cerevisiae, intracellular phosphate levels are maintained by the PHO pathway, activation of which is assayed by increased phosphatase activity. The PHO pathway of Schizosaccharomyces pombe upregulates phosphatase activity (encoded by pho1 +) during low extracellular phosphate levels, but the underlying mechanism is poorly understood. We utilized an alternate repressor of pho1 + expression (adenine supplementation) along with epistasis analysis to develop a model of how S. pombe PHO pathway components interact. Analyzing Pho1 activity in S. pombe PHO pathway deletion mutants during adenine starvation, we observed most mutants with a phosphatase defect in phosphate starvation also had a defect in adenine starvation. Pho7, a transcription factor in the PHO pathway, is necessary for an adenine starvation-mediated increase in Pho1 activity. Comparing adenine starvation to phosphate starvation, there are differences in the degree to which individual mutants regulate the two responses. Through epistasis studies, we identified two positive regulatory arms and one repressive arm of the PHO pathway. PKA activation is a positive regulator of Pho1 activity under both environmental conditions and is critical for transducing adenine concentrations in the cell. The synthesis of IP7 also appears critical for the induction of Pho1 activity during adenine starvation, but IP7 is not critical during phosphate starvation, which differs from S. cerevisiae. Finally, Csk1 is critical for repression of pho1 + expression during phosphate starvation. We believe all of these regulatory arms converge to increase transcription of pho1 + and some of the regulation acts through pho7 +.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proper nutrient sensing is required for cell survival. Therefore, organisms have evolved sensing mechanisms for essential nutrients, such as inorganic phosphate, which is a ubiquitous component of cellular processes (Karthikeyan et al. 2007; Wilson et al. 2013). In the yeast Saccharomyces cerevisiae, cellular homeostasis of intracellular phosphate levels is maintained by the phosphate signal transduction pathway (PHO pathway) (Wykoff and O’Shea 2001). When subjected to low extracellular phosphate concentrations, the PHO pathway is activated. This activation leads to an increase in high-affinity phosphate uptake and to the secretion of phosphatases, which scavenge inorganic phosphate from extracellular organic phosphate-containing compounds (Henry et al. 2011; Kerwin and Wykoff 2009; Secco et al. 2012). The PHO pathway of S. cerevisiae is a relatively simple system of positive and negative regulatory components (Lenburg and O’Shea 1996). Upstream in the PHO pathway of S. cerevisiae is a cyclin–cyclin dependent kinase (CDK) complex regulated primarily by a metabolite, inositol pyrophosphate (IP7) (Lee et al. 2008). The cause of increased intracellular IP7 during external phosphate starvation is unknown, but IP7 is the most upstream metabolite currently identified and regulates the CDK complex through allostery (Lee et al. 2007, 2008).
The PHO pathway in Schizosaccharomyces pombe also upregulates acid phosphatase expression in response to low extracellular phosphate levels (Elliott et al. 1986; Schweingruber et al. 1992). However, the mechanism by which phosphate starvation increases expression of the acid phosphatase and the phosphate transporter is much less characterized in S. pombe relative to S. cerevisiae (Carter-O’Connell et al. 2012; Henry et al. 2011). We have identified a number of genes that appear to regulate the activity of Pho7, as a pho7∆ strain is epistatic to the known regulatory mutants in the PHO pathway (Henry et al. 2011). Recently, it was demonstrated that a long non-coding RNA is transcribed at the pho1 + locus during high external phosphate conditions, leading to the recruitment of RNAi machinery and repression of pho1 + through methylation (H3K9me2) (Shah et al. 2014), although how the signal transduction pathway and this recruitment mechanism interact is currently unknown.
Cell growth is also dependent on the regulation of de novo synthesis of nucleotides, nucleotide recycling, and degradation of macromolecules that contain nucleotides. Nucleotide synthesis, such as adenine biosynthesis, is an energy-intensive process and is highly regulated (Ljungdahl and Daignan-Fornier 2012). Work in S. cerevisiae and S. pombe have demonstrated a coupling of adenine synthesis with a phosphate starvation response (Gauthier et al. 2008; Schweingruber et al. 1992). The signaling factors that control adenine biosynthesis in S. cerevisiae include the transcription factors Pho2, Bas1, Gcn4, and certain metabolites, although many mechanistic details remain to be elucidated (Bhoite et al. 2002; Joo et al. 2009; Pinson et al. 2009; Rebora et al. 2005). In contrast, the signaling pathways regulating adenine biosynthesis in S. pombe are unknown.
In S. cerevisiae, low adenine concentrations in growth medium affect the expression of PHO regulon genes (Gauthier et al. 2008). Expression of the S. cerevisiae high-affinity phosphate transporter, PHO84, increased ~3-fold in medium limited for adenine relative to adenine-replete conditions, even with excess inorganic phosphate present. Since an increase in ScPHO84 expression is considered an indicator of PHO pathway activation in S. cerevisiae, the low adenine condition activates the PHO pathway (Gauthier et al. 2008). The mechanism behind this cross-regulation in S. cerevisiae is unknown. Despite S. pombe and S. cerevisiae being very distantly related (Wapinski et al. 2007), S. pombe also exhibits PHO pathway activation in low adenine conditions with greater than a threefold increase in Pho1 phosphatase activity, even when phosphate is not limiting (Schweingruber et al. 1992). Through the use of adenine repression of the pho1 + gene, we conducted epistatic analysis of previously identified PHO pathway components. Our findings allowed us to develop a model of how S. pombe PHO pathway components interact and provide an initial understanding of the mechanisms underlying the cross-regulation of the S. pombe PHO pathway by adenine starvation.
Materials and methods
Growth conditions and strains
S. pombe cells were maintained in previously described YES and EMM media (Forsburg and Rhind 2006). Wild-type strains h − (DP1) and h + (DP2) were derived between 972 and 975 and generously provided by D. Moazed. Strains mutant in csg1 + , cgs2 +, or gpa2 + were generously provided by Charles Hoffman. The mutant strains should accumulate cAMP and they additionally contained suppressor mutations allowing for them to be backcrossed to wild-type or pho7∆ strains. After backcrossing, spores were plated on EMM medium lacking amino acids (to select for prototrophy) and the mutant phenotypes were identified by poor viability in stationary phase and by the inability to mate after backcrossing. Multiple isolates were examined. Deletion strains were generated through standard replacement of a gene of interest with a marker for G-418 or nourseothricin resistance (Henry et al. 2011), and mating types for strains were determined by PCR (Forsburg and Rhind 2006). The complete list of strains can be found in Supplemental Table 1. All adenine auxotrophic S. pombe strains (ade6 −) were backcrossed into a wild-type ade6 + background to enable the deletion strains to be tested in low adenine conditions (ade − mutants are defective in the induction of pho1 +). S. pombe strains were tested for phosphatase activity using two modified mediums: 90 % SD-10 % EMM (with and without phosphate) and YE and YE5S (which is 5× the standard SP addition) (Forsburg and Rhind 2006; Henry et al. 2011). Formulations of all modified mediums are provided in Supplemental Table 2. For solid plates, 2 % Bacto-agar (Difco) was included.
PNPP assay
To test for phosphatase activity in high and low adenine conditions, strains were grown in YE5S at 30 °C to an optical density of 0.15–0.3 at 600 nm (OD600) (mid-logarithmic phase). Cultures were washed with sterile water, and inoculated into 5 mL of YE5S or YE-ade medium at a low density and grown for 10 h at 30 °C. Cells were centrifuged, suspended in water, and assayed for phosphatase activity for 10 min at pH 4 as described previously (Kerwin and Wykoff 2009). PNPP (ρ-nitrophenyl phosphate) hydrolysis was measured at OD400. The OD400/OD600 ratio for each reaction was calculated, accounting for dilutions.
Phosphatase response to high and low phosphate conditions was tested by growing strains in 90 % SD-10 % EMM with phosphate at 30 °C to an OD600 of 0.15–0.3. Cultures were harvested, washed, and resuspended in sterile water to an OD600 of approximately 0.1. Cells were inoculated into 90 % SD-10 % EMM medium with or without phosphate at a low density and grown for 4 h at 30 °C. Cells were harvested and phosphatase activity assayed as described above.
Epistasis
To observe the phenotype of double mutants, we crossed single deletion strains to create S. pombe strains containing two gene deletions in an ade6 + genotype. Strains of opposite mating type, each containing one gene deletion, were crossed on ME solid medium (Forsburg and Rhind 2006). Spores were chosen for the appropriate antibiotic resistance. PCR was used to confirm the appropriate gene deletions and check mating type.
Time course of pho1 + and pho84 + transcript abundance
Wild-type strains were grown in YE5S medium at 30 °C to OD600 of 0.2–0.5. Cells were pelleted by centrifugation, washed three times, transferred to YE-ade medium, and grown at 30 °C for 10 h. Quantitative reverse-transcription PCR (RT-qPCR) was used to measure the amount of pho84 +, pho1 +, and act1 + transcript at the selected time points during adenine starvation. The cells harvested during the time course were stored at −80 °C until the time of RNA extraction.
RT-qPCR
RNA was purified by an acid phenol protocol (Kerwin and Wykoff 2009) and quantified with a Nanodrop 2000. 1 µg of RNA was reverse transcribed using an iScript cDNA synthesis kit (Bio-Rad), followed by a 1:5 dilution. Transcripts from 3 µl of the diluted cDNA were quantified using a Bio-Rad Chromo-4 with 50 µl Sybr green I reaction mixtures. pho84 + and pho1 + transcript levels were normalized to act1 + transcript, which does not change in abundance during phosphate starvation (Henry et al. 2011).
Results
To determine the responsiveness of wild-type S. pombe to phosphate and adenine starvation, we tested for phosphatase activity in both growth conditions (Fig. 1a). In no phosphate, there is a de-repression of Pho1 acid phosphatase activity, as seen in previous studies (Henry et al. 2011). In low adenine, there is also de-repression of pho1 +, which was observed previously (Schweingruber et al. 1992). We asked whether the two starvation conditions were additive by examining wild-type cells grown during adenine starvation, phosphate starvation, and both starvation conditions together (Supplemental Fig. 1). It appears that adenine starvation is not additive with phosphate starvation alone, suggesting that the strong activation by phosphate starvation masks the weaker activation by adenine starvation. To determine if the increase in Pho1 activity was due to an increase in mRNA expression or an alteration of enzymatic activity, we quantified pho1 + transcript and found that pho1 + mRNA abundance increased during adenine starvation (Supplemental Fig. 2), suggesting that increased Pho1 activity is due to increased gene expression.
a Phosphatase assay of wild-type S. pombe in high and no phosphate and high and low adenine conditions. Phosphatase activity in this figure and all following figures was measured as the amount of PNPP hydrolyzed (OD400) normalized to cell density (OD600) and was performed on cells grown in the same medium under the same conditions on the same day. In this figure and all future figures at least three independent cultures were grown and the bars are standard deviation of the mean. Repetitions on different days had identical trends. Phosphate starvation leads to a much stronger induction of phosphatase activity. b De-repression of Pho1 activity by adenine starvation requires both pho7 + and snf5 +. As only adenine starvation was being measured, the scale on the y axis differs from (a)
We previously identified pho7 +, a zinc-finger transcription factor, and snf5 +, a component of a chromatin remodeling complex, as the downstream effectors of the S. pombe PHO pathway (Carter-O’Connell et al. 2012; Henry et al. 2011) and we tested for acid phosphatase activity in pho7∆ and snf5∆ strains in high and low adenine conditions (Fig. 1b). Wild-type S. pombe increases phosphatase activity in low adenine conditions, and this increase is eliminated in the pho7∆ and snf5∆ mutants. Thus, pho7 + and snf5 + are necessary for both the adenine and phosphate starvation-mediated increase in Pho1 activity. We further hypothesized that regulators of pho1 + during phosphate starvation would act during adenine starvation and therefore analyzed Pho1 activity in S. pombe PHO pathway deletion mutants during adenine starvation (Fig. 2). When comparing adenine starvation (Fig. 2) relative to phosphate starvation (Fig. 2 in Henry et al. 2011 paper) there are differences in the degree to which individual mutants regulate the two responses, suggesting cross-regulation as well as divergent regulation. For example, the vph2∆ and ypa1∆ strains have close to wild-type Pho1 phenotypes with regards to adenine starvation conditions, but are less inducible relative to wild type with regards to phosphate starvation (Henry et al. 2011).
Measurement of Pho1 activity in known PHO pathway mutants in adenine replete and adenine starvation conditions. These mutants were chosen based on Henry et al. (2011), backcrossed to remove auxotrophic markers, and assayed for phosphatase activity in response to adenine starvation. The mutants are sorted according to overall Pho1 activity in low adenine conditions
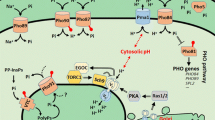
Based on the phenotypes of the S. pombe mutants in high and low adenine conditions, we characterized the PHO pathway components as positive or negative regulators of S. pombe Pho1 activity. Through epistasis studies of the S. pombe gene deletions, we were able to determine the interactions between these positive and negative regulators and developed a model of the S. pombe PHO pathway (Fig. 3). Although our results indicate that additional factors (e.g., ado1 +) are likely involved in the PHO pathway, we were not able to conclusively place these factors within the proposed model (see Supplemental Fig. 3).
Model of regulation of pho1 +. Positive and negative regulation is indicated by arrows. It is unclear how direct any of the actions are, but the IP7 arm appears to act as an amplifier and not a sensor per se. The PKA arm appears more important during adenine starvation and Csk1 appears more important for repression during high phosphate conditions. We assume that the zinc finger protein, Pho7, activates chromatin remodeling (of which Snf5 is a member), but that in most cases both Pho7 and Snf5 are required for Pho1 expression. There is no direct evidence that the PKA pathway acts directly on Pho7, but we hypothesize that Pho7 phosphorylation status regulates activity. Additionally, it is appealing to expect that cAMP activates PKA activity in this regulatory pathway, but our results demonstrate that elevated cAMP levels alone do not lead to increased pho1 + expression
PKA activation is part of a positive regulatory arm of the PHO model (Fig. 3), and is composed of gpa2 + and pka1 +. gpa2 +, a heterotrimeric G protein alpha-2 subunit associated with the activation of adenylate cyclase activity, and pka1 +, a cAMP-dependent protein kinase catalytic subunit, play a central role in PKA signal transduction (Gancedo 2013; Kim et al. 2013). PKA signaling is generally transduced through the gpa2 +-driven cAMP accumulation, then activation of pka1 + (Gupta et al. 2011). Both gpa2 + and pka1 + are positive regulators of pho1 + expression during phosphate starvation in S. pombe (Henry et al. 2011). In addition, gpa2∆ and pka1∆ S. pombe mutants have extremely low levels of pho1 + expression during adenine starvation (Fig. 2). Therefore, active PKA signaling is a positive regulator of Pho1 under both environmental conditions.
Regulation of the PHO pathway in S. cerevisiae and S. pombe is known to be dependent on inositol phosphates, such as IP7 (Henry et al. 2011; Lee et al. 2007). IP7 is synthesized from IP6 by the actions of asp1 +, an IP6 kinase, and degraded by aps1 +, an IP7 phosphatase (Mulugu et al. 2007). The asp1∆ mutant has low Pho1 activity regardless of adenine concentration and the aps1∆ mutant has constitutive Pho1 activity (Fig. 2). Therefore, IP7 is acting as a positive regulator of pho1 + expression during adenine starvation. Previous work has shown that deletion of asp1 + in S. pombe does not produce an obvious phenotype in phosphate starvation conditions based on a phosphatase plate assay (Henry et al. 2011). To determine whether the asp1∆ mutant had a divergent phenotype between phosphate and adenine starvation, we examined Pho1 activity under the replete and starvation conditions (Fig. 4). Interestingly, even though IP7 is required for both responses as indicated by the aps1∆ strain being relatively constitutive under both conditions (Fig. 4), the differing phenotypes of the asp1∆ strain in both conditions suggests the synthesis of IP7 is critical for the induction of Pho1 activity during adenine starvation, but not during phosphate starvation (Fig. 4). Perhaps because phosphate starvation results in a strong induction relative to adenine starvation, the requirement for IP7 synthesis is circumvented by other unknown mechanisms.
csk1 + is a CDK activating kinase which was identified as a negative regulator of pho1 + expression in S. pombe during phosphate starvation (Henry et al. 2011). The csk1∆ mutant has a high level of phosphatase activity independent of adenine concentrations (Figs. 2, 5a). Interestingly, the csk1∆ strain in high and no phosphate expresses at the same level as a wild-type strain in no phosphate conditions (Henry et al. 2011), but expresses twice the level of wild-type adenine starved cells (compare the wild-type and csk1∆ strains in Fig. 5a). This result suggests that loss of repression by Csk1 mimics phosphate starvation. Because most experiments with the csk1∆ strain demonstrate a slight repressibility of Pho1 activity by adenine, we surmise that adenine sensing does not primarily act through csk1 + and acts through csk1 +-independent pathways.
a Epistasis with csk1 + signaling and PKA signaling mutants. Phosphatase activity was measured in mutants lacking csk1 +, PKA signaling components, or both. b Epistasis with the aps1∆ strain defective in IP7 phosphatase activity and PKA signaling. Pho1 phosphatase activity was measured in mutants lacking aps1 +, PKA signaling components, or both
To assess the interactions of csk1 + with PKA signaling, we assayed the phosphatase activity of csk1∆ pka1∆ and csk1∆ gpa2∆ mutants (Fig. 5a). Loss of PKA signaling did not eliminate the high level of pho1 + activity in the csk1∆ mutant but reduced phosphatase activity by half. The constitutive phenotype of csk1∆ pka1∆ and the csk1∆ gpa2∆ strains suggest activating signals of pka1 + act independently from the repressing effects of csk1 +. The phenotypes of csk1∆ gpa2∆ and csk1∆ pka1∆ mutants support a model in which Gpa2 and Pka1 are acting together during adenine starvation and the repressing effects of Csk1 do not act through Pka1.
To determine the effect of IP7 levels on PKA signaling, we analyzed Pho1 activity of the aps1∆ pka1∆ and aps1∆ gpa2∆ strains. Double mutants of the components of the PKA signaling pathway and the IP6 kinase, asp1 +, were not analyzed, as the Pho1 phosphatase phenotypes of both are uninducible and epistasis analysis is uninformative. Deletion of the IP7 phosphatase aps1 + produced an increase in Pho1 activity even with the loss of PKA signaling (Fig. 5b), suggesting that PKA signaling is not required for IP7-dependent pho1 + expression. Neither the aps1∆ gpa2∆ nor the aps1∆ pka1∆ mutant exhibited an increase in phosphatase activity in low adenine relative to high adenine. This phenotype suggests that the elevated IP7 levels increase basal pho1 + expression, but to de-repress the PHO pathway during adenine starvation, activation of PKA signaling is required.
We also investigated the interactions of Csk1 with IP7. The phenotype of the aps1∆ csk1∆ strain is constitutive, with Pho1 levels greater than that of aps1∆ or csk1∆ alone (Fig. 6a), indicating that the removal of Csk1, combined with IP7 accumulation in the Aps1 phosphatase deletion, strongly de-represses pho1 + activity. The additive phenotype of aps1∆ csk1∆ suggests that Csk1 and IP7 serve distinct roles in pho1 + expression regulation and act independent of one another. Analysis of Csk1 with the IP6 kinase Asp1 also supports the establishment of separate roles. The Pho1 phosphatase activity of the asp1∆ csk1∆ mutant (Fig. 6b) exhibits a phenotype intermediate to asp1∆ and csk1∆, suggesting that loss of IP7 can only impact approximately half of the starvation signal. Interestingly, the phosphatase expression of the asp1∆ csk1∆ mutant was more similar to that of asp1∆ than csk1∆, suggesting that the loss of the repressor (Csk1) is not able to efficiently release Pho1 repression if IP7 levels are insufficient. One possible hypothesis is that Csk1 and IP7 act through a common factor, such as Pho7, and the removal of Csk1 is insufficient to fully activate Pho7 in the absence of IP7.
Discussion
The S. pombe PHO pathway utilizes both positive and negative regulators of pho1 + expression and its pathway architecture is dramatically different from S. cerevisiae. The positive regulators are PKA signaling and accumulation of IP7, with the negative regulator being Csk1. Our data suggest that IP7 and Csk1 regulatory signals converge upon Pho7 and the SWI/SNF chromatin remodeling complex (Fig. 3). We provide evidence that the mechanism by which adenine starvation activates the PHO pathway is mediated by PKA signaling, is dependent on the availability of IP7, and is repressed by Csk1 activity. It is worth noting that Pho1 de-repression is likely more dependent on pho7 + than snf5 +, as accumulation of IP7 activates pho1 + expression when snf5 + is deleted, but not when pho7 + is deleted (Supplemental Fig. 4). These results suggest that pho7 is essential for the induction of pho1 +, but that the snf5 + requirement can be circumvented by activation of other factors (presumably Pho7). pho7 + mediates a number of starvation responses in S. pombe, although the regulation of pho1 + in particular is sensitive to pho7 + (Carter-O’Connell et al. 2012). We conclude that pho7 + is absolutely essential for the pho1 + de-repression and is the site of regulation by phosphate and adenine, while chromatin remodeling is subsequently required for efficient gene expression.
The PHO pathways of S. cerevisiae and S. pombe have been shown to exhibit a de-repression of pho1 + expression during both phosphate and adenine starvation (Carter-O’Connell et al. 2012; Gauthier et al. 2008; Henry et al. 2011; Schweingruber et al. 1992). Although the evolutionary significance of this cross-regulation of the PHO pathway by adenine is unknown, we speculate that it may be a molecular “anticipatory” mechanism. As ATP is generated from both adenine and phosphate, adenine and phosphate metabolism are intimately tied to cellular metabolism (Chapman and Atkinson 1977; Lagunas 1986). An adenine deficiency may indicate that a cell is also likely to be phosphate deficient, preemptively activating the PHO pathway. Alternatively, an adenine deficiency may indicate that phosphate will be required in the near future to manufacture ATP from newly manufactured adenosine, also validating the activation of the PHO pathway.
Our data suggest that the mechanism by which adenine starvation activates the S. pombe PHO pathway involves PKA signaling and is also IP7 dependent. The PHO pathway of S. cerevisiae utilizes IP7 to regulate expression of PHO genes through non-covalent interactions with the Pho80/Pho85/Pho81 CDK complex (Lee et al. 2008). Although the PHO pathways of both species converge upon common orthologous gene targets, S. pombe appears to have evolved most of the regulatory interactions of the PHO pathway independently from that of S. cerevisiae, as there is no CDK complex present in S. pombe that regulates PHO genes (Henry et al. 2011; Tanaka and Okayama 2000). Although both species use IP7 as a signaling component, the usage or “sensing” of IP7 may have developed independently, as IP7 is central to the phosphate starvation response in S. cerevisiae, but IP7 signaling is somewhat disposable in S. pombe. In support of this is the observation that an asp1∆ strain in S. cerevisiae (gene is called VIP1) is uninducible for phosphatase activity (Lee et al. 2007), whereas the same deletion in S. pombe does not have an observable phenotype (Fig. 4). However, IP7 is important in signaling in S. pombe, just not as central to the PHO regulatory pathway. Similarly, the PKA pathway appears to play a more peripheral role in the PHO pathway in S. cerevisiae, but a more central role in S. pombe.
S. cerevisiae has interactions between PKA signaling and the PHO pathway. After phosphate starvation, phosphate addition rapidly activates PKA activity independent of cAMP production and independent of phosphate transport through the transceptor Pho84 (Giots et al. 2003; Popova et al. 2010). However, others have recently indicated detectable cAMP increases upon phosphate feeding (Conway et al. 2012). Regardless, studies indicate that lower cAMP levels are correlated with multiple nutrient starvation conditions in S. cerevisiae, suggesting that cAMP levels should be lower during phosphate starvation (Conway et al. 2012; Ma et al. 1997; Markwardt et al. 1995). PKA signaling does not appear to be a core signaling component of the PHO pathway in S. cerevisiae, but is a core requirement for the S. pombe PHO pathway. To determine if elevated cAMP levels activate the expression of pho1 +, we examined three mutants (cgs1, cgs2, and an activated gpa2 allele) that were likely to elevate cAMP levels in the cell and determined those mutants effect on Pho1 activity (Supplemental Fig. 5). Surprisingly, all three were able to increase pho1 + expression during adenine starvation, indicating that while PKA (and gpa2 +) activity is likely required for induction, elevated cAMP levels alone are not sufficient to increase expression of pho1 +. Future studies are required to determine if there is indeed a cAMP signaling difference between the two species or if this is just a complication of different experimental regimes.
Even though both adenine and phosphate starvation act either directly or indirectly through pho7 +, the mechanism by which phosphate starvation activates the S. pombe PHO pathway differs from that of adenine starvation. For example, depletion of IP7 does not alter Pho1 phosphatase activity during phosphate starvation as it does in adenine starvation. Additionally, in S. cerevisiae, adenylate kinase (Adk1) and adenosine kinase (Ado1) have both been shown to impact purine as well as phosphate metabolism, an effect that may be acting through metabolic intermediates or Pho2, a transcription factor (Gauthier et al. 2008). Our data indicate ado1 + signaling requires IP7 synthesis (Supplemental Fig. 3). Further dissection of the adenine/phosphate cross-regulation requires knowledge of the adenine starvation responsive transcription factors, which we are currently attempting to identify in S. pombe.
Adenine starvation is a tool that can be utilized in future studies of the S. pombe PHO pathway. We propose a model of the regulation of PHO promoters based on epistatic interactions and de-repression by two different environmental signals. This model facilitates the comparison of phosphate metabolism between S. pombe and other yeast species. Additionally, this model is a starting point for the clarification of mechanistic details, such as Pho7 phosphorylation, and determination of whether Csk1 is acting, directly or indirectly, on Pho7 or Snf5. By better understanding the evolutionary changes in the metabolic pathways of single-celled eukaryotes, we may better understand the interactions of metabolic pathways in more complex eukaryotes.
References
Bhoite LT, Allen JM, Garcia E, Thomas LR, Gregory ID, Voth WP, Whelihan K, Rolfes RJ, Stillman DJ (2002) Mutations in the pho2 (bas2) transcription factor that differentially affect activation with its partner proteins bas1, pho4, and swi5. J Biol Chem 277:37612–37618. doi:10.1074/jbc.M206125200
Carter-O’Connell I, Peel MT, Wykoff DD, O’Shea EK (2012) Genome-wide characterization of the phosphate starvation response in Schizosaccharomyces pombe. BMC Genom 13:697. doi:10.1186/1471-2164-13-697
Chapman AG, Atkinson DE (1977) Adenine nucleotide concentrations and turnover rates. Their correlation with biological activity in bacteria and yeast. Adv Microb Physiol 15:253–306
Conway MK, Grunwald D, Heideman W (2012) Glucose, nitrogen, and phosphate repletion in Saccharomyces cerevisiae: common transcriptional responses to different nutrient signals. G3 (Bethesda) 2:1003–1017. doi:10.1534/g3.112.002808
Elliott S, Chang CW, Schweingruber ME, Schaller J, Rickli EE, Carbon J (1986) Isolation and characterization of the structural gene for secreted acid phosphatase from Schizosaccharomyces pombe. J Biol Chem 261:2936–2941
Forsburg SL, Rhind N (2006) Basic methods for fission yeast. Yeast 23:173–183. doi:10.1002/yea.1347
Gancedo JM (2013) Biological roles of cAMP: variations on a theme in the different kingdoms of life. Biol Rev Camb Philos Soc 88:645–668. doi:10.1111/brv.12020
Gauthier S, Coulpier F, Jourdren L, Merle M, Beck S, Konrad M, Daignan-Fornier B, Pinson B (2008) Co-regulation of yeast purine and phosphate pathways in response to adenylic nucleotide variations. Mol Microbiol 68:1583–1594. doi:10.1111/j.1365-2958.2008.06261.x
Giots F, Donaton MC, Thevelein JM (2003) Inorganic phosphate is sensed by specific phosphate carriers and acts in concert with glucose as a nutrient signal for activation of the protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol 47:1163–1181
Gupta DR, Paul SK, Oowatari Y, Matsuo Y, Kawamukai M (2011) Multistep regulation of protein kinase A in its localization, phosphorylation and binding with a regulatory subunit in fission yeast. Curr Genet 57:353–365. doi:10.1007/s00294-011-0354-2
Henry TC, Power JE, Kerwin CL, Mohammed A, Weissman JS, Cameron DM, Wykoff DD (2011) Systematic screen of Schizosaccharomyces pombe deletion collection uncovers parallel evolution of the phosphate signal transduction pathway in yeasts. Eukaryot Cell 10:198–206. doi:10.1128/EC.00216-10
Hoffman CS, Winston F (1991) Glucose repression of transcription of the Schizosaccharomyces pombe fbp1 gene occurs by a cAMP signaling pathway. Genes Dev 5:561–571
Joo YJ, Kim JA, Baek JH, Seong KM, Han KD, Song JM, Choi JY, Kim J (2009) Cooperative regulation of ADE3 transcription by Gcn4p and Bas1p in Saccharomyces cerevisiae. Eukaryot Cell 8:1268–1277. doi:10.1128/EC.00116-09
Kao RS, Morreale E, Wang L, Ivey FD, Hoffman CS (2006) Schizosaccharomyces pombe Git1 is a C2-domain protein required for glucose activation of adenylate cyclase. Genetics 173:49–61 doi:10.1534/genetics.106.055699
Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG (2007) Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta 225:907–918. doi:10.1007/s00425-006-0408-8
Kerwin CL, Wykoff DD (2009) Candida glabrata PHO4 Is necessary and sufficient for Pho2-independent transcription of phosphate starvation genes. Genetics 182:471–479. doi:10.1534/genetics.109.101063
Kim JH, Roy A, Jouandot D 2nd, Cho KH (2013) The glucose signaling network in yeast. Biochim Biophys Acta 1830:5204–5210. doi:10.1016/j.bbagen.2013.07.025
Lagunas R (1986) Misconceptions about the energy metabolism of Saccharomyces cerevisiae. Yeast 2:221–228. doi:10.1002/yea.320020403
Lecoq K, Belloc I, Desgranges C, Daignan-Fornier B (2001) Role of adenosine kinase in Saccharomyces cerevisiae: identification of the ADO1 gene and study of the mutant phenotypes. Yeast 18:335–342. doi:10.1002/1097-0061(20010315)18:4<335:AID-YEA674>3.0.CO;2-X
Lee YS, Mulugu S, York JD, O’Shea EK (2007) Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science 316:109–112. doi:10.1126/science.1139080
Lee YS, Huang K, Quiocho FA, O’Shea EK (2008) Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat Chem Biol 4:25–32. doi:10.1038/nchembio.2007.52
Lenburg ME, O’Shea EK (1996) Signaling phosphate starvation. Trends Biochem Sci 21:383–387
Ljungdahl PO, Daignan-Fornier B (2012) Regulation of amino acid, nucleotide, and phosphate metabolism in Saccharomyces cerevisiae. Genetics 190:885–929. doi:10.1534/genetics.111.133306
Ma P, Goncalves T, Maretzek A, Dias MC, Thevelein JM (1997) The lag phase rather than the exponential-growth phase on glucose is associated with a higher cAMP level in wild-type and cAPK-attenuated strains of the yeast Saccharomyces cerevisiae. Microbiology 143(Pt 11):3451–3459
Markwardt DD, Garrett JM, Eberhardy S, Heideman W (1995) Activation of the Ras/cyclic AMP pathway in the yeast Saccharomyces cerevisiae does not prevent G1 arrest in response to nitrogen starvation. J Bacteriol 177:6761–6765
Mulugu S, Bai W, Fridy PC, Bastidas RJ, Otto JC, Dollins DE, Haystead TA, Ribeiro AA, York JD (2007) A conserved family of enzymes that phosphorylate inositol hexakisphosphate. Science 316:106–109. doi:10.1126/science.1139099
Pinson B, Vaur S, Sagot I, Coulpier F, Lemoine S, Daignan-Fornier B (2009) Metabolic intermediates selectively stimulate transcription factor interaction and modulate phosphate and purine pathways. Genes Dev 23:1399–1407. doi:10.1101/gad.521809
Popova Y, Thayumanavan P, Lonati E, Agrochao M, Thevelein JM (2010) Transport and signaling through the phosphate-binding site of the yeast Pho84 phosphate transceptor. Proc Natl Acad Sci USA 107:2890–2895. doi:10.1073/pnas.0906546107
Rebora K, Laloo B, Daignan-Fornier B (2005) Revisiting purine-histidine cross-pathway regulation in Saccharomyces cerevisiae: a central role for a small molecule. Genetics 170:61–70. doi:10.1534/genetics.104.039396
Schweingruber ME, Edenharter E, Zurlinden A, Stockmaier KM (1992) Regulation of pho1-encoded acid phosphatase of Schizosaccharomyces pombe by adenine and phosphate. Curr Genet 22:289–292
Secco D, Wang C, Shou H, Whelan J (2012) Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins. FEBS Lett 586:289–295. doi:10.1016/j.febslet.2012.01.036
Shah S, Wittmann S, Kilchert C, Vasiljeva L (2014) lncRNA recruits RNAi and the exosome to dynamically regulate pho1 expression in response to phosphate levels in fission yeast. Genes Dev 28:231–244. doi:10.1101/gad.230177.113
Tanaka K, Okayama H (2000) A pcl-like cyclin activates the Res2p-Cdc10p cell cycle “start” transcriptional factor complex in fission yeast. Mol Biol Cell 11:2845–2862
Wapinski I, Pfeffer A, Friedman N, Regev A (2007) Natural history and evolutionary principles of gene duplication in fungi. Nature 449:54–61. doi:10.1038/nature06107
Wilson MS, Livermore TM, Saiardi A (2013) Inositol pyrophosphates: between signalling and metabolism. Biochem J 452:369–379. doi:10.1042/BJ20130118
Wykoff DD, O’Shea EK (2001) Phosphate transport and sensing in Saccharomyces cerevisiae. Genetics 159:1491–1499
Acknowledgments
This work was supported by the National Science Foundation grant MCB-1121714, and MCB-1412582, the Dennis M. Cook Endowed Gregor Mendel Chair in Genetics, the Villanova College of Liberal Arts and Sciences, and the Villanova Department of Biology. We also appreciate the suggested experiments from anonymous reviewers that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. S. Hoffman.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1 Measurement of phosphatase activity in a wild-type strain in varying phosphate and adenine conditions to demonstrate that de-repression of Pho1 activity in response to adenine starvation is not additive to phosphate starvation.
Supplemental Fig. 2 Transcript abundance of pho1 +, ade1 +, and pho84 +, after shifting from high to low adenine at time = 0 h, measured by quantitative PCR of reverse transcribed RNA. Transcripts were normalized to act1 +, and are presented as a ratio of expression. The average and standard deviation of triplicate measurements are given for each time point.
Supplemental Fig. 3 Measurement of phosphatase activity in mutants lacking ado1 +, an adenosine kinase, and asp1 +, the IP6 kinase. Phosphatase measurements suggest that Ado1 is a negative regulator of PHO1 expression, which is consistent with previous studies (Henry, et al. 2011, Lecoq, et al. 2001), and that Ado1 may be acting independently of all arms of the proposed pathway save for IP7, as the constitutive phenotype of ado1∆ is dependent on the presence of IP7.
Supplemental Fig. 4 Measurement of phosphatase activity in wild-type and pho7 + or snf5 + deletion strains and selected constitutive deletion strains. Deletion of pho7 + or snf5 + in strains deleted for aps1 + or csk1 + resulted in low phosphatase expression. Note that Pho1 expression may be more dependent on Pho7 than Snf5, suggesting that the loss of chromatin remodeling does not completely eliminate expression of PHO genes.
Supplemental Fig. 5 Measurement of phosphatase activity in strains with increased accumulation of cAMP. Mutants gpa2 R176H, cgs1-1, and cgs2-2 were generated, grown, and assayed as described in the Materials and Methods section (Hoffman and Winston 1991, Kao, et al. 2006). The cgs1-1 and cgs2-2 mutants have a slight constitutive appearance (higher level of phosphatase activity than wild-type in high adenine conditions) when grown on solid medium, but that qualitative appearance is not detectable using the quantitative liquid phosphatase assay used in this work. The discrepancy between the qualitative and quantitative results may indicate that factors in addition to cAMP are required for elevated Pho1 activity.
294_2014_466_MOESM1_ESM.pdf
Supplementary material 1 (PDF 92 kb) Supplemental Fig. 1 Measurement of phosphatase activity in a wild-type strain in varying phosphate and adenine conditions to demonstrate that de-repression of Pho1 activity in response to adenine starvation is not additive to phosphate starvation. Supplemental Fig. 2 Transcript abundance of pho1 +, ade1 +, and pho84 +, after shifting from high to low adenine at time = 0 hours, measured by quantitative PCR of reverse transcribed RNA. Transcripts were normalized to act1 +, and are presented as a ratio of expression. The average and standard deviation of triplicate measurements are given for each time point. Supplemental Fig. 3 Measurement of phosphatase activity in mutants lacking ado1 +, an adenosine kinase, and asp1 +, the IP6 kinase. Phosphatase measurements suggest that Ado1 is a negative regulator of PHO1 expression, which is consistent with previous studies (Henry, et al. 2011, Lecoq, et al. 2001), and that Ado1 may be acting independently of all arms of the proposed pathway save for IP7, as the constitutive phenotype of ado1Δ is dependent on the presence of IP7. Supplemental Fig. 4 Measurement of phosphatase activity in wild-type and pho7 + or snf5 + deletion strains and selected constitutive deletion strains. Deletion of pho7 + or snf5 + in strains deleted for aps1 + or csk1 + resulted in low phosphatase expression. Note that Pho1 expression may be more dependent on Pho7 than Snf5, suggesting that the loss of chromatin remodeling does not completely eliminate expression of PHO genes. Supplemental Fig. 5 Measurement of phosphatase activity in strains with increased accumulation of cAMP. Mutants gpa2 R176H, cgs1-1, and cgs2-2 were generated, grown, and assayed as described in the Materials and Methods section (Hoffman and Winston 1991, Kao, et al. 2006). The cgs1-1 and cgs2-2 mutants have a slight constitutive appearance (higher level of phosphatase activity than wild-type in high adenine conditions) when grown on solid medium, but that qualitative appearance is not detectable using the quantitative liquid phosphatase assay used in this work. The discrepancy between the qualitative and quantitative results may indicate that factors in addition to cAMP are required for elevated Pho1 activity.
Rights and permissions
About this article
Cite this article
Estill, M., Kerwin-Iosue, C.L. & Wykoff, D.D. Dissection of the PHO pathway in Schizosaccharomyces pombe using epistasis and the alternate repressor adenine. Curr Genet 61, 175–183 (2015). https://doi.org/10.1007/s00294-014-0466-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-014-0466-6