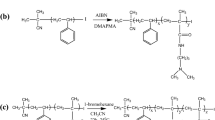
Abstract
Diblock copolymers consisting of higher hydrophobic styrene and hydrophilic N-hydroxyethylacrylamide (HEAAm) segments were successfully synthesized via direct two-step atom transfer radical polymerization. Polystyrene (PS) homopolymers were synthesized using methyl 4-(bromo-methyl) benzoate initiator in N,N′- dimethylformamide (DMF) in the presence of Cu(I)Br/pentamethyldiethylene tetramine (PMDETA) catalyst system at 90 and 110 °C in nitrogen atmosphere. PS was used as macro-initiator for the synthesis of PS-b-PHEAAm polymer. Block copolymerizations were carried out in pure DMF in the presence of the same catalyst system at 110 °C while argon was used for deoxygenation and inert environment. Block copolymer was purified through dialysis into deionized water using a dialysis tubing (MWCO 3500, cellulose membrane). The number average molecular weight of PS polymers (Mn = 2240, 3250 and 10,800 Da) was determined by size exclusion chromatography using tetrahydrofuran (THF) as eluent. The chemical structures, functional groups and actual compositions of copolymer were determined using instrumental data, c.f. elemental analysis, attenuated total reflectance infra-red and proton—nuclear magnetic resonance (1H-NMR) spectroscopy analysis. Thermo-gravimetric analysis confirms that diblock copolymer has higher thermal stability than PS homopolymer. Co-solvent effect on particle formation was investigated by regulating dielectric constant. It is revealed that THF/DMF (9:1) provides susceptible environment for the formation of smallest size (13 ± 4 nm) particle. This synthetic route would establish a direct synthesis of diblock copolymer with antagonistic segments for advanced technological applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Amphiphilic block copolymers with diversified characteristics have found great research interest due to their unique self-assembling behavior in suitable solvents which provide ultimate possibilities to generate and control novel geometries and structures avoiding complicated chemistry [1,2,3]. Amphiphilic block copolymers offer ample opportunities to regulate self-assembly affinity due to their inherent interaction properties for formation of different morphological structures in solutions [4]. Applications of advanced materials based on amphiphilic block copolymer in the field of nanoscience and nanotechnology mainly depend on its behavior in solvent c.f. solubility [5, 6], stable nanoparticles formation by micellization [7, 8] and their phase separation with various polymer matrixes [9].
Various geometries of self-assembly such as spheres, cylinders, tubes, and bilayers are produced due to interfacial energies of polymer–solvent and polymer–polymer interactions [1, 5, 6]. In the past, many authors reported different successful stories for the design and synthesis of numerous new polymers in terms of size, shape, monomer types with stimuli properties [2, 3, 10, 11], hydrophilicity and lipophilicity that have fascinating assembling structures and properties, [11,12,13] supra-molecular assemblies [14] based on non-covalent interactions. The synthesis of amphiphilic block copolymer is still a crucial issue, especially in case of higher hydrophilic and hydrophobic monomers.
The synthesis of block copolymers through different techniques [3, 10,11,12,13,14,15,16] has already been established. Among them controlled/living radical polymerization [17,18,19] (CRP) methods have offered an efficient way to prepare well-defined polymers. This process offers a predetermined molecular weights, narrow molecular weight distributions, and well-controlled architectures, compositions, and terminal functionalities in systems where the contributions of side reactions are negligible. Especially, single electron transfer radical polymerization (SET-RP) and atom transfer radical polymerization (ATRP) [20,21,22,23,24,25,26,27] have been made toward better control of polymerization. A chart of polystyrene-based block copolymers is mentioned in Table 1, where several authors synthesized different block copolymers [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
Amphiphilic block copolymers can be produced from various combinations of hydrophobic and hydrophilic monomers. CRP techniques allow us to prepare their possible combination and structure with specific aims. However, many problems are still concerning factors in synthesis of amphiphilic block copolymers directly from the antagonistic behavioral monomers. The selection of a suitable solvent for all blocks, applicability of CRP techniques to each monomer, and copolymers isolation are the prime obstructions in preparing such structures.
The synthesis of amphiphilic block copolymers is still a critical issue, especially in terms of higher hydrophilic and hydrophobic monomers. Therefore, we set our target for direct synthesis of amphiphilic diblock copolymers, poly(styrene-b-HEAAm) using higher hydrophobic styrene and higher hydrophilic HEAAm monomers by ATRP and studies on mechanistic solvent induced interactions on nanocorona. The influence of cosolvents on the aggregation and thermal stability of poly(styrene-b-HEAAm) diblock copolymer is widely investigated. This study has been instigated by fundamental interests, which include the polar co-solvents that we have chosen to study (DMSO, DMF and THF) which resemble the monomers of the polystyrene blocks and are less polar than water.
Materials and methods
Materials
The styrene and N-hydroxyethylacrylamide (HEAAm) monomers and the methyl-4-(bromomethyl) benzoate (MBMB, 98%) initiator were procured from Aldrich, Japan. Styrene was purified through distillation under vacuum using hydroquinone to eliminate inhibitor and HEAAm was used as received. Cuprous bromide (Cu(I)Br, 98%), pentamethyldiethylene- tetramine (PMDETA) and other chemicals and solvents were also purchased from Aldrich, Japan, and used as received.
Synthesis of PS-Br macroinitiator
Styrene monomer (10.0 g, 11.1 mL, 96 mmol) and DMF (3 mL) were charged into a 50-mL reaction flask. By purging nitrogen, the mixture was deoxygenated for 30 min. While purging continued, PMDETA (210 µL/0.17 g, 0.96 mmol) and Cu(I)Br (138 mg, 0.96 mmol) were introduced successively as a result the solution turned into slightly green. Thereafter, the greenish solution was deoxygenated again for 10 min and methyl 4-(bromo-methyl) benzoate (0.225 g, 0.96 mmol) was charged into the flask an ambient temperature (10 °C) for initiating the reaction.
Then, the reaction flask with final solution was subjected to degas by a vacuum pump and backfilled several times with nitrogen from a balloon for 10 min and kept into a preheated oil bath at 110 °C. This set of apparatus was fitted with a condenser. Before quenching the polymerization reaction by allowing air leaving a deep green opaque viscous solution, a predesigned reaction time was framed.
The viscous polymer solution was dropped into methanol (250 mL), resulting in a green jelly-precipitated polymer onto the bottom of the beaker, and a mixture of unwanted copper/ligand complex and DMF with methanol was decanted. The jelly polymer was washed with fresh methanol (100 mL); then, washed polymer was dissolved in dichloromethane (200 mL) and passed through an activated alumina (neutral) column (1 inch diameter and 5 inch length). Dichloromethane solvent was drifted partially from colorless polymer solution through a rotary evaporator. A thicker polymer solution was then precipitated into methanol (250 mL), and finally, a white polymer was obtained which was dried under vacuum at 40 °C for 48 h.
Synthesis of PS-b-PHEAAm diblock copolymers
PS with DP = 30 (0.54 g, 0.16 mmol) was dissolved in 10 mL DMF in a 50-mL reaction flask, and HEAAm (5.15 g, 43.48 mmol) monomer was charged into it. By purging argon, the solution was deoxygenated for 30 min; therefore, a colorless, transparent solution was appeared in the flask. Then, a catalyst Cu(I)Br (23 mg, 0.16 mmol) was added to the solution and developed a very light green color solution. When the ligand, PMDETA (44µL, i.e., 3.6 mg, 0.20 mmol) was charged into the flask and slowly the solution color was turned into light bluish green. The solution in the flask was degassed and backfilled with inert argon gas several times by vacuum pump for 10 min and dipped into a preheated (110 °C) oil bath. With magnetically vigorous stirring, the reaction was conducted for 24 h. Finally, a dark brown color solution was observed.
The resultant final polymer solution was slowly cooled to room temperature and transferred into cellulosic dialysis tubing. The dialysis was performed against deionized water and changing frequently for first 12 h. Then, the dialysis was conducted for at least 5 day to ensure the removal of unreacted HEAAm monomer. From the milky white polymer in the tubing, water was removed by rotary vacuum evaporator at 70 °C. The product was dried in a vacuum dryer at 40 °C for 48 h, and 0.96 g dried polymer was found.
Characterization
The chemical bonds of polymers were determined by analyzing the spectra (4000–600 cm−1) recorded by ATR-IR spectroscopy. The instrument used was a PerkinElmer 2000, spectrum one FT-IR spectrometer with a diamond (USA), single bounce foundation series ATR accessory and a 45° angle of incidence. At a resolution of 4 cm−1, each spectrum was obtained by cumulating 32 scans. Thermo-Electron Corp. CHNS analyzer (Flash-EA-1112) (Japan) was used for elemental analysis (EA). Structural analyses of the synthesized polymers were performed on a JEOL 400 MHz 1H-NMR (Japan) where CDCl3 and DMSO-d6 were used as solvents.
The optical absorption spectra of rhodamin B, PS-b-PHEAAm with and without rhodamin B were observed separately using UV–vis spectrometer (UV-1601, Shimadzu, Japan) for investigation of encapsulation of rhodamin B dye into PS-b-PHEAAm micelle in different environments.
The average molecular weight of PS was measured by SEC with a column (Shodex KF-806 M, Mw 500–20,000,000) in THF using a HPLC pump (JASCO 880 PU), a column thermostat (JASCO CO-2060) and a UV detector (JASCO) equipped with a Chromatopac (C-R6A). The flow rate, 1 mL/min and temperature of the column oven at 45 °C were maintained. Polystyrene standard samples were used to develop the calibration curve. The polymer solution was purified to pass through a 20 µM filter and a 20 µL solution was injected. Using Agilent 1260 Infinity II-MDS instrument with two PLgel Mixed-D columns operating in DMF, SEC of the block copolymers were measured. The refractive index detector (RID) was used in case of PS-b-PHEAAm diblock copolymers. The column used was calibrated using a polystyrene standard with a weight-average molecular weight (Mw) ranging from 1,000 to 3,040,000, and a root means square value R2 = 0.9995 was obtained.
Thermo-gravimetric analysis (TGA) was performed using a Mettler Toledo TGA/STDA 851 (Switzerland). Heating was performed at 10 °C min−1 in the presence of air.
An electrophoretic light scattering spectrophotometer (Otsuka Electronics Co., Ltd., Osaka, Japan) with a 90° scattering angle was used to collect the dynamic light scattering (DLS) data. The light source of DLS was a 10 mW He–Ne laser. Millipore filter (0.45 μm) was used to prepare all the sample solutions by filtration, and the samples were kept at given temperatures to reach equilibrium before the measurements. The data were collected at 25 °C and analyzed using the Marquardt method. On the basis of our intuition, we adjusted concentration of the block copolymer in the DMF or DMSO so that the final solution contained 1 mg polymer per mL of solvent or solvent mixture for DLS measurements.
Results and discussion
Synthesis of block copolymers using higher hydrophilic and higher hydrophobic monomers is still very challenging. Direct diblock copolymer synthesis is more difficult than modified techniques. Before moving forward, here we demonstrate an overview on some more recent results, including the author’s contributions, concerning the synthesis of well-defined block copolymers, the preparation and characterization techniques. In our previous article, [27] a well-defined structure of PMMA-b-PHEAAm diblock copolymer was reported where direct ATRP in DMF and DMF-water solvents system was applied separately. The effect of solvent on the formation of second PHEAAm block has also been discussed elaborately. Block copolymers, poly(styrene-b-methyl methacrylate) [28, 29], polystyrene-b-poly(4-vinyl pyridine) [30], polystyrene-b-poly(2-vinyl pyridine) [31] and polystyrene-b-poly(acrylic acid) [32] were reported by different research groups separately. Very recently, Bicak et al. [33] have reported an excellent synthetic procedure of PHEAAm-b-PS diblock polymer by combination of ATRP and aminolysis. Poly(ethyl acrylate) (PEA) was synthesized instead of PHEAAm, and then, the PEA-b-PS diblock copolymer was produced via sequential ATRP. Finally, the block copolymer was treated through aminolysis using ethanolamine results the targeted PHEAAm-b-PS diblock copolymer.
The synthesis of diblock copolymer PS-b-PHEAAm by sequential ATRP is outlined in reaction scheme. Well-defined PS chains were grown by stimulating from initiator, and the resulting end-capped PS chains with bromide acted as polymeric macro-initiator for controlled radical polymerization of HEAAm monomers.
The reaction parameters and characteristics for polystyrene homopolymers are summarized in Table 2. MBMB for homopolymerization of styrene gives excellent PS yield at 90 and 110 °C. At 100:1 molar ratio of styrene monomer and initiator, the DPs of PS were found exactly 100 measured from 1H-NMR and SEC analysis after 5 h of reaction time at 110 °C. SEC traces of PS homopolymers with different monomer and initiator and reaction time are presented in Fig. 1. Since PS is more lipophilic than PMMA, we envisaged to synthesize smaller DP of PS and therefore reduce molar ratio to 30:1 for second trial. It is important to mention that we have already successfully synthesized PMMA-b-HEAAm diblock copolymers [27] by direct ATRP. Till today, no body yet reported also to synthesize PS-b-PHEAAm by ATRP only. The second trial also gave similar result with DPs = ca 31 of PS within 3 h reaction time at 90 °C. Therefore, the polymerization time was further decreased 1 h and conducted reaction for 2 h with maintaining the same reaction conditions, results PS DPs of 18 or 20. Polydispersity index (PDI) of PS obtained from the reaction at 110 °C is 2.14, whereas at 90 °C PDI value was found 1.24. This result shows that at high temperature, reaction gave polydispersed polymers compared to the polymers obtained from polymerization carried out at low temperature.
Effect of solvent on ATRP of PS-b-PHEAAm
Among the hydrophilic polymers poly(hydroxyethylacrylamide) (PHEAAm) is one of the relatively higher hydrophilic polymers. Based on water holding capacity, i.e., hydration number (4 mol of water per repeating unit), PHEAAm is the highest one ever known water-soluble polymers [33]. Therefore, this polymer has been described as a promising material for advanced potential applications in variety of fields, [5, 7] including biology, biomedical science, surface chemistry, and electrochemistry, due to its unique properties, such as solubility and flexibility of the chains. Because of biocompatibility, solubility in water and organic solvents, this polymer is widely used as a carrier polymer.
The ATRP of HEAAm from PS-Br macroinitiator was successfully carried out using Cu(I)Br/PMDETA as catalyst in DMF as shown in Fig. 2A. Our first attempt was to search suitable solvent to perform reaction of block polymerization. Therefore, at first we used dioxane as a solvent, since Nykänen et al. [34] have reported a successful polymerization condition for PS-b-PNIPAAm-b-PS polymer via RAFT. Then, dichloromethane as the second choice of solvent for polymerization was chosen because Shinde et al. [42] have reported a synthetic procedure for PS-b-NIPAAm diblock copolymers in dichloromethane through ATRP in the presence of Cu(I)Br/HMTETA catalytic system. These solvents did not work for block polymerization from PS-Br with HEAAm, although we tried several experiments with varying DP’s of PS-Br and temperature, in the presence of Cu(I)Br with PMDETA or HMTETA systems. Besides, the block polymerization did not proceed even at 110 °C in DMF, when we used PS-Br of 10,800 Da. The possible reason of unsuccessful polymerization would be the higher lipophilic characteristic of polystyrene became insoluble in presence of the higher hydrophilic HEAAm monomer producing a white precipitate in DMF. Therefore, we used PS-Br of DP = 30 to conduct ATRP in the presence of Cu(I)Br and PMDETA at 110 °C and obtained the desired block copolymer. In order to confirm the polymerization condition, another reaction was carried out using PS-Br of DP = 18 without changing the earlier reaction condition, resulting in a block copolymer with high content of PHEAAm segment. This phenomenon indicates that the smaller the PS-Br, the easier the polymerization. Interestingly, the growth of PHEAAm segments for both of the two polymers after 24 h was found almost same, which indicates that the rate of polymerization was well controlled. Initially, the purification of polymer by precipitation into solvent was difficult, because this block copolymer is amphiphilic in nature, no solvent looks better for precipitation. Besides, the polymer solution was too dilute to effective precipitation. Therefore, we introduced dialysis technique for purification of block copolymers using cellulose membrane tubing with MWCO 3,500. The kinetics of the block polymerization was not studied, rather than we emphasized to screen out a suitable solvent for ATRP polymerization. These results suggest that Cu(I)Br/PMDETA catalyst in only pure DMF at around 110 °C would be suitable for steadily growth of PHEAAm segment as shown in Table 3. The result of EA also reveals an increment of nitrogen content from 6.94 to 8.22% when the DP of macroinitiator was changed from 30 to 18 and the resulting polymers with DP of HEAAm segment were obtained 39 and 41, respectively.
Spectral analysis
ATR-IR spectroscopic analysis was conducted to determine the functional group of polymers as shown in Fig. 2B. The hydroxyl, amide and alkyl functional groups present in PHEAAm (Fig. 2B-I) attributed the bands at 3282 and 1060, 1635 and 2925 cm−1, respectively. The monosubstituted phenyl ring and unsaturated carbon = carbon double bond in the ring of polystyrene (Fig. 2B–III) showed bands at 697, 755 and 1600 cm−1, respectively. The block copolymers (Fig. 2B–II) showed almost all bands present in each homopolymer, polystyrene and PHEAAm, namely at 1600 and 697 cm−1 for phenyl and at 1639 cm−1 for amide functional groups.
The number average molecular weight of PS was also determined by 1H-NMR analysis in CDCl3 at 25 °C demonstrated in Fig. 2C–I. Although the proton signals of initiator such as methylprotons (–CH3, δ = 3.9–4.0) and two phenyl protons (2H, δ = 7.8–8.0) produced very clear peak, we have estimated Mn values from the area ratio of methyl (3H) protons of initiator indicated as “a” and the phenyl (3H) protons present in the monomer units identified as “h and i” as shown in Fig. 2a. The number average molecular weight of PS-b-PHEAAm diblock copolymers was determined by 1H-NMR analysis in deuterated DMSO-d6 at 25 °C depicted in Fig. 2C–II. The protons presence in the structural formula of block copolymers was identified and labeled. Since the proton signals of initiator’s methyl group were detected clearly, here we have calculated the Mn values by comparing from the area ratio with hydroxyl group (δ = 4.75–5.2) of HEAAm units. Furthermore, we have cross checked from the area of phenyl ring protons denoted as “h and i” of PS segments and hydroxyl group of HEAAm units. Interestingly, both of the two systems ensure the calculated DP values of HEAAm segment are same.
Thermal analysis
Thermal stability of polymers was explored by TGA in the range of 30 to 600 °C. As shown in Fig. 3b, the TGA analysis of PS30 shows a one-step thermal decomposition at 350 °C, with a maximum point at 400 °C in the differentiating curve of PS. Besides, TGA analysis of the block copolymer shows a two-step segmental thermal decomposition pointing two maximum points at 300 and 460 °C as shown in Fig. 3b. The block copolymer PS18-b-PHEAAm41 with lower number of PS units showed relatively lower decomposition temperature than the block polymer PS30-b-PHEAAm39 with higher PS unit.
It may be explained from the antagonistic behavior of PS and HEAAm segment as due to higher repulsive forces between the blocks, help to stack tightly the segments separately which is governed by the π-electrons of phenyl ring and hydrogen bonds produced from hydroxyl groups. The two maxima for the PS-b-PHEAAm copolymers can be assigned to the decomposition of the PS and PHEAAm segments, respectively.
Co-solvent effect on aggregation
The robust inherent ability of amphiphilic block copolymers to generate morphologically diverse molecular nanoarchitectures by self-assembly process has extensively been exploited by scientific community in the last decades and it is no surprise to notice these technologically advanced copolymers to find potential applications in laboratory, biology, catalysts, medicine, microelectronics, biomaterials, photoelectric materials and other industrial arena. Linear amphiphilic block copolymers have extensively been studied which contain two or more frequently immiscible and chemically distinct blocks that are connected through covalent bonds. This immiscibility offers the block copolymers to self-assemble in a selective solvent. A thermodynamically good solvent for one block which at the same time acts as a precipitant for another block forces the copolymer chains to organize in a reversible fashion leading to the formation of micellar aggregates of nanoscopic dimensions. As a result, different shapes are generated similar to those obtained from low molecular weight surfactants. In this micellar arrangement, a more or less swollen core of the insoluble blocks are surrounded by a flexible corona of the soluble blocks. The aggregation of insoluble blocks to form spherical micelle is observed when the soluble blocks are predominant. In order to minimize the thermodynamically unfavorable solvophobic interaction, the self-assembly of copolymer chains arises. The formation and growth of the micelle are controlled by a combination of two forces: attractive force between insoluble blocks and repulsive force between soluble blocks. However, the micelles are ultimately stabilized by the interaction of soluble blocks with the solvent [1, 34, 44] (Fig. 4).
In order to investigate the effect of solvents on the morphological transformation, we have employed four different protic (H2O) and aprotic (DMSO, DMF, THF) solvents. As it has been well established that the dielectric constant of solvent and/or solvent pairs largely controls the nature and size of the macromolecular assemblies, we have used four types of co-solvents/solvent pairs such as H2O/DMSO (1:1), H2O/DMF (1:1), THF/DMF (9:1) and THF/DMSO (9:1). Among these four solvents, H2O has the highest dielectric constant (80.1) while THF has lowest dielectric constant (7.5). This value is higher for DMSO (47) compared to DMF (38). As governed by this value, the solvent pairs H2O/DMSO (1:1) and H2O/DMF (1:1) create hydrophilic environment for polymeric macromolecules whereas THF/DMF (9:1) and THF/DMSO (9:1) create lipophilic environment. Both of the hydrophilic and lipophilic co-solvents are responsible for the generation of two different reversible core–shell assembly known as micelles. When amphiphilic block copolymer is placed in H2O/DMSO (1:1), the higher hydrophobic PS block encounters an extremely hostile hydrophilic environment due to high dielectric constant of the medium. Consequently, rapid aggregation of several macromolecular chains occurs leading to the formation of larger size spherical micelle (355 ± 110 nm). However, due to the low dielectric constant of H2O/DMF (1:1) compared to H2O/DMSO (1:1), a relatively smaller size micelle (272 ± 70 nm) is formed. In both of these cases, the core of the micelle is formed by styrene whereas the shell is constituted from HEAAm. On the contrary, opposite morphological transformation occurs in lipophilic environment, i.e., formation of micelle with HEAAm in the core and styrene in the shell. Larger size particle (26 ± 7 nm) is generated in THF/DMSO (9:1) due to high dielectric constant while low dielectric constant in THF/DMF (9:1) is responsible for the formation of smallest size (13 ± 4 nm) particle. This sort of observation opens plenty of room at the bottom for the investigation of several amphiphilic block co-polymers in different solvent pairs having variation in their dielectric constants (Fig. 5).
Interaction between PS18-b-PHEAAm41 and Rhodamin B
In Fig. 6A and B, it is revealed that an interaction between a textile dye Rhodamin B and PS18-b-PHEAAm41 polymer was investigated into different co-solvents. Here, ATR-IR spectroscopic analysis was conducted using aggregates produced from THF/DMSO (9:1) and H2O/DMSO (1:1) and compared with the spectrum of poly(styrene-b-HEAAm). A band at 1720 cm−1 observed from the aggregates assembled in the environment H2O/DMSO (1:1) for carbonyl group of Rhodamin B was significantly larger than that aggregates produced from THF/DMSO (9:1) co-solvent. Carbonyl group band for neat polymer was almost negligible and was enlarged when interacted with Rhodamin B during formation of aggregates.
Rhodamin B in DMSO, Rhodamin B and PS18-b-PHEAAm41 polymer in DMSO and two different co-solvents [e.g., THF/DMSO (9:1) and H2O/DMSO (1:1)] were investigated using UV–vis spectroscopy. It is revealed that the absorbance at maxima recorded from Rhodamin B and PS18-b-PHEAAm41 polymer in DMSO was highest even compared to that of Rhodamin B in DMSO. This can be explained as Rhodamin B interact with polymer specially with styrene segment, it became more soluble into solution and the higher solubility ensure absorption of light easily. It means that the polymer also assists to create a suitable environment with required dielectric constant. Similarly, THF/DMSO (9:1) produced smaller aggregates and the filtrate solution gave higher absorption maxima compared to that of H2O/DMSO (1:1). It can be easily explained that the bigger aggregates entrapped larger amount of Rhodamin B, therefore the remaining lower concentrated Rhodamin B in the filtrate showed the lowest absorption maxima.
Conclusions
We have successfully established a new synthetic route for synthesis for diblock copolymer, PS-b-PHEAAm through two-step direct ATRP technique. CuBr/PMDETA catalyst system in pure DMF solvent at 110 °C would be a suitable condition for advanced kinetic study of block polymerization. The block copolymer containing hydrophobic polystyrene (PS) and hydrophilic N-Hydroxyethylacrylamide (PHEAAm) segments was confirmed from ATR-IR, 1H-NMR, and DLS measurements. The TGA trace for the PS-b-PHEAAm diblock copolymers shows that the decomposition temperature varies with the size of PS segment. This functional amphiphilic block copolymer is attractive for biomedical applications for preparations of vesicles, micelles, nanoparticles for gene carrier, etc. This polymer would also be crucial for the study of surface modification by means of film preparation and micro-phase separation. The proposed new synthetic pathway permits the design of well-defined multiblock copolymers via atom transfer radical polymerization (Scheme 1).
Data availability
No datasets were generated or analysed during the current study.
References
Riess G (2003) Micellization of block copolymers. Prog Polym Sci 28:1107–1170
Virtanen J, Holappa S, Lemmetyinen H, Tenhu H (2002) Aggregation in aqueous poly (N-isopropylacrylamide)-block-poly (ethylene oxide) solutions studied by fluorescence spectroscopy and light scattering. Macromolecules 35:4763
Matyjaszewski K, Xia J (2001) Atom transfer radical polymerization. Chem Rev 101:2921
Zhengyu D, Shiyong L (2020) Emerging trends in solution self-assembly of block copolymers. Polymer 207:122914
Hule RA, Pochan DJ (2007) Polymer nanocomposites for biomedical applications. MRS Bull 32:354
Hurter PN, Alexandridis P, Hatton TA (1995) Solubilization in Surfactant Aggregates. In: Christian SD, Scamehorn JF (eds) Surfactant Science Series. Marcel Decker, New York
Bockstaller M, Kolb R, Thomas EL (2001) Metallodielectric photonic crystals based on diblock copolymer systems. Adv Mater 13(23):1783–1786
Jeng US, Sun YS, Lee HY, Hsu CH, Liang KS, Yeh SW, Wei KH (2004) Binding effect of surface-modified cadmium sulfide on the microstructure of PS-b-PEO block copolymers. Macromolecules 37:4617
Sriprom W, James M, Perrier S, Neto C (2009) Ordered microphase separation in thin films of PMMA-PBA synthesized by RAFT: effect of block polydispersity. Macromol 42:3138–3146
Heish HL, Quirk RP (1996) Anionic polymerization: principles and practical applications. Marcel Dekker, New York
Matyjaszewski K (1996) Cationic polymerizations: mechanisms, synthesis and applications. Marcel Dekker, New York
Novak BM, Risse W, Grubbs RH (1992) The development of well-defined catalysts for ring opening olefin metathesis polymerization (ROMP). Adv Polym Sci 102:47–71
Nagarajan S, Srinivasan KSV (1995) Redox polymerization process: an efficient tool for the synthesis of block copolymers. J Polym Sci Part A: Polym Chem 33:2925
Ozturk T, Cakmak I (2007) Synthesis of block copolymers via redox polymerization process: a critical review. Iran Polym J 16(8):561–581
Temel Ö, Mahmut Y, Melahat G, Baki H (2016) One-step synthesis of triarm block copolymers by simultaneous atom transfer radical and ring-opening polymerization. Polym Bull 73(6):1497–1513. https://doi.org/10.1007/s00289-015-1558-2
Temel Ö, Cansu Y (2020) Synthesis of block copolymer including polyepichlorohydrin and polyethylene glycol by “click” chemistry: evaluation of primary parameters of copolymerization. Polym Bull 77:4773–4788. https://doi.org/10.1007/s00289-019-02989-4
Kamigaito M, Ando T, Sawamoto M (2001) Metal-catalyzed living radical polymerization. Chem Rev 101:3689
Hawker CJ, Bosmon AW, Harth E (2001) New polymer synthesis by nitroxide mediated living radical polymerizations. Chem Rev 101:3661
Delaittre G, Save M, Gaborieau M, Castignolles RPJ, Charleux B (2012) Synthesis by nitroxide-mediated aqueous dispersion polymerization, characterization, and physical core-crosslinking of pH–and thermoresponsive dynamic diblock copolymer micelles. Polym Chem 3:1526–1538
Hawker CJ (1994) Molecular-weight control by a living free-radical polymerization process. J Am Chem Soc 116:11185
Wayland BB, Poszmik G, Mukerjee SL, Fryd M (1994) Living radical polymerization of acrylates by organocobalt porphyrin complexes. J Am Chem Soc 116:7943
Chiefari J, Chong YK, Ercole F, Krstina J, Jeffery J, Le TPT, Mayadunne RTA, Meijs GF, Moad CL, Moad G, Rizzardo E, Thang SH (1998) Living free-radical polymerization by reversible addition−fragmentation chain transfer: the RAFT process. Macromol 31:5559
Kato M, Kamigaito M, Sawamoto M, Higashimura T (1995) Polymerization of methyl methacrylate with the carbon tetrachloride/dichlorotris (triphenylphosphine)–ruthenium (II)/methylaluminum bis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromol 28:1721
Wang JS, Matyjaszewski K (1995) Controlled living radical polymerization atom transfer radical polymerization in the presence of transition-metal complexes. J Am Chem Soc 117(20):5614–5615
Zhang X, Matyjaszewski K (1999) Synthesis of well-defined amphiphilic block copolymers with 2-(dimethylamino)ethyl methacrylate by controlled radical polymerization. Macromol 32:1763
Kotani Y, Kato M, Kamigaito M, Sawamoto M (1996) Living radical polymerization of alkyl methacrylates and synthesis of their block copolymers. Macromol 29:6979
Ashaduzzaman M, Kunitake M (2013) Poly(methylmethacrylate)-block-poly(N hydroxylethylacrylamide) diblock copolymers: direct ATRP synthesis and characterization. Iran Polym J 22:493–499. https://doi.org/10.1007/s13726-013-0150-6
Melahat GÖKTAŞ (2019) Synthesis and characterization of poly(styrene-b-methyl methacrylate) block copolymers via ATRP and RAFT. J Inst Sci Tech 9(1):139–149
Temel Ö, Sevil SY, Baki H, Yusuf ZM (2010) ATRP of methyl methacrylate initiated with a bifunctional initiator bearing bromomethyl functional groups: synthesis of the block and graft copolymers. J Polym Sci A 48(6):1364–1373. https://doi.org/10.1002/pola.23898
Chih-Feng H, Shiao-Wei K, Jem-Kun C, Feng-Chih C (2005) Synthesis and characterization of polystyrene-b-Poly(4-vinyl pyridine) block copolymers by atom transfer radical polymerization. J Polym Res 12:449–456
Ramkrishnan A, Dhamodharan R (2000) A novel and simple method of preparation of poly(styrene-b-2-vinylpyridine) block copolymer of narrow molecular weight distribution: living anionic polymerization followed by mechanism transfer to controlled/”living” radical polymerization (ATRP). J Macromol Sci Part A Pure App Chem 37(6):621–631
Guang HL, Chang GC (2002) Synthesis and characterization of poly(vinyl alcohol-b-styrene) via atom transfer radical polymerization and saponification. Macromol Res 10(6):339–344
Gunes D, Kurtarel OB, Bicak N (2012) Poly(N-hydroxyethyl acrylamide)-b-polystyrene by combination of ATRP and aminolysis processes. J Appl Polym Sci. https://doi.org/10.1002/APP.37604
Nykänen A, Nuopponen M, Laukkanen A, Sami-Pekka H, Marjaana R, Turunen O, Tenhu H, Mezzenga R, Ikkala O, Ruokolainen J (2007) Phase behavior and temperature-responsive molecular filters based on self-assembly of polystyrene-block-poly(N-isopropylacrylamide)-block-polystyrene. Macromol 40(16):5827–5834
Bingyi L, Yan S, Wanchao Z, Zhifeng F, Wantai Y (2006) Synthesis of amphiphilic poly(styrene-b-acrylicacid) diblock copolymers by iodide–mediated radical polymerization. Polym J 38:387–394
Hyun JJ, Ji HY (2010) Synthesis of Poly(vinyl acetate)-b-polystyrene and poly(vinyl alcohol)-b-polystyrene copolymers by a combination of cobalt-mediated radical polymerization and RAFT polymerization. Macromolecules 43:2184–2189
Guillaume D, Bernadette C (2008) Kinetics of in-Situ formation of poly(acrylic acid)-b-polystyrene amphiphilic block copolymers via nitroxide-mediated controlled free-radical emulsion polymerization. Discussion on the effect of compartmentalization on the polymerization rate. Macromolecules 41:2361–2367
Cheng ZC, Edward MB, Yang Q, Frieder J (2010) Synthesis and solvent-dependent micellization of the amphiphilic block copolymer poly(styreneboronic acid)-block-polystyrene. J Polym Sci Part A Polym Chem 48:2438–2445
Khalid I, Arto S, Susanna H, Kirsi K, Henna L, Barbro L, Janne L, Jukka S (2006) Preparation and characterization of polystyrene–poly(ethylene oxide) amphiphilic block copolymers via atom transfer radical polymerization: potential application as paper coating materials. J Appl Polym Sci 102:4304–4313
Kok HW, Thomas PD, Christopher B-K, Martina HS (2007) Honeycomb structured porous films from amphiphilic block copolymers prepared via RAFT polymerization. Polymer 48:4950-e4965
Ashaduzzaman M, Kunitake M (2013) Polystyrene-block-poly(N-isopropylacrylamide) thermo-responsive diblock copolymers: synthesis and characterization. Int J Mater Sci Innov (IJMSI) 1(3):124–132
Shinde VS, Girme MR, Pawar VU (2011) Thermoresponsive polystyrene-b-poly(N-isopropylacrylamide) copolymers by atom transfer radical polymerization. Ind J Chem 50:781–787
Temel Ö, Ismail C (2008) Synthesis of poly(ethylene glycol-b-styrene) block copolymers by reverse atom transfer radical polymerization. J Polym Res 15(3):241–247. https://doi.org/10.1007/s10965-007-9164-0
Karayianni M, Stergios P (2016) Self-assembly of amphiphilic block copolymers in selective solvents. Fluoresc Stud Polym Contain Syst. https://doi.org/10.1007/978-3-319-26788-3_2
Acknowledgements
The authors express gratitude to the Centre for Advanced Research in Sciences (CARS), University of Dhaka, Bangladesh, for partial analytical support. We also thank the department of Applied Chemistry and Chemical Engineering, University of Dhaka for laboratory facilities to carry out this research. For providing a collaboration platform under its R&D World Links, the authors thank the International Association of Advanced Materials (IAAM), Sweden.
Author information
Authors and Affiliations
Contributions
Md. Ashaduzzaman contributed to conception of the work, data analysis, and writing—review and editing; Shaikat Chandra Dey contributed to data collection and analysis, writing—review and editing; Md. Kaium Hossain contributed to data collection and drafting the article; Ashutosh Tiwari contributed to writing—review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ashaduzzaman, M., Dey, S.C., Hossain, M.K. et al. Mechanistic interactions in polystyrene-block-poly(N-hydroxyethylacrylamide) diblock copolymer-based nano-corona toward elucidation of solvation responses. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05424-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05424-5