Abstract
Arsenic (As), a hazardous and carcinogenic heavy metal, poses a significant threat to both human health and the environment. Given its toxicity even at low concentrations, there is a pressing need for a low-cost technology for arsenic removal from environmental samples. In this study, a highly selective and reusable ion-imprinted polymer was synthesized to efficiently remove As from various environmental samples. The synthesis involved using methacrylic acid as a functional monomer, As(III) as a template, and multi-walled carbon nanotubes as a supporting material (MWCNT-IIP). To confirm the specificity of MWCNT-IIP for As(III), three other types of sorbents were also prepared: IIP, MWCNT-NIP, and NIP (non-imprinted polymer). Various parameters, including the initial concentration of metal ions, time, pH, and solvent medium, were systematically checked and optimized. In the adsorption study, the total capacity of the developed system for As(III) reached a linear range of 1–5 ppm, with an adsorption capacity of 27.14 mg/g. At pH 5, with a 40 min binding time, MWCNT-IIP demonstrated itself as an effective sorbent with a high percentage of removal efficiency. Inter- and intrachain interactions of the fabricated sorbents were calculated using the Equilibrium Water Content (EWC) method. In conclusion, MWCNT-IIP presents itself as an excellent alternative approach for removing As(III) from various environmental samples, including those collected from lakes, pigments, mining, and insecticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As) is one of the world's major environmental concerns due to the significant potential health hazards associated with its contamination of groundwater sources. Arsenic poisoning has become a major environmental problem globally. It is classified as a Group 1 carcinogenic substance for humans. When arsenic species are soluble in water, they can exist in both inorganic and organic forms, such as As(III) arsenite and As(V) arsenate [1]. The toxicity of arsenic species varies depending on the oxidative state and their presence in the environment [2]. Arsenite exists mostly in a completely protonated form, whereas arsenate is an anion that can be found in many ionic forms in dissolved species. Millions of people have been exposed to excessive arsenic through polluted groundwater used for drinking. In small quantities, arsenic is essential as a nutrient for our bodies; if ingested beyond the permissible limit, it becomes highly toxic [3,4,5]. According to the guidelines recommended by the World Health Organization, the maximum limit of arsenic in drinking water should not exceed 10 ppb. Arsenic has been used in various fields such as mining, glassmaking, fertilizers, wood preservatives, pharmaceuticals, and semiconductor industries.
The intake of arsenic (As) has severe carcinogenic effects on the kidneys, brain, lungs, and nervous and respiratory systems [6]. Therefore, there is a critical need to extract arsenic ions from environmental samples. Various methods have been reported for the detection of arsenic ions, such as atomic absorption spectrometry [7], inductively coupled plasma mass spectrometry [8], and chromatographic separation [9]. Despite the introduction of newer and more advanced equipment like ICP-AES and ICP-MS, Flame Atomic Absorption Spectroscopy (FAAS) has remained a preferred technique for the sequential determination of elements for many years. Thus, the development of novel adsorbents for enhanced selectivity has been the primary driver of analytical speed.
Ion-imprinted polymers (IIPs) represent a novel class of adsorbents with notable advantages, including easy and cost-effective synthesis, excellent stability and robustness, tolerance to high temperatures and pressures, and applicability to a wide range of target molecules. The ion imprinting polymer (IIP) has gained significant attention as a sorbent due to its high selectivity, sensitivity, and durability against various chemical environments [10].
The principle behind ion imprinting polymers is rooted in the lock-and-key concept of supramolecular chemistry. In this process, the polymerizable binding group is bonded to a metal ion, which is then copolymerized in the presence of a crosslinking agent, resulting in a crosslinked polymer. The removal of the metal ion creates a binding cavity that mimics that of the metal ion. The resulting polymer contains precise binding cavities, exhibiting high selectivity and sensitivity for the targeted metal ions used in the polymer preparation.
Despite significant efforts invested in IIPs, a certain degree of attenuation is still associated with their highly crosslinked structure [10, 11]. The heterogeneous distribution of binding sites results in the thorough incorporation of the template and functional monomer inside the polymer network, leading to disadvantages such as insignificant binding capacity, slow mass transfer, and small recognition sites [12, 13].
To overcome these challenges and expose the recognition sites, functional groups are grafted onto the surface of solid matrices during synthesis. IIP structures have been immobilized on various supports, such as Fe3O4 [14], TiO2 nanoparticles [15]. Selecting appropriate support is crucial; in this regard, multi-walled carbon nanotubes (MWCNTs) stand out as ideal support materials due to their high surface area, thermal, electrical, chemical, and mechanical stability, along with strong interactions for grafting onto the IIP [16].
Numerous researchers have dedicated their efforts to devising innovative strategies for eliminating toxic anions and cations from water, aiming to ensure the safety of drinking water and preserve natural aquatic ecosystems. The material proposed in this study is constructed from polymers and has demonstrated stability to changes in pH and contact time. Importantly, the estimated cost of the proposed material is not prohibitively expensive.
The research goals can be divided into two main objectives: (1) to explore the feasibility of removing arsenic from environmental samples using a synthesized ion-imprinted polymer with As(III) as a template, methacrylic acid (MAA) as the monomer, and vinyl-functionalized multi-walled carbon nanotubes (MWCNT) as nanolayer support, with N, N-methylene-bis-acrylamide (NNMBA) serving as the crosslinking agent [17, 18] and (2) to characterize the MWCNT-IIP in batch mode, encompassing binding studies and optimization. Following successful characterization and optimization, the system was applied for selectivity studies involving other metal ions. Ultimately, a highly selective and reusable MWCNT-based ion-imprinted polymer proved effective in extracting arsenic from waste ecological samples.
Experimental
Materials
MWCNT with a diameter of 10–15 nm was obtained from Reinsto Nano Ventures Private Limited in India. Methacrylic acid (MAA) and metal chlorides were purchased from SRL (India), while N, N’ Methylene-bis-acrylamide (NNMBA) and potassium peroxydisulfate (K2S2O8) were obtained from Sigma-Aldrich (Germany). Thionyl chloride (SOCl2), dimethylformamide (DMF), and tetrahydrofuran (THF) were acquired from Merck (India). All chemicals used in this study are of analytical grade. For the synthesis and washing purposes of the polymer, double-distilled water was used.
Instruments
The FTIR spectra were analyzed using a PerkinElmer 400 FTIR spectrophotometer. X-ray diffractograms of the adsorbent were obtained using a PANalytical XPERT-PRO instrument. Morphology and elemental composition studies of the synthesized nanolayered polymer were conducted with a JEOL-2100 model tunneling electron microscope equipped with EDX. Absorption spectra of As(III) ions were measured using a PerkinElmer Atomic Absorption Analyzer 300. Thermogravimetric analysis was performed using the NETZCH STA449C instrument. All studies in this paper were conducted at room temperature.
Preparation of MWCNT functionalized with a vinyl group
Carboxylic acid functionalization of MWCNT was carried out by mixing 0.5 g of MWCNT with 60 ml concentrated HNO3 and subjected to sonication for 10 min. The mixture was then refluxed for 24 h while maintaining a minimum temperature of 60 °C with constant stirring. Afterward, the mixture was diluted with distilled water and filtered through a Whatman No. 41 filter paper. Continuous washing with distilled water was performed until the solution pH became neutral. The solid obtained after washing was then dried under a vacuum at 80 °C to obtain MWCNT functionalized with a carboxyl group [19] (MWCNT-COOH).
The next step involved the synthesis of acid chloride functionalization. For this, MWCNT functionalized with a carboxyl group (0.4 g) was treated with a mixture of thionyl chloride (10 ml) and chloroform (30 ml), and the mixture was refluxed at 60 °C for 24 h with constant stirring. After cooling, the mixture was continuously washed with anhydrous THF to remove excess thionyl chloride. The solid obtained was then dried under a vacuum for 24 h to obtain COCl functionalized MWCNT (MWCNT-COCl).
The production of vinyl-functionalized MWCNT was carried out using the following procedure. Acid chloride functionalized MWCNT (0.3 g) was dispersed in 30 ml THF, followed by adding 20 ml allylamine with 10 ml of DMF in a round-bottom flask, and allowed to react at 80 °C for 24 h with continuous stirring [20]. The mixture was then allowed to cool and washed frequently with anhydrous THF to remove excess reagent. The obtained MWCNT-CH = CH2 solid was then dried under vacuum and used for further studies in this paper.
Synthesis of As(III) ion-imprinted and non-imprinted polymers
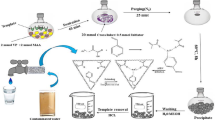
Ion-imprinted polymer loaded with As(III) ions was produced through free radical polymerization of the ion-imprinted layer on the vinyl-functionalized MWCNT surface (MWCNT-IIP). For the preparation of MWCNT-IIP, 0.6 g of vinyl group functionalized MWCNT was added to water in a 250 ml round-bottom flask and purged with N2 under constant magnetic stirring. The template ion, arsenic chloride (AsCl3, 0.6 g), and methacrylic acid (MAA, 0.025 mmol) were added to the reactor and mixed well for half an hour to form a template molecule and functional monomer complex. Then, 8 g N, N’ Methylene-bis-acrylamide (NNMBA), and 100 mg potassium peroxy disulfate (K2S2O8) were also poured into the template molecule and functional monomer complex. The temperature was maintained at 70 °C with constant stirring for 12 h. The synthesized polymer was systematically cleaned with soxhlet extraction, first with normal water followed by distilled water, to remove arsenic ions from the polymer surface. The elution process was continued for a period of one month to remove traces of As(III) ions from As(III) ion-bound MWCNT-IIP. It was then dried at 80 °C under a vacuum to obtain As(III) ion-imprinted MWCNT-IIP [24]. To understand the significance of MWCNT, an ion-imprinted polymer (IIP) was also prepared without using MWCNT, using the same procedure. For comparison studies, non-imprinted polymers were also prepared using the same procedure without using As(III) ions (MWCNT-NIP, NIP) (Scheme 1).
Procedure for extraction
Optimization studies of As(III) ions
For the extraction studies, different sorbents such as MWCNT-IIP, MWCNT-NIP, IIP, and NIP (10 mg) were mixed with 10 ml of As(III) ion solutions at different concentrations ranging from 1 to 5 ppm. The mixtures were continuously stirred for 2 h. The concentration of As(III) ions was determined from a standard calibration curve. The binding mixtures were then filtered, and the concentration of As(III) ions collected from the eluent was checked using atomic absorption spectroscopy (AAS). The adsorption capability of As(III) ions on the synthesized nanolayered imprinted and non-imprinted polymers was calculated using Eq. (1)
where Q (m mol/g) is the adsorption capacity of the adsorbent, V (ml) is the volume of the metal ion solution, M (g) is the weight of the polymer, and Co and Ce (mmol.−1) are the primary and equilibrium concentration of As (III) ion in the aqueous solution [21]. The kinetic studies of As(III) ions with imprinted and non-imprinted polymers were assessed at different time intervals, ranging from 0 to 100 min, without changing the concentration of As(III) ions. The effect of pH on As(III) adsorption was studied over a pH range of 3.0–7.0 while keeping the concentration of As(III) ions constant. Selectivity studies of the nanolayered imprinted and non-imprinted polymers were conducted using different metal ions, such as Cr(III), Pb(II), and Cd(II) ions, following the batch experiment method. Factors influencing adsorption, such as mass and solvent effects, were also studied using the synthesized imprinted and non-imprinted polymers. Reusability studies were carried out through adsorption–desorption studies. Swelling studies were also conducted, and the Equilibrium Water Content (EWC%) was determined using the following Eq. (2)
Application of the extraction method
The environmental samples collected from a lake, pigments, mining, and insecticides were filtered using Whatman No. 1 filter paper. The As(III) ion extraction studies of these samples were carried out using synthesized nanolayered imprinted and non-imprinted polymers, following the procedure explained above.
Results and discussion
Characterization techniques
FTIR analysis
The FTIR analysis of vinyl-functionalized MWCNT, IIP, and MWCNT-IIP is presented in Fig. 1. Vinyl-functionalized MWCNT exhibits peaks at 1756 cm−1 and 1638 cm−1, corresponding to the C = O and C = C stretching vibrations, respectively. The peak observed at 1249 cm−1 indicates the presence of the C–O bond, characteristic of MWCNT functionalized with the carboxyl group [22]. This confirms the successful layering of a vinyl group on the surface of MWCNTs. In the case of MWCNT-IIP and IIP, characteristic peaks appear at 3339 cm−1 and 1719 cm−1, resembling the groups present in methacrylic acid, which is used as the monomer in polymer production. This confirms the formation of the ion-imprinted polymer layer on the vinyl-functionalized MWCNT surface. Furthermore, the main peaks of IIP and vinyl-functionalized MWCNT are detected in the spectrum of MWCNT-IIP, establishing the development of an ion-imprinted polymer layer on MWCNT-IIP.
X-Ray diffraction spectroscopy (XRD)
The X-ray diffractogram represents the crystallinity of the MWCNT and sorbents. In Fig. 2, the XRD spectra of MWCNT, MWCNT-IIP, and IIP are displayed. The presence of a peak at 2θ = 28° corresponding to the (002) plane and 45° corresponding to the (100) plane (JCPDS 96-101-1061) confirms the crystalline nature of MWCNT [23]. Moreover, MWCNT-IIP exhibits some intense peaks in addition to the broad peak of the polymer. The results indicate that the imprinted layer was successfully coated over the nanostructure. It can be concluded that the crystalline nature of MWCNT was preserved in MWCNT-IIP.
Thermogravimetric analysis (TGA)
The thermal strengths of MWCNT, IIP, and MWCNT-IIP were examined using thermogravimetric studies. The thermogram of MWCNTs exhibits a linear graph up to 800 °C without any considerable mass loss, indicating the very high-thermal stability of MWCNTs. Moreover, the degradation temperature of MWCNT-IIP was much higher than that of IIP. The mass loss for IIP was 45%, while for MWCNT-IIP, it was 20% (Fig. 3). The mass loss of the ion-imprinted polymer was almost double that of MWCNT-IIP. The higher thermal strength of MWCNT-IIP implies that the nanolayered structures can shield the materials from rapid decomposition.
TEM analysis
HR-TEM was applied for the complete morphological investigation; the outcomes are exposed in Fig. 4. As in the case of MWCNT-IIP, the coating layer formed on the surface of vinyl-functionalized MWCNTs is very clearly detected. Moreover, for IIP, the tubular morphology is absent subsequently the absence of MWCNTs and a complex rigid structure is verified. A coagulated image was exhibited by the bulk IIP due to the agglomeration on the polymer surface.
EDX analysis
The presence and elution of As(III) ion-bound and imprinted polymers were examined by energy-dispersive X-ray analysis, as shown in Fig. 5. A clear, sharp EDAX signal exists in As(III) ion-bound sorbents due to the presence of As(III) ions which is also evident from the EDAX data table (Table 1). However, that sharp EDAX peak vanished in the case of As(III) ion-imprinted sorbents, the outcome confirmed that the As(III) ions were removed from the MWCNT-IIP, leaving behind the occupied space and forming As(III) ion-imprinted sorbents.
Swelling study
The swelling studies of prepared As(III) ion-imprinted and non-imprinted polymers were carried out using water by soaking a known amount of sorbent in water for 24 h. The results attained are tabulated in Table 2. The nature of the prepared sorbents and their inter and intrachain interactions play a very important role in swelling studies. The result clarified that the swelling of nanolayered ion-imprinted polymer was higher than other polymers without nanolayers. It is mainly due to the presence of a nanostructured layer along with the huge availability of binding sites in it. Due to the nonexistence of binding cavities, swelling studies of other polymers exhibit very poor results. Thus, we can conclude the order of swelling property as MWCNT-IIP > IIP > MWCNT-NIP > NIP.
Dispersion test
The dispersibility of MWCNT, vinyl-functionalized MWCNT and MWCNT-IIP was examined as shown in Fig. 6. The whole dispersion test of all three samples was done with a sonication reaction. The dispersibility was investigated after 24 h. Dispersion pictures of MWCNTs, MWCNT-CH = CH2, and MWCNT-IIP, fully settled after 24 h. It displays that there is no direct interaction between the MWCNT and the solvent taken for the reaction. But vinyl-functionalized MWCNT, a slight increase occurred for its dispersibility. But, MWCNT-IIP is very effortlessly dispersed in water. And it did not settle down after 24 h. Thus, the chemical alteration of MWCNTs supports to increase in its contact with an aqueous medium and thus supports its dispersibility.
Optimization of extraction studies
Effect of concentration
The adsorption measurements of all the prepared systems (imprinted/non-imprinted) were examined with various amounts of As(III) ions ranging from 1 to 5 ppm. The adsorption rate increased with the concentration of nanolayered sorbent, reaching a maximum at 5 ppm and then, decreased (Fig. 7a). Similarly, MWCNT-IIP exhibited a significantly higher binding rate toward As(III) ions due to the presence of IIP/As-covered MWCNTs. This assembly offers a dual effect with imprinted sites along with nanostructures. The exceptional properties, such as the high surface-to-volume ratio of nanolayered structures, contribute to the increased binding rate. In contrast, for IIP, where these nanolayered structures are absent, the degree of binding decreased. Additionally, in the case of MWCNT-NIP and NIP, where the imprinted sites are absent, certain As(III) ions enter into the holes of MWCNTs and exhibit a very low binding rate [20, 24]. Thus, the nanolayered sorbents can be ordered in terms of the binding rate as follows: MWCNT-IIP > IIP > MWCNT-NIP > NIP.
The binding isotherm provides qualitative knowledge about the nature of solute–surface contacts and the specific relationship between the concentration of the nanolayered sorbent and the amount of binding on the nanolayered sorbent surface at a constant temperature. The binding isotherm of As(III) ions is better fitted with the Langmuir isotherm model. The Langmuir model accounts for monolayer binding and homogeneous coverage of the nanolayered sorbent surface. As shown in Fig. 7b, a straight-line graph with a correlation coefficient (R2) of 0.9971 was achieved for MWCNT-IIP by plotting Ce/Q against Ce, and the adsorption capacity is found to be 27.14 mg/g, and it is found to be more active adsorbent for the adsorption of arsenic compared with other adsorbent (Table 3). The Langmuir isotherm model is a better fit than the Freundlich isotherm model.
Effect of pH
The pH control from 3 to 6.5 for the binding of As(III) ion by all the fabricated sorbent systems was investigated and illustrated in Fig. 8. Based on remarks, it is found that the binding amount of As(III) ion increases with pH and achieves a maximum of 5.0 with ABS. So, the binding represents a greater result in an acidic medium than in an alkaline medium. In an alkaline medium, As(III) ions get settled as precipitate that may affect the adsorption rate. Thus, pH 5.0 was considered optimal for further binding studies.
Effect of time
In binding kinetics, the rate of binding was calculated as a function of time. From Fig. 9a, it is very clear that the rate of binding of As(III) ion by As(III) ion-imprinted and the non-imprinted systems were explored. As time increases, the rate of binding initially increases. At the initial stage, the imprinted polymers keep a very large number of free active binding sites and the binding rate shows a sudden increase within 20 min then, it reaches a binding maximum of 40 min. But non-imprinted sorbents do not have a large number of binding sites. So the amount of binding was inferior to that of imprinted sorbents. The maximum binding capacity was touched within 40 min, and it reached equilibrium. Moreover, the pseudo-second-order rate equation was useful to study the kinetics of As(III) ion adsorption on MWCNT-IIP. Binding kinetics displays a straight-line graph of a pseudo-second-order rate equation with a correlation coefficient of 0.9984. The outcomes certified that the pseudo-second-order kinetic equation provided an enhanced rate as compared to the further kinetic rate equation. The rate constant of the pseudo-second-order of MWCNT-IIP is 0.5526 min−1, and for IIP, it is 0.0172 min−1 (Fig. 9b).
Effect of binding medium
The binding medium plays a very important role in polymerization reactions. The binding medium for the fabrication of imprinted sorbents is to generate the porous structure. And all the polymerization reaction elements must be soluble in the binding medium. The effect of the binding medium on As(III) ion binding was studied with three different solvents as represented in Fig. 10. As an outcome, water was considered a good solvent for the adsorption of As(III) metal ions. Methanol and acetonitrile show very low binding capacity toward As(III) metal ions because these solvents are not supposed to create porosity on the sorbent surface and that will reduce the adsorption rate. So based on the outcomes, water is selected as a suitable binding medium for all further examinations.
Effect of sorbent dosage
The influence of nanolayered sorbent dosage of As(III) ion adsorption was studied by changing the sorbent dosage from 10 to 50 mg as shown in Fig. 11. From the investigation data, it was found that the competence of metal ions removal increases with nanolayered adsorbent dosage. The high ability of adsorption with adsorbent dosage is because of the existence of a very large number of binding active sites. The rate of binding higher for MWCNT-IIP may be because of the higher surface-to-volume ratio of the existing nanostructures. The adsorption rate of non-imprinted sorbents was too low as compared to imprinted sorbents because of the unavailability of specific binding sites.
Selectivity studies
The applicability of the developed sorbents toward As(III) ion binding was checked with a binary mixture of competitive metal ions As(III)-Cr(III), As(III)-Pb(II), As(III)-Cd(II) at optimal pH. Figure 12 shows the high selectivity of the fabricated systems, the sorbents represent maximum selectivity toward As(III) ion since the imprinting sites of the fabricated sorbents are very comfortable for As(III) ion trapping. Due to some physical changes, some of the binding sites are effective for competitive metal ions.
Reusability
Metal elution is more economical if the sorbents developed can be reusable. To examine the reusability of fabricated systems, the experiment was repeated for 5successive cycles as represented in Fig. 13. The outcome demonstrates that the As(III) ion-imprinted system can be applied numerous times without a considerable decrease in its binding efficiency. The experiment confirmed that after 5 cycles, the reusability of the developed system slightly decreased but was not noticeable. This may be because of the destruction of a few recognition binding sites in the sorbent network at the time of rewashing; thus, some binding sites were not fit for metal ion template accumulation. So, the results approved the reusability and selectivity efficiency of synthesized nanolayered sorbent.
Extraction of As(III) ion using imprinted and non-imprinted polymers from environmental samples
The application of the synthesized imprinted and non-imprinted polymers was carried out using environmental samples collected from the lake, pigments, mining, and insecticides. The collected samples were filtered well to eliminate floating sewages and analysis of these samples were carried out by using AAS. The outcome of the studies was tabulated in Table 4. The recovered percentage signifies the extraction efficiency of the developed nanosystems.
Conclusion
In conclusion, MWCNT-IIP was effectively synthesized with a high arsenite capacity. The characterization results demonstrate that our proposed strategies successfully create an imprinted cavity corresponding to arsenic. The impact of various factors on the capture of As(III) from the aqueous solution was thoroughly examined. The presence of specific binding cavities for As(III) ions in the ion-imprinted layer increases selectivity and sensitivity in the presence of different metal ions. Under different optimized conditions, As(III) ions could be extracted within a linear range from 1 to 5 ppm. The optimized MWCNT-IIP exhibited a high As(III) adsorption capacity of 27.14 mg/g and a short equilibration period of 40 min at pH 5.0. Moreover, the results confirm that the system strictly follows the Langmuir adsorption isotherm and pseudo-second-order kinetic equation, with correlation coefficients of 0.9971 and 0.9984, respectively. After successful optimization, the selectivity and reusability of the system were verified. Finally, the robustness and efficiency of the fabricated sorbents were tested with various environmental samples.
References
Rajakovic L, Rajakovic-Ognjanovic V (2018) Arsenic in water: determination and removal. Arsenic: analytical toxicological studies. IntechOpen 25:9–24
Tolkou AK, Kyzas GZ, Katsoyiannis IA (2022) Arsenic (III) and arsenic (V) removal from water sources by molecularly imprinted polymers (MIPs): a mini review of recent developments. Sustainability 14(9):5222
Kirisenage PM et al (2022) Development of adsorptive membranes for selective removal of contaminants in water. Polymers 14(15):3146
Hashemi SA, Mousavi SM, Ramakrishna S (2019) Effective removal of mercury, arsenic and lead from aqueous media using Polyaniline-Fe3O4-silver diethyldithiocarbamate nanostructures. J Cleaner Prod 239:118023
Turkmen D et al (2022) Development of ion imprinted based magnetic nanoparticles for selective removal of arsenic (III) and arsenic (V) from wastewater. Sep Sci Technol 57(6):990–999
Behari JR, Prakash R (2006) Determination of total arsenic content in water by atomic absorption spectroscopy (AAS) using vapor generation assembly (VGA). Chemosphere 63(1):17–21
Rajakovic L, Rajakovic-Ognjanovic V, Antanasijevic D (2012) Review: The approaches for estimation of limit of detection for ICP-MS trace analysis of arsenic. Talanta 102:79–87
Niedzielski P, Siepak M (2003) Analytical methods for determining arsenic, antimony and selenium in environmental samples. Polish J Environ Stud 12(6):653–667.
Sedghi R, Heidari B, Kazemi S (2018) Novel magnetic ion-imprinted polymer: an efficient polymeric nanocomposite for selective separation and determination of Pb ions in aqueous media. Environ Sci Pollut Res 25(26):26297–26306
Aravind A, Mathew B (2020) Nano layered ion imprinted polymer based electrochemical sensor and sorbent for Mn (II) ions from real samples. J Macromol Sci, Part A 57(4):256–265
Fayazi M et al (2016) Fe3O4 and MnO2 assembled on halloysite nanotubes: a highly efficient solid-phase extractant for electrochemical detection of mercury (II) ions. Sens Actuators, B Chem 228:1–9
Deng F et al (2012) Preparation of conductive polypyrrole/TiO2 nanocomposite via surface molecular imprinting technique and its photocatalytic activity under simulated solar light irradiation. Colloids Surf A: Physicochem Eng Aspects 395:183–189
Aravind A, Mathew B (2019) An electrochemical sensor and sorbent based on mutiwalled carbon nanotube supported ion imprinting technique for Ni (II) ion from electroplating and steel industries. SN Appl Sci 1:1–11
Bahrani S, Aslani R, Hashemi SA, Mousavi SM, Ghaedi M (2021) Introduction to molecularly imprinted polymer. In: Interface Science and Technology, vol 33. Elsevier, pp 511–556
Hashemi SA, Bahrani S, Mousavi SM, Omidifar N, Behbahan NGG, Arjmand M, Firoozsani M (2021) Graphene-based femtogram-level sensitive molecularly imprinted polymer of SARS-CoV-2. Adv Mater Interfaces 8(24):2101466
Kumar D, Sharma R (2022) Plasmonic Nanosensors for Detection of Aqueous Toxic Metals. CRC Press, Boca Raton
Aravind A, Chacko AR, Mathew B (2022) Multi-walled carbon nanotubes based molecular imprinted polymers for sensing. Nanocomposite Mater Sens 2(14):96–109
Aravind A, Mathew B (2018) Tailoring of nanostructured material as an electrochemical sensor and sorbent for toxic Cd (II) ions from various real samples. J Anal Sci Tech 9(1):1–17
Abdul Halim A et al (2022) Systematic review study on application of Ion Imprinted Polymer (IIP) in heavy metals detection. Int J Environ Anal Chem 15:1–25
Islam A, Rais S (2022) A facile approach for grafting Ion Imprinted polymer onto magnetic multi-walled carbon nanotubes for selective removal and preconcentration of cadmium in food and wastewater samples prior to atomic spectrometric determination. Food Chem 405, Part A:134751
Ho YS (2006) Second-order kinetic model for the sorption of cadmium onto tree fern: a comparison of linear and nonlinear methods. Water Res 40:119–125. https://doi.org/10.1016/j.watres.2005.10.040
Sebastian M, Mathew B (2018) Multiwalled carbon nanotube based ion imprinted polymer as sensor and sorbent for environmental hazardous cobalt ion. J Macromol Sci A 55:455–465
Sebastian M, Mathew B (2018) Carbon nanotube-based ion imprinted polymer as electrochemical sensor and sorbent for Zn(II) ion from paint industry wastewater. Int J Polym Anal Char 23:18–28
Sooraj MP, Mathew B (2014) Structure-specific sorbent based on nanostructures for selective recognition of cimetidine from its structural analogues. J Appl Polym Sci 131(20):40947
Singh J, Kumar A, Pathak A, Palai T (2023) Adsorptive removal of Arsenic (III) from contaminated water using rice husk, tea waste and sugarcane bagasse bio-adsorbents. Water Air Soil Pollut 234(5):308
Biftu, WK, Ravindhranath K (2022) Novel adsorptive methods for the effective arsenic (III) removal from polluted water. Biomass Convers Biorefinery 1–13
Sherlala AIA, Raman AAA, Bello MM, Buthiyappan A (2019) Adsorption of arsenic using chitosan magnetic graphene oxide nanocomposite. J Environ Manage 246:547–556
Ahmed W, Mehmood S, Núñez-Delgado A, Ali S, Qaswar M, Shakoor A, Chen DY (2021) Adsorption of arsenic (III) from aqueous solution by a novel phosphorus-modified biochar obtained from Taraxacum mongolicum Hand-Mazz: adsorption behavior and mechanistic analysis. J Environ Manage 292(2021):112764
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aravind, A., Sebastian, M. & Nair, V.R. Highly selective reusable nanolayered sorbent for the elution of hazardous As (III) from environmental samples. Polym. Bull. 81, 11911–11929 (2024). https://doi.org/10.1007/s00289-024-05271-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-024-05271-4