Abstract
Using cheap epichlorohydrin and dimethylamine as monomers in order to introduce cationic groups, acrylamide as another monomer, and ammonium persulfate as initiator through free radical copolymerization in aqueous solution, cationic sacrifice agent (SA1) was synthesized. Similarly, an amphoteric sacrifice agent (SA2) containing both quaternary amine and carboxylate functional group was synthesized. The terpolymers were used as a sacrifice material for polycarboxylate superplasticizer (PCE), and its reactive mechanism in cement–PCE–water with clay system was studied, which is desirable to solve the problem of PCE sensitivity to clay. The flow behavior of cement paste containing PCE in the presence and absence of clay was assessed. It was pointed out that the presence of montmorillonite (MMT) or kaolin significantly reduced the dispersion effect of PCE, eventually decreased the slurry fluidity. Fortunately, the addition of the sacrifice agents hindered this disadvantage. The fluidity of slurry increased with the increase in the amount compared with the lack of sacrificial agent. The sacrifice agent has a positive effect on retaining the fluidity of cement pastes, and to some extent makes up for the poor dispersion of PCE in the presence of clay impurities. The adsorption of SA1 or SA2 by MMT particles was studied by total organic carbon (TOC) analyzer. The results show that SA1 or SA2 can be preferentially adsorbed on particles by MMT compared with conventional PCE which result in more releasing PCE macromolecules and less useless loss. The sacrifice agent provides a possibility for improving the adaptability of PCE in low-quality cementing materials and has a potential application.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycarboxylate superplasticizer (PCE) is one of the most commonly used admixtures in concrete [1]. It has attracted wide attention because of its advantages such as low dosage, high water reduction, excellent dispersion, and environment friendliness [2]. With the rapid development of infrastructure construction on the world, the consumption of high-quality sand and stone aggregates is rising and the use of poor-quality aggregates with clay will be inevitable [3]. Nevertheless, the clay can cause a serious negative impact on PCE, making it difficult to play its function, thus reducing the performance of concrete products [4].
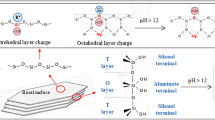
The PCE comb molecule consists of a carbon–carbon backbone bearing carboxylate groups and a polyethylene glycol side chain. By means of electrostatic interaction and steric hindrance to cement particles, PCE exists an efficient dispersing role, thereby improving the workability of concrete and reducing water volume [5]. In engineering applications, there are adaptability problems between PCE and clay such as montmorillonite (MMT), kaolin, and illite [6]. The structure of kaolin belongs to the 1:1 type clay mineral connected by aluminum–oxygen octahedron and silicon–oxygen tetrahedron which have strong interlayer bonding force and small interlayer spacing followed with almost no lattice substitution. The crystal structure of montmorillonite belongs to 2:1 type with weak interlayer bonding force, large interlayer spacing and lattice substitution. Illite layer spacing and interlayer force are in between. Different types and contents of clay have a great impact on the dispersion of commercial PCE [7]. Generally, the main reason is the surface adsorption and inserted interlayer adsorption of comb PCE molecules by clay [8].
In order to solve the above problems, scholars have carried out advanced research. One way is to increase the amount of PCE, which is costly and so undesirable. Recently, researchers have synthesized clay-resistant PCE from the perspective of molecular architecture design, which is the second strategy [9]. For example, Plank et al. designed and synthesized a series of new PCE with different anchoring groups, through maleic anhydride, 2-(methacryloyloxy)ethyl phosphate and other monomers, which led to modified chemical structure to replace traditional PCEs [10, 11]. Lei et al. prepared a series of PCE with different side chain lengths via grafting based on methoxy polyethylene glycol (MPEG) and investigated their clay resistance. The results showed that PCE held shorter PEG side chains and exhibited better clay resistance compared to its counterpart possessing a longer PEG pendant [12]. Zheng et al. reported that lignin-based PCE, which benefit from its claw-shaped structure, can wrap MMT and disperse cement well [13]. Li et al. found that an amphoteric polycarboxylate water reducer with anionic, cationic, and nonionic groups could significantly hinder the adsorption of PCE by clay. The zwitterion offers a good impedance clay property compared to conventional PCE [14]. Otherwise, photocatalysis is a promising technology in clay-resistance PCE synthesis instead of traditional initiator [15, 16]. Although these efforts have yielded some valuable results, actual difficulties still exist.
The third strategy is to combine the sacrifice agent with PCE to address the clay adaptability of PCE. Compared with the synthetic clay-resistant PCE, the prepared technology of the sacrifice agent is more economical and convenient in engineering practice [17]. For instance, Zheng et al. obtained the sacrifice agent quaternized lignosulfonate (QL) by quaternization of sulfonated lignin. QL was quickly adsorbed by MMT relying on cationic groups, thus successfully preventing the adsorption of PCE by MMT. After adding 0.010 wt% QL, the workability of cement paste with MMT is obviously improved [18]. Tan et al. tried to enhance the compatibility of PCE and clay by adding polymeric ferric sulfate (PFS). In cement paste containing MMT, PFS can form a cover layer that completely hinders the insertion of the polyoxyethylene side chain between layers. The positive charge of PFS is similar to a bridge to achieve the aggregation of MMT particles, so that the dispersion effect of PCE in mud cement can be maintained [19]. Li et al. prepared a new starch-based sacrifice agent (SSB) through sulfonation and quaternization. When the amount of SSB is 0.02wt%, the fluidity of MMT mixed cement slurry reaches 222 mm. Moreover, the introduction of SSB also improved the rheological properties of the cementing material [20]. The above studies mainly focus on the clay with single-component, which have some deviations in the practical engineering, while the studies on the multi-component clay are few. At present, the harm of clay can only be reduced instead of eliminating. In the future, the research needs to be combined with the actual engineering application, so as to better serve the engineering practice.
Organic cationic polymers are as clay anti-swelling agent, water injection, acidification, cracking, perforating fluid, drilling fluid, etc. It has excellent properties that inorganic salt, inorganic alkali and nonionic polymer cannot compare. It can inhibit the hydration expansion, dispersion and migration of shale, and has long-term stability. Based on this, in the cement–MMT slurry, MMT particles adsorbed by the sacrifice polymer are likely to be wrapped and agglomerated. In engineering practice, when using sand and stone with high mud content, the combination of PCE and sacrifice polymer in the cementitious system can maintain the dispersion effect of PCE and broaden the application range of PCE. In this work, the objective is to synthesize two novel polymer sacrifice agents with cation or amphoteric groups, respectively, via simple radical polymerization under mild conditions. The new sacrifice agents possessing height cation density for enhanced robustness toward clay by electrostatic neutralization and hydrogen bonding have been scarcely investigated. The chemical structure was analyzed by FTIR and 1H NMR spectrometer. The flow properties were tested in the presence or absence of clay in the cement slurry. The role of synthetic polymers in cement–PCE systems with clay will be discussed. The prepared sacrifice polymers are expected to enhance the clay tolerance of PCE and provide a feasible strategy for PCE adaptability to inferior aggregates.
Experimental
Materials
Acrylamide (AM) and dimethylamine (33% aqueous solution) (DMA) are supplied by Beijing Chemical Plant in China for direct use. Acrylic acid (AA) was supplied by Aladdin Reagents. Epichlorohydrin (ECH) was purchased from Tianjin Yongsheng Fine Chemical Co., Ltd., China. The ammonium persulfate (APS) and anhydrous sodium sulfite were obtained from Aladdin (Shanghai). Deionized water was used throughout this work. The Ordinary Portland cement (P.O 42.5) was provided by Jilin Yatai Cement Company. A commercial polycarboxylate ether (PCE) with 40% solid content and the 30% water-reducing ratio was produced by Hunan Zhongyan Building Materials Technology Co., Ltd., China. Kaolin and montmorillonite were purchased from Shijiazhuang Winbond Mineral Products Co., Ltd., Hebei China.
Synthesis of sacrificial agents
The first type of sacrifice polymer (SA1) comes from AM, ECH, and DMA raw materials. The typical synthesis steps are as follows: 7.108 g (0.1 mol) of acrylamide and deionized water are added into a 250-mL three-necked flask with a round bottom, stir until dissolution. Then, the pH was adjusted to 8–9 with 10% NaOH aqueous solution by weight, and the temperature was raised to 40 °C. Subsequently, 9.252 g (0.1 mol) epichlorohydrin was dropped with a constant pressure funnel within 30 min, and then, the pH was adjusted to 4 ~ 5 with hydrochloric acid. After that, 9.153 g (0.067 mol) of dimethylamine aqueous solution was dropped, followed by 0.05 g ammonium persulfate, and heated to 60 °C. Afterward, 0.05 g anhydrous sodium sulfite was added to the above solution and the reaction was carried out at 60 °C for 3 h. Finally, a transparent viscous polymer solution with 40% solid content was obtained and designated as a sacrifice agent (SA1).
The second type of sacrifice polymer (SA2) comes from AA, ECH, and DMA monomers. The method is similar to the above, except that the reaction was carried out at room temperature. Finally, the resulting light yellow clear solution was precipitated in acetone, dried at 50℃, and labeled as a sacrifice agent (SA2).
Polymer characterization
The molecular structure of the polymers was confirmed by Fourier transform infrared spectroscopy (FTIR) with Bruker Vertex-70 (Germany). The polymers were precipitated in acetone and dried overnight at 50 °C, ground, and pressed together with KBr particles into tablets. 1H NMR spectra were measured on a 400 MHz ARX-400 spectrometer (Bruker Co., Germany) using tetramethylsilane as the internal standard and deuterated water as the solvent. The relative molecular weight and polydispersity index (PDI) of the polymers were measured by gel permeation chromatography (GPC) equipped with waters and an automatic sampler. It was calibrated with polystyrene standard, and NaNO3 aqueous solution (0.1 mol/L) was used as the mobile phase.
Dispersion analysis
The fluidity of cement paste was tested using a mini-slump cone by GB/T 8077-2012 (China). The cement was partially replaced by MMT using the internal mixing method, while PCE was added at 0.2% of the cement mass. For example, the test process for SA1-M4 is as follows: a mixture of 291 g cement, 9 g clay, 87 g water, 1.5 g PCE stock solution, and 0.60 g sacrifice agent (SA1) was installed in a cement slurry mixer and slowly agitated for 1 mim. After standing for 15 s, it was quickly stirred again for 1 min. SA1 had been previously dissolved in the PCE stock solution. A truncated cone (60 mm height, 36 mm upper diameter, and 60 mm bottom diameter) was placed on a glass plate and the above mixture was poured into it before being lifted vertically. After 30 s, the spread diameters were evaluated for the initial fluidity, and the result was the average value of two perpendicular direction diffusion. In addition, the fluidity of the cement paste was measured for 30 min, 1 h, and 2 h, respectively. The formula of the cement system is shown in Table 1.
Total organic carbon (TOC)
A TOC analyzer was used to test the adsorption properties of the samples with the added PCE or sacrificial agent. TOC-L-CPN analyzer (Shimadzu Co., LTD., Japan) was used to measure the carbon content. The adsorption quantity Qad (mg/g) of clay particles on polymer was calculated according to the following formula: \({\mathbf{Q}}_{{\varvec{a}}{\varvec{d}}}=\frac{\left({\mathbf{c}}_{0}-{\mathbf{c}}_{{\varvec{t}}}\right)\mathbf{V}}{\mathbf{m}}\), where C0 is the initial concentration of organic carbon (mg/L), and Ct is the residual concentration after adsorption t min (mg/L). V and m represent the total volume of solution (L) and added mass of clay (g), respectively.
Results and discussion
Polymer analysis
To improve the adaptability of PCE to clay, the design and compounding of sacrificial agents have become a good method. Synthetic reactions of sacrificial polymers are classified as free radical copolymerization. The typical polymerization process is described in Scheme 1 with ammonium persulfate and sodium sulfite as the oxidation–reduction initiator system. The cationic ionomer P(ECH-DMA) is derived from ECH and DMA monomers. On this basis, cationic and zwitterionic polymers were obtained by ternary copolymerization with acrylamide and acrylic monomer, respectively.
The chemical structure of the synthesized polymers was analyzed by FTIR as shown in Fig. 1. Figure 1a represents the infrared curve of binary copolymer P(ECH-DMA). The wide peak of the polymer at 3400 cm−1 is attributed to the vibration of the N–H bond, and the double peak comes from its symmetric and antisymmetric stretching vibration. The absorption peaks appear on 2930 cm−1 and 2850 cm−1 in accord with the stretching vibration of –CH3 and –CH2– groups on DMA and ECH. Signals at 1634 cm−1 and 1460 cm−1 correspond to the stretching vibration peak of the C-O bond and the bending vibration peak of –CH2–, respectively. The absorption peak at 982 cm−1 should be attributed to the stretching vibration of the quaternary ammonium group [21]. The absorption peak at 1107 cm−1 belongs to the stretching vibration of the C–N bond. In Fig. 1b, PAM/P(ECH-DMA) shows an obvious vibration peak in the range of 1650–1645 cm−1, which corresponds to the characteristic absorption peak of a carbonyl group in amide. There is no double peak about 3400 cm−1, which should be caused by the overlapping vibrations of O–H and N–H. It is inferred that random copolymerization of AM with ECH and DMA occurs. Comparing Fig. 1a, Fig. 1c shows a clear absorption peak at 1734 cm−1, which is attributed to the characteristic absorption of sodium carboxylate on PAA/P(ECH-DMA). The characteristic absorption peak of the carbonyl group is at 1600 cm−1. The absorption peak of quaternary ammonium salt at 982 cm−1 still exists. These signals imply that the zwitterionic polymer of PAA/P(ECH-DMA) was synthesized successfully.
The structural information of the synthesized polymers can be further verified by 1H NMR spectra. Figure 2 shows 1H NMR spectra of PAM/P(ECH-DMA) and PAA/P(ECH-DMA). As can be seen in Fig. 2a, the peaks at 4.51 ppm and 3.23 ppm represent the chemical shifts of methyl protons (N–CH2) and methylene protons (N-CH3) linked to the quaternary ammonium ion, respectively. The proton chemical shifts of other groups on ionomers were 4.18, 3.89, 2.08, and 1.53 ppm, respectively. The double peaks appeared distinctly at 3.57 ppm and 3.51 ppm, which are corresponding to the hydrogen atoms of the amide group. These displayed characteristic peaks indicate conformity to the target molecular structure.
In contrast, the new peaks at 3.00 ppm and 2.80 ppm correspond to the protons of sodium polyacrylate on the copolymer, which are also consistent with its structural formula in Fig. 2b. Besides, the peaks at 3.53 ppm and 3.29 ppm correspond to two H atoms, which are from cationic ionomer portion in the copolymer. Other proton peaks show slightly discrepancy positions because the same group exhibits different peaks in NMR in different chemical environments. All this information means that the expected structural sacrifice polymer is obtained.
The relative molecular weight and its distribution are important parameters to control the molecular structure of polymers [22]. The molecular weight of the polymers was determined by GPC and its PDI values were calculated. In Fig. 3, the GPC curve shows a major peak region, which represents the linear polymer part, and the sample appears to be relatively pure. As shown in Table 2, the number and weight average molecular weights of the SA2 are 3782 and 10,629, respectively. The PDI values of the SA1 and SA2 are between 2.8 and 3.0. Compared with commercial PCE, the sacrifice polymers SA1 and SA2 have smaller molecular weights and similar linear structure result in faster diffusion. Therefore, when it is combined with PCE, the sacrifice agent may have the advantage of preferential adsorption of clay so as to restoring the workability of the cementing system containing mud cement.
Flow behavior
The fluidity of cement paste was determined in the absence and presence of 3% clay content (by cement mass), and the water/cement ratio (w/c) was set as 0.29. The cement paste was added with 0.2% dosage PCE (by weight of cementitious material) and its mix design is shown in Table 1. According to Fig. 4, R0 is a simple cement–PCE–water system, in which PCE plays an excellent dispersive role own to its long polyethylene oxide side chain contributing to high steric hindrance effects [23]. Anions on the PCE skeleton act as anchors and are absorbed on the cement surface, generating electrostatic repulsion. In addition, the long side chains of polyoxyethylene extend into the cement slurry. This kind of unique conformation means that they occupy more space outside the particle, thus forming a barrier that prevents the adjacent cement particles from clumping together. Compared with the reference sample R0, the fluidity of Rf-K cement slurry was reduced by the addition of kaolin, while the fluidity of Rf-M cement slurry was reduced more significantly by the addition of montmorillonite, with a reduction rate of 38%. When kaolin or MMT is present in the cement paste, the dispersion of PCE in cement particles is greatly weakened due to the stronger adsorption of PCE by clay particles. For Rf-M samples, PCE is not only adsorbed on the surface of MMT particles, but also its side chains embedded in the interlayer space of MMT, thus greatly consuming PCE and reducing its dispersion efficiency for cement. The interaction between PCE and kaolin is only through surface electrostatic adsorption because of the strong interlayer force produced by hydrogen bonds. Instead, PCE and MMT with weak interlayer force can produce stronger interlayer adsorption, so the sensitivity of PCE to MMT is much higher than that to kaolinite.
To evaluate the effect of the sacrifice agent on the clay tolerance of PCE, the dispersion ability of MMT-containing cement paste was analyzed. Figure 5 shows the evaluation of the flow behaviors of cement slurry with 0.2%PCE at different dosages of the sacrifice polymer, which are calculated by the mass sum of cement and clay. Figure 5a shows the neat R0 sample without clay, which has a relatively prominent fluidity. Fortunately, despite the presence of clay, the fluidity of the cement slurry has a tendency to improve own to the introduction of sacrifice polymer SA1, as shown in Fig. 5b. It gradually increased with the increase in SA1 dosage. When the SA1 dosage is 0.15%, the fluidity reached 94% of R0 value, and when the SA1 dosage was 0.20%, the fluidity reached 285 mm, which was close to R0. From an economic point of view, it is appropriate to choose within this range. Figure 5c shows the cement–PCE–water system mixed with 3% MMT and the zwitterionic sacrifice polymer SA2. Clearly, the fluidity increases almost linearly with the addition of SA2. When the content of SA2 is 0.20%, the fluidity of the sample can reach 279 mm.
The cationic sacrifice agent SA1 has exhibited a favorable positive effect on the fluidity, which maybe own to the electrostatic interaction between the positively charged SA1 and the negatively charged MMT. SA1 formed a preferential occupation position and thus hindered the adsorption of the PCE by clay. Moreover, the zwitterionic sacrifice agent SA2 also showed a positive effect on initial mobility, especially during the high-dose stage. The SA2 polymer with both quaternary ammonium and carboxylic acid anchoring groups preempts the MMT surface relying on electrostatic interaction and short chain structure, while the PCE polymer with long chain structure is less competitive. Therefore, both the SA1 and SA2 polymers mentioned above can sacrifice themselves through electrostatic and barrier effects, alleviating the negative effect of clay on PCE and allowing PCE macromolecules to be “released.”
To further evaluate the effect of the sacrifice agent on the fluidity of the cement paste, SA1-M4 was selected as a representative sample and used in subsequent tests. The flow retention performance tested over 2 h is shown in Fig. 6, with a fixed w/b ratio of 0.29 and 0.2%PCE content. Figure 6c shows that the Rf-M sample is a cement–PCE–water–MMT system lacking the sacrifice agent, and its fluidity is lost seriously over time. The fluidity loss of the cement paste mixed with 3% MMT is very fast and the fluidity is almost lost completely after 30 min.
In Fig. 6a, SA1-M4 is the case of adding 0.20% cationic sacrifice agent SA1 in the cement–PCE–water–MMT system. In contrast to Rf-M, there is a loss of fluidity as time increases, but the decline in the curve is quite gentle. This means that for low-quality cement systems, the cationic sacrifice agent (SA1) not only improves the initial dispersion effect of PCE but also can continuously suppress the negative effect of clay on PCE. On the one hand, the protective film formed by the SA1 neutralized the charge on the clay surface, which reduced the interlayer repulsion and made the compression of the layers. On the other hand, the film prevents MMT particles from contacting PCE. Moreover, because of the difficulty of multi-site adsorption and desorption, the impact of the sacrifice polymer on MMT can be long-lasting. In Fig. 6b, the curve of SA2-M4 is similar to that of SA1-M4, and the fluidity only decreases moderately, not sharply, during the 120 min period. This phenomenon can be attributed to the incorporation of amphoteric polymers, which improves the clay adaptability of PCE and has a good clay-resistance efficacy.
It is very necessary to evaluate the adsorption performance of clay particles to polymers with different molecular structures, which is closely related to the anti-mud mechanism. To compare the differences between the PCE adsorption capacity of MMT and sacrifice agent, different concentrations of SA solutions were configured with deionized water and tested with a TOC analyzer.
Figure 7 represents the effect of sacrifice agent concentration on adsorption behavior. As the concentration of SA1 increases, the adsorption of PCE by MMT decreased. This is mainly due to the existence of SA1, which blocks the adsorption path between MMT layers and inhibits the insertion of PCE side chains into MMT layers, which will benefit PCE to play its role in water reduction and dispersion. In addition, MMT adsorbed a little more PCE-doped SA2 than SA1, which may be due to the different cationic density. These results explain the important reason that the sacrifice agent can improve the clay tolerance of PCE. It means that the introduction of a sacrifice agent can improve the dispersion retention ability of PCE in the cementing material system with clay.
Figure 8 shows the comparison of the adsorption behavior of sacrifice agents and commercial PCE. As can be seen in Fig. 8, the adsorption rate of PCE by MMT is very fast, and then basically reached a plateau after 10 min. There are abundant active sites on the surface of clay particles at the beginning, where the sacrifice polymer can easily attach and formed an adsorption film. With the extension of time, the increase in adsorption capacity tended to moderate due to the coverage of the active site. Moreover, the adsorption of PCE on MMT was significantly lower than that of alone PCE on MMT after the addition of sacrifice agent SA1. It shows that the PCE adsorption capacity of MMT is very strong, and the addition of a sacrifice agent can effectively reduce the PCE adsorption of MMT, thus strengthening the dispersion of PCE to cement. The sacrifice polymer has cationic groups and a linear structure, so it diffuses faster in the slurry than the comb PCE macromolecules and the cationic groups are more readily electrostatically adsorbed by the negatively charged clay. Compared with PCE, the adsorption rate of PCE with SA1 is reduced by 53%, implying that there is competitive adsorption between SA1 and PCE. The introduction of SA1 polymer prevents the PCE side chain from entering the aluminum silicate layer, which can be explained to be self-sacrificing for releasing the PCE molecule and is also consistent with the fluidity results.
Conclusions
The failure of PCE fluidizing in concrete has many reasons, one of the reasons is its interaction with clays such as montmorillonite. Here, two new clay-resistant sacrifice agents (SA1 and SA2) have been successfully prepared, which have positive and amphoteric charges, respectively. Generally, the affinity of sacrifice agent to clay originates from two different mechanisms: first, through the adsorption and neutralization of cationic polymer SA1 on MMT surface, the clay particles are coated with macromolecules. Second, due to the hanging hydroxyl group on the sacrifice polymer chain, it can form hydrogen bonds with the oxygen or hydroxyl group on the clay surface to strengthen the adsorption strength. Because the SA2 is cationized slightly lower than SA1, the mud inhibition is slightly inferior to the former. TOC tests showed that PCE with SA1 had a lower adsorption capacity than pure PCE on MMT over time. It means avoiding PCE deactivation was the main reason for improving adaptability of PCE to MMT. The sacrifice agents preferentially occupy the clay and prevent the PCE side chain from inserting into the interlayer, thus avoiding the useless consumption of PCE on the MMT. All the results indicate that SA1 or SA2 can be used as an effective and convenient sacrifice agents to alleviate the negative effects caused by poor aggregate when combined with PCE.
References
Yoshioka K, Sakai E, Daimon M, Kitahara A (1997) Role of steric hindrance in the performance of superplasticizers for concrete. J Am Ceram Soc 80(10):2667–2671
Marchon D, Sulser U, Eberhardt A, Flatt RJ (2013) Molecular design of comb-shaped polycarboxylate dispersants for environmentally friendly concrete. Soft Matter 9(45):10719–10728
Ayati F-M, Newport D, Cheeseman C (2018) Use of clay in the manufacture of lightweight aggregate. Constr Build Mater 162:124–131
Courard L, Michel F, Pierard J (2011) Influence of clay in limestone fillers for self-compacting cement based composites. Constr Build Mater 25(3):1356–1631
Yamada K, Takahashi T, Hanehara S, Matsuhisa M (2000) Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem Concr Res 30(2):197–207
Tan HB, Gu BQ, Ma BG, Li X, Lin CL, Li XG (2016) Mechanism of intercalation of polycarboxylate superplasticizer into montmorillonite. Appl Clay Sci 129:40–46
Nehdi ML (2014) Clay in cement-based materials: critical overview of state-of-the-art. Constr Build Mater 51:372–382
Ng S, Plank J (2012) Interaction mechanisms between Na montmorillonite clay and MPEG-based polycarboxylate superplasticizers. Cem Concr Res 42:847–854
Xu HJ, Sun SM, Wei JX, Yu QJ, Lin C (2015) β-Cyclodextrin as pendant groups of a polycarboxylate superplasticizer for enhancing clay tolerance. Ind Eng Chem Res 54(37):9081–9088
Lei L, Plank J (2014) Synthesis and properties of a vinyl ether-based polycarboxylate superplasticizer for concrete possessing clay tolerance. Ind Eng Chem Res 53(3):1048–1055
Stecher J, Plank J (2019) Novel concrete superplasticizers based on phosphate esters. Cem Concr Res 119:36–43
Werani M, Lei L (2021) Influence of side chain length of MPEG-based polycarboxylate superplasticizers on their resistance towards intercalation into clay structures. Constr Build Mater 281:122621
Zheng T, Zheng DF, Qiu XQ, Yang DJ, Fan L, Zheng JM (2019) A novel branched claw-shape lignin-based polycarboxylate superplasticizer: preparation, performance and mechanism. Cem Concr Res 119:89–101
Li SM, Pang H, Zhang JF, Meng YY, Huang JH, Lin XJ, Liao B (2019) Synthesis and performance of a novel amphoteric polycarboxylate superplasticizer with hydrolysable ester group. Colloids Surf A 564:78–88
Yousefi R, Amiri O, Salavati-Niasari M (2019) Control sonochemical parameter to prepare pure Zn0.35Fe2.65O nanostructures and study their photocatalytic activity. Ultrason Sonochemistry 58:104619
Yousefi R, Alshamsi HA, Amiri O, Salavati-Niasari M (2021) Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J Mol Liq 337:116405
Li XK, Zheng DF, Zheng T, Cai C, Lou HM, Liu WF, Qiu XQ (2017) Enhancement clay tolerance of PCE by lignin-based polyoxyethylene ether in montmorillonite-contained paste. J Ind Eng Chem 49:168–175
Zheng T, Zheng DF, Li XK, Cai C, Lou HM, Liu WF, Qiu XQ (2017) Synthesis of quaternized lignin and its clay-tolerance properties in montmorillonite-containing cement paste. ACS Sustain Chem Eng 5(9):7743–7750
Tan H, Gu B, Guo Y (2018) Improvement in compatibility of polycarboxylate superplasticizer with poor-quality aggregate containing montmorillonite by incorporating polymeric ferric sulfate. Constr Build Mater 162:566–575
Li B, Gao RJ, Wang L (2021) Synthesis and properties of a starch-based clay tolerance sacrificial agent. Starch-Starke 73:2000223
Ren QY, Zou HW, Liang M, Wang YM, Wang JC (2014) Preparation and characterization of amphoteric polycarboxylate and the hydration mechanism study used in Portland cement. RSC Adv 4(83):44018
Zhao H, Liao B, Nian F, Wang K, Guo Y, Pang H (2018) Investigation of the clay sensitivity and cement hydration process of modified HPEG-type polycarboxylate superplasticizers. J Appl Polym Sci 135(32):46572
Hu KY, Sun ZP, Yang HJ (2019) Effects of polycarboxylate superplasticizers with different functional units on early hydration behavior of cement paste. J Mater Civ Eng 31(5):04019041
Acknowledgements
We thank the Jilin Provincial Education Department Project (Nos.JJKH20240355KJ) and the Jilin Provincial College Student Innovation Engineering Project (Nos.S202310191042) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Luo, J. & Zhu, H. Synthesis of novel polyelectrolyte sacrifice agents and their effects on the adaptability of polycarboxylate superplasticizer with clay-containing cement paste. Polym. Bull. 81, 8029–8043 (2024). https://doi.org/10.1007/s00289-023-05078-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-023-05078-9