Abstract
Polyvinyl alcohol (PVA) is a low-cost biocompatible polymer with potential applications in the textile industry. Similarly, zinc oxide (ZnO) is a unique material that exhibits semiconducting, optical, piezoelectric, pyroelectric, and antibacterial properties. Electrospinning is a technique that allows the formation of nano- to micro-sized fibers. Therefore, the development of ZnO-containing PVA fibers using electrospinning is a useful proposition. In this work, freeze-dried ZnO powder and as-precipitated ZnO suspension obtained from a novel flow synthesis methodology were used. Varying concentrations of ZnO were then used to obtain PVA/ZnO electrospun fibrous mats. Synthesized ZnO was assessed for phase purity using X-ray diffraction. The as-prepared composite nanofibers were characterized using Fourier transform infra-red spectroscopy and scanning electron microscopy. Tensile properties of PVA/ZnO mats were also measured. It is herein shown that antibacterial fibrous PVA/ZnO composites can be successfully synthesized using electrospinning. Antibacterial studies of the fabricated electrospun mats were carried out using zone inhibition method on Gram-positive S. aureus bacteria. The results showed that with increasing concentration of ZnO, antibacterial properties were improved.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Research has recently witnessed a steady rise in interest in tunable tissue engineered scaffolds using biodegradable and biocompatible polymers. The key requirements from these scaffolds are porous structure and high surface area. This condition promotes cell adhesion, proliferation, differentiation and retention of its phenotype [1], making the interconnected porosity an important characteristic for scaffold integration, but on the other hand, reducing the mechanical strength [1].
Undoubtedly, various polymers including hydrogels have been investigated for tissue engineering applications [2]. Poly(vinyl alcohol) (PVA) is a hydrophilic polymer with a simple chemical structure. It is biocompatible, non-toxic, non-immunogenic and inert in physiological environments [3,4,5,6]. Several studies have focused on the applications of PVA in the biomedical and pharmaceutical fields [7, 8]. Due to its excellent biocompatibility, PVA has found its use in wound dressings and management [9, 10], drug delivery systems [11], artificial organs [12] and contact lenses [13]. PVA has been found to have unique biological properties such as non-mutagenic and non-carcinogenic for human cells [14,15,16] and widely used in multiple applications as emulsifier, paper adhesives, photosensitive coating and finishing chemicals for wood and leather. PVA has a dense closely packed monoclinic crystallite which makes it a good gas barrier in various applications. Due to its unique properties such as high tensile strength, flexibility, water solubility and high thermal and chemical stability makes it important in textile industry and paper coatings. PVA is the only barrier that exhibits flexibility, and transparency within economic feasibility [17].
Polymeric matrices containing inorganic reinforcements offer the best combination of organic nanofibers’ properties such as flexibility and processability. The reinforced materials also provide high strength, thermal stability and chemical resistance which comes in handy for a number of applications [18,19,20,21,22,23]. PVA combined with inorganic materials such as silica [19], gold [24] and silver (Ag) nanoparticles [25] are very well investigated for biomedical applications. The introduction of these inorganic materials in PVA makes it resilient in harsh processing conditions, also are safe for human use if used under threshold levels [26].
Zinc oxide (ZnO) exhibits semiconducting, optical, piezoelectric and pyroelectric properties, making it unique and useful for a number of applications such as solar cells [27, 28], gas sensors [29], photocatalysts [30], photodetectors [31], and biosensors [32]. ZnO was recently investigated to have potential biomedical application due to its chemical stability and antimicrobial activity [33,34,35]. Padmavathy et al. [36] and Raghupathi et al. [37], in their separate studies, observed that ZnO nano particles have better antimicrobial activity than microparticles due to high surface-to-volume ratio, making it an effective antimicrobial agent than other metal oxides, such as SiO2, MgO or TiO2 [37]. Farouk et al. [38] investigated the antimicrobial effect of coated cotton fabric with ZnO nanoparticle–chitosan composite for combined antimicrobial response of ZnO and chitosan. It was revealed that lower molecular weight chitosan–ZnO composite was a better antibacterial agent. The research concluded that the reason might be the improved migration of ZnO nanoparticles to the medium as a result of reduced molecular weight of the composite [38]. Besides, ZnO has been found non-toxic to humans and environment, hence, suitable for biomedical research and applications [39, 40].
Electrospinning, an electrostatic-based process offers the possibility of obtaining fibrous polymeric materials with appropriate morphologies for biomedical applications since the porosity of the produced scaffolds allows for tissue growth and regeneration. It is a suitable method for preparing ultrathin fibers having diameters in the range of tens of nanometers to micrometres [41, 42]. Attempts have been made by several researchers to develop nanofibers using electrospinning from suspensions of inorganic materials in polymeric solutions. For instance, Shao et al. [19] successfully synthesised PVA/silica composite nonwoven nanofiber mats using electrospinning. In another report, fibers of PVA/carboxymethyl chitosan composite substituted with silver nanoparticles were developed by electrospinning. This material inhibited the growth of Escherichia coli (E. coli) [43]. Furthermore, Augustine et al. [44, 45] reported the development of poly(caprolactone) (PCL)/ZnO membranes using electrospinning technique. ZnO concentration was reported to have a strong influence on the fibrous morphology and antibacterial activity. An optimal level of ZnO is necessary for reducing inflammation and infection in a defect site in animals. The PCL/ZnO composite membranes demonstrated excellent fibroblast attachment, which is crucial for the wound healing applications.
Textile industry is commonly known for clothing products. However, they also play vital roles in domestic home furnishings, water purification systems, food packaging, automotive, air filters, medical devices, mechanical and protection, sports gear, healthcare and hygienic applications [46, 47]. Large surface areas present in these textile materials are prone bacteria and fungi contamination, which are present everywhere and can readily multiply [48]. Hence, the demand for antimicrobial textiles has attracted the attention of textile scientists and researchers over the last few years due to growing consumers awareness on health hazards and personal hygiene linked with some microorganisms [48, 49].
The antimicrobial applications in field of textile have been studied which include introduction of leachable antimicrobial compounds to polymeric fibers. The process involves physical modification of the surface of fibers or grafting certain moieties on the surface of the polymers [47, 50, 51]. The antimicrobial treatment in textile must be specifically effective against microorganisms suitable for textile processing; durable to laundering, dry cleaning and hot pressing with favorable safety and environmental profile; and it should not affect the textile quality or appearance [48, 49].
Different metal oxides or salts based on silver, zinc, titanium and cobalt have been used for biocidal activity applications in field of textile, healthcare and other industries [52]. Such compounds in nano-range present higher surface area and ease of embedment into fibers’ polymeric matrices, making them unique by allowing release of metal ions with stronger antimicrobial effect [51,52,53].
Keeping in view the requirements of industry, we composed a project in which antibacterial electrospun fiber mats were fabricated using electrospinning and incorporated with ZnO particles. Although the ZnO can be synthesized using batch synthesis method, we used a novel flow synthesis technology. This technology is based on ambient temperature, continuous production, and has a high throughput. PVA/ZnO mats obtained were characterized using the universal tensile machine (UTM), FTIR spectroscopy, scanning electron microscopy (SEM) and X-ray diffraction (XRD). Its antimicrobial activity against Gram-positive (Staphylococcus aureus) was also determined.
Experimental
Materials
Polyvinyl alcohol (PVA) (MW = 72,000) powder (Merck, Germany), zinc nitrate hexahydrate (Merck, Germany) and sodium hydroxide (Sigma-Aldrich) were used as received.
Synthesis of ZnO nanoparticles
ZnO particles were synthesized using a novel flow synthesis system [54] wherein solutions of zinc nitrate hexahydrate and sodium hydroxide were pumped through a single pump at a flow rate of 45 ml/min at room temperature, mixed in a metallic T-piece and result in suspensions. Suspensions were freeze-dried (at − 40 ℃ for 48 h) to obtain ZnO in powdered form.
Preparation of PVA/ZnO blend solution
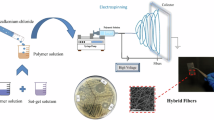
PVA and ZnO were used as starting materials. 8% (w/v) PVA solutions were prepared using 18.2MΩ deionized water (under vigorous stirring and 85 °C temperature). Two groups of composites were developed. Group 1 involved addition of ZnO in powdered form. Group 2 involved of ZnO in the form of as-synthesized suspension. 2.16 wt% of PVA/ZnO was prepared by adding 0.0088 g of ZnO in the 5 mL 8% PVA solution. Similarly, 6.16 wt% and 11.6 wt% of PVA/ZnO was prepared by adding 0.0264 g and 0.0528 g of ZnO powder in 5 mL PVA (8%) solution, respectively (see Table 1).
Electrospinning of PVA/ZnO nanofibers
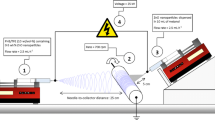
The suspension was loaded into a 10 mL plastic syringe having a needle made of stainless steel. The needle was connected to a high-voltage power supply with aluminium foil serving as a counter electrode and fiber collector. The suspension was delivered via needle at flow rate of 0.5 mL per hour. The distance between needle tip and collecting foil was 12 cm. The application of 20 kV and a flow rate of 0.5 mL per hour resulted in the deposition of fibers. The collected fibers were dried at 80 °C for 24 h in a vacuum drying oven.
Material characterization
The electrospun fibers were observed under scanning electron microscopy (SEM) (TESCAN-VEGA 3 LMU, Czech Republic) for morphological analysis. For obtaining the SEM micrographs, the dried electrospun fibers were mounted on a metal stub using a double-sided adhesive carbon tape. Elemental mapping was carried out using an Oxford Instruments Energy-Dispersive Spectrometer connected to the SEM. The phase purity of ZnO was assessed using X-ray diffraction (X-Pert Powder, PANalytical, Netherlands). Fourier transform infrared (FTIR) spectrophotometer (FTIR, Nicolet 6700) was used to record FTIR spectra. Tensile properties of the deposited mats were measured using a Walter Bai 1.5 kN Fatigue Testing Unit in static mode with crosshead speed of 2 mm/min.
Antimicrobial assays
Antimicrobial activities were carried out on the nanofiber mats according to the method [55]. Nanofiber mats with nanoparticles of known dimensions were sterilized in an oven for 60 min at 80 °C. Fibrous PVA mat without any antimicrobial nanoparticles was used as a negative control specimen whereas Chloramphenicol disc was used as positive control. Antibacterial activity was evaluated by placing all the samples into the inoculated Petri dish followed by incubation at 37 °C for 24 h with S. aureus.
Results and discussion
Scanning electron microscopy
Figure 1a and b represents the SEM images of electrospun mats of PVA and ZnO particles, respectively. Figure 1a reveals that the fibers were randomly oriented of uniform thickness (measured 25 fibers [ca. 862 nm (± 116 nm)]. Figure 2 shows the electrospun fibers with ZnO powder addition in three different concentrations and Fig. 3 shows ZnO added in suspension form with three different concentrations. Free-standing nanoparticles were observed on sample 1P-ZnO/PVA (Fig. 2a). Increasing the percentage of the ZnO in sample 2P/ZnO/PVA, the free-standing particles observed increased in number (Fig. 2b). A further increase in the ZnO percentage in sample 3P-ZnO/PVA showed a further increase in the free-standing nanoparticles (Fig. 2c). Based on SEM images, it is suggested that increasing the ZnO percentage, increases the incidence of particles on electrospun fibers. Furthermore, the morphology of electrospun fibers remained the same when compared to control fibers (Fig. 1a). In Fig. 2, the ZnO was looking like embedded on the surface of the fibers; however, in the figure, the ZnO particles were observed in the form of aggregates embedded in pockets. With the increase in suspension percentage sample 2S-ZnO/PVA, the size of pockets increased, and fibers became fused (Fig. 3b). With further increase in sample 3S-ZnO/PVA, the pockets cannot be distinguished, fiber fusion markedly increased (Fig. 3c). The reason of fusion might be due to low viscosity because the water content of solution increases with the addition of suspension (as suspension is water based); however, in case of powder no further water is added which results in an increase in viscosity and more distinct fibers (Fig. 2).
SEM image (a–c) of composites at magnification 10.0 kx. a 1P-ZnO/PVA, b 2P-ZnO/PVA, c 3P-ZnO/PVA. Energy-dispersive spectrum and elemental mapping d–g of PVA/ZnO nanofibres; d, f 3% suspension and e, g 6% suspension. Red, green and blue colors are in spectra are for carbon, oxygen and zinc, respectively
More so, there is no doubt that at higher concentrations, ZnO powder may optimize the viscosity of PVA to obtain a suitable viscosity necessary to initiate a high degree of entanglement during solvent evaporation in the jet (Fig. 2). This entanglement is necessary for fiber formation. The solvent evaporation occurs locally leading to increased charged density. Sometimes, the presence of metallic particles can alter the solution conductivity, which could equally affect fiber formation [56]. We believe that the ZnO nanoparticle used here might have changed the solution conductivity which affected the fiber formation (Fig. 4).
The fiber diameters were calculated using image analysis software, Image J. Compared to control PVA fibers (having no ceramic added) (Fig. 1), all the composite fibers show slightly lower fiber diameter (Figs. 2 and 3). The average diameters of fibers are described in Table 2. The image analysis further demonstrated that the fiber diameter decreases with increase in ZnO concentration. It was hypothesized that the ZnO in suspension form would offer a good mix with PVA; however, SEM results demonstrated the presence of agglomeration and fiber fusion in response to suspension addition (Fig. 3).
X-ray diffraction
The XRD analysis was performed to evaluate the phase purity and composition of the ZnO nanoparticles (Fig. 5). All the diffraction peaks of the sample corresponding to the unique hexagonal wurtzite structure when compared with literature with ICDD pattern 036–1451 [57]. A similar pattern of X-ray diffraction has been reported by Yadav [58], Chen et al. [59], and Pong et al. [60]. No characteristic peaks of impurity phases except ZnO were present, hence proving the production of production of pure crystalline ZnO nanoparticles.
FTIR analysis
Figure 6 presents the FT-IR spectra of the PVA and PVA/ZnO electrospun samples measured in the range of 4000–500 cm−1. To be specific, the broadband observed between 3000 and 3200 cm−1reveals the O–H stretch from the intermolecular and intramolecular hydrogen bonds. The vibrational band found between 2880 and 2950 cm−1 is the result of the C–H stretch from alkyl groups and the peaks from 1650 to 1670 cm−1 and from 1540 to 1590 cm−1 are due to the C=O and C–O stretches from the remaining acetate groups in PVA, respectively. The C–O stretches in this region links to saponification reaction of PVA [61, 62]. However, other peaks which are related to PVA include, 1350–1450 cm−1 assigned to (CH)CH2; 1050–1100 cm−1 of the group (C–O)–C–OH; 800–850 cm−1 from alkyl chain backbone [63, 64]. The peak observed in the range of 3400 to 3550 cm−1 may be due to O–H stretching assigned to the water adsorption on the metal surface. The peaks at 1600 and 580 cm−1 correspond to Zn–O stretching and deformation vibration, respectively.
Mechanical properties of electrospun PVA/ZnO nanofibers
The mechanical properties of PVA/ZnO nanofibers with different ZnO concentrations and forms were evaluated using stress–strain analysis. The tensile strength was calculated by dividing the maximum force with the cross-sectional area, the elastic modulus was calculated by dividing stress over strain, and the elongation was calculated by the difference of initial and final length (see Fig. 7). Filler dispersion and agglomeration strongly influenced the mechanical properties of composites. The tensile property plots of different composite fibers in comparison with pure PVA fibers are presented in Fig. 7. The results showed that the addition of ZnO nanoparticles either as suspension or powder causes a change in the mechanical properties of the fibers.
The mechanical analysis demonstrated that the ZnO powder addition improved the mechanical behaviour of the PVA mats when compared to PVA alone and ones added with ZnO suspension. This behaviour suggests better incorporation of ZnO nanoparticle. These results are in agreement with the results obtained from antimicrobial studies and SEM. On the other hand, the elastic modulus of the sample 3S-ZnO/PVA had the highest value (Fig. 7b). It suggests here that ZnO nanoparticle did not predominantly act as a reinforcing filler. Another factor could be due to the porous nature of mats and weak interaction between the filler and the matrix. Elongation at breakpoint was found to gradually increase sample 3P-ZnO/PVA (Fig. 7c). It is reasonable to believe that the addition of fillers should decrease the elongation at break, but such is not the case here. The same factor that led to low elastic modulus might be responsible for the increase in elongation at break. However, in the case of ZnO suspension addition, the trend was reversed in a way that sample 3S-ZnO/PVA showed the lowest elongation at breakpoint (Fig. 7c). The reason might include that with the addition of suspension, the water content increases and during electrospinning the fibers fuse together (see Fig. 3c). Furthermore, in suspension, the nanoparticles are present in agglomerates which can be seen in SEM images as well. The agglomerates do not provide uniformity to electrospun fibers hence resulting in reduced mechanical properties (Fig. 3).
The stress withstood by electrospun fibers was analyzed and it was suggested that with ZnO powder addition the nanoparticles were distributed uniformly (Fig. 2a–c) which results in a gradual increase in maximum stress when the powder percentage was increased. However, in the case of suspension, this is not the case. In sample 1S-ZnO/PVA, it was recorded 7.3 MPa, for in sample 2S-ZnO/PVA it reduced to 4.5 MPa and for 3S-ZnO/PVA it again increased to 7.5 MPa. This shows the random distribution of particles. Moreover, in suspension, the percentage of nanoparticles cannot be calculated precisely as in the case of dried powder. The scenario here corroborates the free-standing particles in case of powder that increase with the percentage increase (Fig. 2a–c) but with a suspension this is not the case (Fig. 3a–c).
Antibacterial activity
Possible inhibition or annihilation of bacteria is an important property of developed electrospun fibers for a number of applications in bacteria-rich areas. The antimicrobial property of the PVA/ZnO fibers against Gram-positive Bacterium S. Aureus, which is a laboratory test organism, is of biological importance. In this, antibacterial activity was studied on synthesized samples. Figure 8 shows that samples 1P-ZnO/PVA, 1S-ZnO/PVA and 2P-ZnO/PVA did not show any antimicrobial activity. However, the inhibition zone for samples 2S-ZnO/PVA, 3P-ZnO/PVA and 3S-ZnO/PVA was found to be in between 11 and 13 mm which are in agreement with the Standard Antibacterial test “SNV 195920-1992”. The Standard Antibacterial test “SNV 195920-1992” specimens showing more than 1 mm microbial zone inhibition are considered as good antibacterial agents [65, 66]. In the present study, the inhibition zones showed by samples 2S-ZnO/PVA, 3S-ZnO/PVA 3P-ZnO/PVA are 11, 11 and 13 mm, respectively. The results demonstrated that increase in the concentration of ZnO lead to increase in antibacterial activity. The samples obtained using ZnO suspension showed greater activity in comparison with the powdered form. The reason might attribute to the enhanced surface area in case of agglomerate exposure to bacterial surface and more interaction capability of the material. Chloramphenicol used as a positive control which shows a 25 mm zone of inhibition against S. aureus. Only PVA containing fibers were used as a negative control and showed no antimicrobial activity. The interaction between bacteria and ZnO is reported to be toxic [67], which has led to its use in many industries for antimicrobial applications. Sawai et al. [68] have reported that the mechanism of antimicrobial activity of ZnO is due to the release of active oxygen species in environment which lead to the production of H2O2 in media. H2O2 can penetrate the bacterial cells, causes harm to the cell membranes, and prevents the growth of bacteria.
Conclusions
PVA/ZnO nanofiber mats were prepared via electrospinning technique. Two forms of ZnO with different concentrations were used for the preparation of the electrospun fiber mats. Fiber mat morphologies and mechanical properties were evaluated and observed to be affected by the type and concentration of ZnO nanoparticles wherein these features were more pronounced for ZnO Powder at higher concentration. Antibacterial studies demonstrated that antibacterial properties (against S. aureus) were improved by increasing the concentration of ZnO.
References
Nkhwa S, Lauriaga KF, Kemal E, Deb S (2017) Conference papers in science, p 7
Hoffman AS (2012) Hydrogels for biomedical applications. Adv Drug Deliv Rev 64:18
Paradossi G, Cavalieri F, Chiessi E, Spagnoli C, Cowman MK (2003) Poly (vinyl alcohol) as versatile biomaterial for potential biomedical application. J Mater Sci Mater Med 14:687
Oka M, Noguchi T, Kumar P, Ikeuchi K, Yamamuro T, Hyon S, Ikada Y (1990) Development of an artificial articular cartilage. Clin Mater 6:361
Yamaoka T, Tabata Y, Ikada Y (1995) Comparison of body distribution of poly (vinyl alcohol) with other water‐soluble polymers after intravenous administration. J Pharm Pharmacol 47:479
Noguchi T, Yamamuro T, Oka M, Kumar P, Kotoura Y, Hyonyt SH, Ikadat Y (1991) Poly(vinyl alcohol) hydrogel as an artificial articular cartilage: evaluation of biocompatibility. J Appl Biomater 2:101
Kaetsu I (1993) Recent trends in radiation polymer chemistry. Springer, Berlin
Hassan CM, Peppas NA (2000) Cellular PVA hydrogels produced by freeze/thawing. J Appl Polym Sci 76:2075
Kenawy E-R, Kamoun EA, Eldin MSM, El-Meligy MA (2014) Physically crosslinked poly(vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: synthesis and characterization for biomedical applications. Arab J Chem 7:372
Zhao L, Mitomo H, Zhai M, Yoshii F, Nagasawa N, Kume T (2003) Synthesis of antibacterial PVA/CM-chitosan blend hydrogels with electron beam irradiation. Carbohydr Polym 53:439
Muggli DS, Burkoth AK, Anseth KS (1999) Crosslinked polyanhydrides for use in orthopedic applications: degradation behavior and mechanics. J Biomed Mater Res 46:271
Yang X, Liu Q, Chen X, Yu F, Zhu Z (2008) Investigation of PVA/ws-chitosan hydrogels prepared by combined γ-irradiation and freeze-thawing. Carbohydr Polym 73:401
Hyon S-H, Cha W-I, Ikada Y, Kita M, Ogura Y, Honda Y (1994) Poly(vinyl alcohol) hydrogels as soft contact lens material. J Biomater Sci Polym Ed 5:397
Burkinshaw S, Kumar N (2008) The reduction clearing of dyed polyester. Part 1: colour strength. Dyes Pigm 76:799
Burkinshaw S, Kumar N (2008) A polyvinyl alcohol after treatment for nylon 6,6. Part 2: complex formation. Dyes Pigm 77:86
Pourciel M, Launay J, Sant W, Conédéra V, Martinez A, Temple-Boyer P (2003) Development of photo-polymerisable polyvinyl alcohol for biotechnological applications. Sens Actuat B Chem 94:330
Peng S, Liu X, Sun J, Gao Z, Yao L, Qiu Y (2010) Influence of absorbed moisture on desizing of poly(vinyl alcohol) on cotton fabrics during atmospheric pressure plasma jet treatment. Appl Surf Sci 256:4103
Hajji P, David L, Gerard J, Pascault J, Vigier G (1999) Synthesis, structure, and morphology of polymer-silica hybrid nanocomposites based on hydroxyethyl methacrylate. J Polym Sci B Polym Phys 37:3172
Shao C, Kim H-Y, Gong J, Ding B, Lee D-R, Park S-J (2003) Fiber mats of poly(vinyl alcohol)/silica composite via electrospinning. Mater Lett 57:1579
Abdolmaleki A, Mallakpour S, Borandeh S (2011) Preparation, characterization and surface morphology of novel optically active poly(ester-amide)/functionalized ZnO bionanocomposites via ultrasonication assisted process. Appl Surf Sci 257:6725
Rathi A, Kundalwal SI (2020) Preparation, characterization and surface morphology of novel optically active poly(ester-amide)/functionalized ZnO bionanocomposites via ultrasonication assisted process. Polym Compos 41:2491
Kundalwal S, Rathi A (2020) Improved mechanical and viscoelastic properties of CNT-composites fabricated using an innovative ultrasonic dual mixing technique. J Mech Behav Mater 29:77
Rathi A, Kundalwal SI, Singh S, Kumar A (2021) Adhesive and viscoelastic response of MWCNT/ZrO2 hybrid epoxy nanocomposites. J Mech Mater Struct 16:281
Wang J, Yao H-B, He D, Zhang C-L, Yu S-H, Appl ACS (2012) Facile Fabrication of gold nanoparticles-poly(vinyl alcohol) electrospun water-stable nanofibrous mats: efficient substrate materials for biosensors. Mater Interfaces 4:1963
Jin W-J, Jeon HJ, Kim JH, Youk JH (2007) A study on the preparation of poly(vinyl alcohol) nanofibers containing silver nanoparticles. Synth Met 157:454
Wang Y, Zhang Q, Zhang C-L, Li P (2012) Characterisation and cooperative antimicrobial properties of chitosan/nano-ZnO composite nanofibrous membranes. Food Chem 132:419
Pang Z, Dai Z, Wang Z (2001) Nanobelts of Semiconducting oxides. Science 291:1947
Rensmo H, Keis K, Lindström H, Södergren S, Solbrand A, Hagfeldt A, Lindquist S-E, Wang L, Muhammed M (1997) High light-to-energy conversion efficiencies for solar cells based on nanostructured ZnO electrodes. J Phys Chem B 101:2598
Cheng X, Zhao H, Huo L, Gao S, Zhao J (2004) ZnO nanoparticulate thin film: preparation, characterization and gas-sensing property. Sens Actuat B Chem 102:248
Kamat PV, Huehn R, Nicolaescu R (2002) A “sense and shoot” approach for photocatalytic degradation of organic contaminants in water. J Phys Chem B 106:788
Sharma P, Sreenivas K, Rao K (2003) Analysis of ultraviolet photoconductivity in ZnO films prepared by unbalanced magnetron sputtering. J Appl Phys 93:3963
Topoglidis E, Cass AE, O’Regan B, Durrant JR (2001) Immobilisation and bioelectrochemistry of proteins on nanoporous TiO2 and ZnO films. J Electroanal Chem 517:20
Wang C, Liu L-L, Zhang A-T, Xie P, Lu J-J, Zou X-T (2016) Optical and microstructure of thin film of Ag-doped ZnO synthesized by sol-gel. Afr J Biotechnol 11:10248
Li SC, Li YN (2010) Mechanical and antibacterial properties of modified nano‐ZnO/high‐density polyethylene composite films with a low doped content of nano‐ZnO. J Appl Polym Sci 116:2965
Vicentini DS, Smania A, Laranjeira MC (2010) Chitosan/poly (vinyl alcohol) films containing ZnO nanoparticles and plasticizers. Mater Sci Eng C 30:503
Padmavathy N, Vijayaraghavan R (2016) Chemical manipulation of oxygen vacancy and antibacterial activity in ZnO. Sci Technol Adv Mater 77:1027–1034
Raghupathi KR, Koodali RT, Manna AC (2011) Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 27:4020
Farouk A, Moussa S, Ulbricht M, Textor T (2012) ZnO nanoparticles-chitosan composite as antibacterial finish for textiles. Int J Carbohydr Chem 2012:1
Zhou J, Xu NS, Wang ZL (2006) Dissolving behavior and stability of ZnO wires in biofluids: a study on biodegradability and biocompatibility of ZnO nanostructures. Adv Mater 18:2432
Sangkhaprom N, Supaphol P, Pavarajarn V (2010) Fibrous zinc oxide prepared by combined electrospinning and solvothermal techniques. Ceram Int 36:357
Li D, Xia Y (2004) Electrospinning of nanofibers: reinventing the wheel? Adv Mater 16:1151
Dzenis YA (2004) Spinning continuous fibers for nanotechnology. Science 304:1917
Zhao Y, Zhou Y, Wu X, Wang L, Xu L, Wei S (2012) A facile method for electrospinning of Ag nanoparticles/poly (vinyl alcohol)/carboxymethyl-chitosan nanofibers. Appl Surf Sci 258:8867
Augustine R, Malik HN, Singhal DK, Mukherjee A, Malakar D, Kalarikkal N, Thomas S (2014) Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J Polym Res 21:1
Augustine R, Dominic EA, Reju I, Kaimal B, Kalarikkal N, Thomas S (2014) Electrospun polycaprolactone membranes incorporated with ZnO nanoparticles as skin substitutes with enhanced fibroblast proliferation and wound healing. RSC Adv 4:24777
Singleton J (2013) World textile industry. Routledge, London
Shahidi S, Wiener J (2012) Antimicrobial agents. InTech, New York
Gao Y, Cranston R (2008) Recent advances in antimicrobial treatments of textiles. Text Res J 78:60
Windler L, Height M, Nowack B (2013) Comparative evaluation of antimicrobials for textile applications. Environ Int 53:62
Simoncic B, Tomsic B (2010) Structures of novel antimicrobial agents for textiles—a review. Text Res J 80:1721
Bshena O, Heunis TD, Dicks LM, Klumperman B (2011) Antimicrobial fibers: therapeutic possibilities and recent advances. Future Med Chem 3:1821
Morais DS, Guedes RM, Lopes MA (2016) Antimicrobial approaches for textiles: from research to market. Materials 9:498
Palza H (2015) Antimicrobial polymers with metal nanoparticles. Int J Mol Sci 16:2099
Ijaz K (2017) Low temperature flow synthesis of nanobioceramics—design, development and proof of concept studies, CIIT, Lahore
Jyoti K, Baunthiyal M, Singh A (2016) Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Radiat Res Appl Sci 9:217
Ghosal K, Thomas S, Kalarikkal N, Gnanamani A (2014) Electrospun polycaprolactone/ZnO nanocomposite membranes as biomaterials with antibacterial and cell adhesion properties. J Polym Res 21:1
File PD (1967) ASTM, Philadelphia, Pa, p 9
Yadav A, Prasad V, Kathe A, Raj S, Yadav D, Sundaramoorthy C, Vigneshwaran N (2006) Functional finishing in cotton fabrics using zinc oxide nanoparticles. Bull Mater Sci 29:641
Chen C, Yu B, Liu P, Liu J, Wang L (2011) Investigation of nano-sized ZnO particles fabricated by various synthesis routes. J Ceram Process Res 12:420
Pung S-Y, Lee W-P, Aziz A (2012) Kinetic study of organic dye degradation using ZnO particles with different morphologies as a photocatalyst. Int J Inorg Chem 2012:1
Wang T, Turhan M, Gunasekaran S (2004) Selected properties of pH-sensitive, biodegradable chitosan–poly(vinyl alcohol) hydrogel. Polym Int 53:911
Mansur HS, Sadahira CM, Souza AN, Mansur AA (2008) Selected properties of pH-sensitive, biodegradable chitosan–poly(vinyl alcohol) hydrogel. Mater Sci Eng C 28:539
Coates J (2000) Encyclopedia of analytical chemistry, interpretation of infrared spectra, a practical approach. Wiley, Chichester
Mansur HS, Oréfice RL, Mansur AA (2004) Characterization of poly(vinyl alcohol)/poly(ethylene glycol) hydrogels and PVA-derived hybrids by small-angle X-ray scattering and FTIR spectroscopy. Polymer 45:7193
Pollini M, Russo M, Licciulli A, Sannino A, Maffezzoli A (2009) Characterization of antibacterial silver coated yarns. J Mater Sci Mater Med 20:2361
Raghavendra GM, Jayaramudu T, Varaprasad K, Ramesh S, Raju KM (2014) Microbial resistant nanocurcumin-gelatin-cellulose fibers for advanced medical applications. RSC Adv 4:3494
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, Hasan H, Mohamad D (2015) Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett 7:219
Sawai J, Shoji S, Igarashi H, Hashimoto A, Kokugan T, Shimizu M, Kojima H (1998) Hydrogen peroxide as an antibacterial factor in zinc oxide powder slurry. J Ferment Bioeng 86:521
Acknowledgements
The authors would like to thank the Higher Education Commission NRPU (Project No. 20-1834/R&D/10-4886), Ministry of Science and Technology (for a developmental grant—PC1 CATBM) CUI-TWAS program for financial support (post-doctoral fellowship and research grant).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obasi, H.C., Ijaz, K., Akhtar, H. et al. Fabrication of antimicrobial electrospun mats using polyvinyl alcohol–zinc oxide blends. Polym. Bull. 80, 2681–2695 (2023). https://doi.org/10.1007/s00289-022-04164-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04164-8