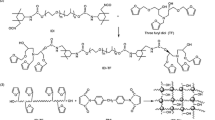
Abstract
In this paper, a self-repairing polyurethane without external stimulation was synthesized. First, a reversible acyl hydrazone bond was introduced into the polyurethane molecular chain. The bond could be dynamically reversible under acidic stimulation to repair the material damage. After that, different contents of bis(2-ethylhexyl) phosphate components were added to modify the material in the synthesis, and its performance was compared to pure polyurethane (PU-0). The polymer was tested using digital viscometer, differential scanning calorimeter (DSC), thermogravimetric analysis (TG), tensile test, etc. The results showed that the addition of bis(2-ethylhexyl) phosphate can promote the healing of the material, so that the synthetic polyurethane material can be repaired to a certain extent without external stimulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Based on its adjustable physical and chemical properties, polyurethane has been widely used in medical, electronic, and architectural fields [1, 2], and can be made into products such as adhesives [3], coatings [4], and foam boards [5]. When the material is subjected to environmental and external forces [6] for a long time, it will generate cracks, which will make the material invalid and unable to meet the requirements of use. It can only be replaced with new materials, causing waste [7].
Based on the self-healing phenomenon of the human body [8], people began to study self-repairing polyurethanes. At present, self-healing materials are mainly divided into two categories: one is external repair agents, including microcapsules [9], fiber tube sealing [10], 3D microvascular [11] and so on. The above-mentioned repairing agent is embedded in the substrate, and the material can be repaired when the material breaks [12]. Another type is to introduce reversible bonds into the polymer chain, including Diels–Alder (DA) bond [13], acyl hydrazone bond [14], N–O bond [15], disulfide bond [16], coumarin [17], hydrogen bond [18], etc. The repair was achieved through the reversible reaction of bonds.
Nowadays, self-healing polyurethanes prepared with DA bonds can be repaired under infrared irradiation [19] or microwave stimulation [20], making polyurethanes promising for sensors, artificial skin [21], etc. By introducing disulfide bonds into polyurethane [22] can achieve self-healing of materials at moderate temperatures, [23, 24] and can prepare smart materials [25] or flexible electronic products [26]. Compared to other reversible bonds, acyl hydrazone bonds have the advantages of high bond energy and good stability. It is often used to prepare elastomers [27], hydrogels [28] and other materials, and can be used in bioengineering [29]. Because the repair of the acyl hydrazone bond must be achieved under acidic or aniline stimulation [14, 30, 31], the material cannot be repaired completely independently, which limits the use of the material [32,33,34,35].
In this work, we introduced the synthesized acyl hydrazone bond chain extender into the polyurethane chain and added different contents of bis(2-ethylhexyl) phosphate components to synthesize a series of self-repairing polyurethanes without external stimulation. The properties of each polyurethane material were studied, and glacial acetic acid was applied to PU-0 to verify the repair ability of the acyl hydrazone bond, and then no stimulation was applied to each spline cutting to test the self-repair ability of the material. The results showed that the addition of bis(2-ethylhexyl) phosphate enables the polyurethane material containing acyl hydrazone bond to undergo a certain degree of self-repair without stimulus.
Experimental
Materials
Dimethyl succinate (CP, Mn = 146.14, colorless liquid) was purchased from Sinopharm Chemical Reagent Co. Ltd. Hydrazine hydrate (AR, 80% colorless liquid) was purchased from Tianjin Fuyu Fine Chemical Co. Ltd. 4-hydroxybenzaldehyde (CP, Mn = 122.12) was purchased from Sinopharm Group Chemical Reagent. Anhydrous ethanol (CP colorless liquid) was purchased from Tianjin Fuyu Fine Chemical Co. Ltd. Hexamethylene diisocyanate (CR colorless liquid) was purchased from Bayer, Germany. Hexamethylene diisocyanate trimer(–NCO = 19.6%) was purchased from Wanhua Chemical Group Co. Ltd. Polyethylene glycol (LR Mn = 1000) was purchased from Tianjin Damao Chemical Co. Ltd. Bis(2-ethylhexyl) phosphate (CP) Canos Technology Co., Ltd. Glacial acetic acid (AR, Mn = 60.05, colorless liquid) was purchased from Nanjing Chemical Reagent Co. Ltd. Dibutyltin dilaurate (DBTDL, AR, 95.0%, Mn = 631.57, colorless oily liquid) was purchased from Tianjin Damao Chemical Reagent factory. Dimethyl sulfoxide (DMSO, AR, Mn = 78.13, colorless liquid) was purchased from Tianjin HengXing Chemical Reagent Co., Ltd. N,N-dimethylformamide (DMF, AR, 99.5%, Mn = 73.09, colorless liquid) as solvent were purchased from Tianjin Fuyu Fine Chemical Co. Ltd.
Synthesis of hydroxyl-terminated small molecule acylhydrazone chain extender(PN)
Synthesis of Succinic Dihydrazide: Dimethyl succinate (29.23 g, 0.2 mol), hydrazine hydrate (30.03 g.0.6 mol) and absolute ethanol (100 g) were added into a flask and refluxed for 2 h in absolute ethanol at 60 ℃. The turbid liquid was filtered and washed with absolute ethanol three times for purification, and dried in vacuum to constant weight to obtain the white powder, yield: 89%.
Synthesis of chain extender (PN): Succinic dihydrazide (14.6 g, 0.2 mol) was dissolved in deionized water (100 g) and add it into a flask. 4-hydroxybenzaldehyde (24.4 g, 0.2 mol) was dissolved in absolute ethanol and slowly add it dropwise to the flask, so that 4-hydroxybenzaldehyde can fully react with succinic dihydrazide at 40 ℃ for 2 h. The crude product was filtered and washed three times with absolute ethanol and deionized water, and dried in vacuum to obtain a white powder, yield: 91%. The schematic of the reaction is shown in Scheme 1.
Synthesis of self-healing polyurethane
A series of polyurethane with different compositions were prepared using the two-step route (Scheme 2), and the feed ratios are presented in Table 1. In the first step, PEG (5.00 g, 5.00 mmol) was added to a vessel and dehydrated at 100 °C under vacuum for 1 h. Then, DMSO (15 g) was poured into the container as solvent in nitrogen atmosphere, and then HDI (5.05 g 0.03 mol) and DBTL (0.10 g) were added to the container, the polyurethane prepolymer (PEG-HDI) was obtained by stirring at 70 ℃ for 4 h. In the second step, chain extender PN (10.63 g ·0.03 mol) was added to the mixture without stopping the reaction. After 4 h of reaction, the solution temperature was lowered to room temperature, a certain amount of bis(2-ethylhexyl) phosphate and HDI trimer (6.43 g) were added and stirred for 10 min. After that, the mixture was poured into a mold and dried in an oven at 80 ℃ for 24 h. In order to carry out the control experiment, polyurethane PU-0 without bis(2-ethylhexyl) phosphate was prepared according to the above steps.
Characterizations
FTIR spectra were recorded using the HQL Fourier Transform Infrared-Raman Spectrometer of Bruker Company in Germany, and attenuated total reflectance (ATR) method was used. 64 scans at a resolution of 2 cm−1 were averaged for each measurement. The digital viscometer (LDV-2 + Pro) tests the viscosity change of the self-healing polyurethane solution at 80 ℃. The rotor type was No.4. Differential scanning calorimeter (TA, Q20) and thermal gravimetric analyzer (TA, Q20) were used to test the thermal properties of polyurethane materials. DSC test conditions: heating rate 10 ℃/min (− 80 ℃ ~ 150 ℃), nitrogen, TG test conditions: heating rate 20 ℃/min (40 ~ 700 ℃), nitrogen.
The mechanical properties were tested by electronic stretching machine (Zwick/005, German, ISO527-2). The tensile speed was 500 mm/min, and the average value of each three samples was taken. The calculation formula of self-repair efficiency (R) was as follows:
Among them, \({\varepsilon }_{0}\) and \({\varepsilon }_{1}\) were the breaking elongation before and after self-repair; \({\sigma }_{0}\) and \({\sigma }_{1}\) were the tensile strength before and after self-repair, respectively.
The equilibrium swelling rate (ESR) and gel rate (GR) of the polyurethane material were tested in order to verify the cross-linked structure of a series of polyurethanes. Samples of a certain quality were cut and soaked in DMF solution. The samples were taken out and weighed every 10 min, and then soaked after recording the quality. Repeat soaking-weighing steps until the sample quality remains unchanged, that is, the swelling balance is reached. Equilibrium swelling ratio (ESR) is calculated according to formula 3. The swelling equilibrium sample was placed in deionized water for 48 h to replace DMF solution. Then, take out the sample and put it in the oven for drying, take out and weigh it at regular intervals until the weight no longer changes, and record the final quality. The gel content (GR) was calculated according to formula 4.
Among them, W0, W1, W2 were the weight of the polyurethane sample before and after swelling and the weight of the sample after the final drying, respectively.
Results and discussion
Synthesis of hydroxyl-terminated small molecule acyl hydrazone chain extender (PN)
Figure 1a shows the infrared spectra of dimethyl succinate and succinyl dihydrazide. In the infrared curve of dimethyl succinate, the C = O stretching vibration absorption peak at 1732 cm−1, the stretching vibration absorption peak of CH in the -CH3 group at 2950 cm−1, and the stretching vibration absorption peak of C–O–C is from 1100 to 1200 cm−1. In the infrared curve of succinyl dihydrazide, the stretching vibration of C = O is at 1619 cm−1, the absorption peaks at 3175 cm−1 and 3288 cm−1 are the symmetric and antisymmetric stretching vibration absorption peaks of N–H in –NH2, respectively. The infrared curve of succinic dihydrazide in the figure does not show the characteristic absorption peaks of –CH3 representing dimethyl succinate and –COC– in the ester group, but –NH2 which has a representative hydrazide group appears. The characteristic absorption peak of –NH and the stretching vibration absorption peak of –CN– bond indicates that the reaction successfully prepared succinic dihydrazide.
Figure 1b is an infrared spectrum of succinyl dihydrazide and acyl hydrazone chain extender (PN). In the molecular spectrum of PN, the stretching vibration peak of C = N is at 1653 cm−1, and the stretching vibration absorption peak of C = O in acyl hydrazone bond is at 1606 cm−1, and at 3231 cm−1 is the stretching vibration absorption peak of O–H in compound molecules. The characteristic peaks of C = N and –H appear in the infrared spectrum of the PN, which proves that the reaction product has been successfully synthesized.
Synthesis of self-healing polyurethane
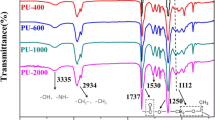
Following the well-designed synthesis route (Scheme 2), the target cross-linked PU was prepared. The FTIR spectra in Fig. 2a support the structure of the produced PEG-HDI. On the PEG-1000 spectrum, 3420 cm−1 is hydroxyl stretching vibration absorption peak. On the IPDI spectrum, 2260 cm−1 is –NCO groups stretching vibration absorption peak. By contrast, on the PEG-IPDI spectrum, the –NCO groups still exist, and it illustrate that the system still contains isocyanate groups after PEG-1000 and IPDI reaction completely. The absorption peak of -NH- was at 3420–3010 cm− 1. The C = O was at 1750 cm-1 and the absorption peak at 1604 cm− 1 was the absorption peak of C–N–H. Above analyses illustrate that PEG-1000 and IPDI could react with each other and produce carbamate groups (–CONH–) [36].
In the Fig. 2b, On the PN spectrum, 3231 cm−1 is hydroxyl stretching vibration absorption peak. On the PEG-IPDI spectrum, 2260 cm−1 is the characteristic absorption peak of isocyanate groups. Moreover, when the chain extender is added, the absorption of –NCO at 2260 cm−1 disappears in the spectrum of PEG-HDI-PN, which means that the chain extender has successfully reacted with the prepolymer. And there is a combined absorption peak of –NH and –OH at 3565–3100 cm−1. These results confirmed that the second step of the reaction was successful [37].
Analysis of viscosity
Figure 3 shows the change of the viscosity of each polyurethane solution with the extension of reaction time at 80 ℃. In the viscosity test curve, it can be seen that with the extension of time, the viscosity of each polyurethane material will increase, and the solution gradually turned into a gel to form a cross-linked polyurethane material. The increase in the amount of bis(2-ethylhexyl) phosphate added made the viscosity of the polyurethane material increase slowly. When the addition is 1.0wt%, the gel time of polyurethane containing bis(2-ethylhexyl) phosphate is ten times longer than that of pure PU without addition, which proves that only an appropriate amount of bis(2-ethylhexyl) phosphate can be added, but not infinitely.
Characterization of cross-linked structure and thermal properties
Differential scanning calorimetry (DSC) analysis
The DSC curves shown in Fig. 4 convey the glass transition temperature (Tg) of the self-healing polyurethane. All the synthesized self-repairing polyurethanes have two glass transition temperatures. The glass transition temperature (Tg1) of the soft segment is below 0 ℃, and the glass transition temperature (Tg2) of the hard segment is above room temperature, which means that the materials are in a high elastic state at room temperature, and the chain segments can move, which is beneficial to the self-healing of self-repairing polyurethane materials. In addition, we also found that with the increase of the amount of bis(2-ethylhexyl) phosphate, the Tg1 and Tg2 of the synthesized self-repairing polyurethane materials decreased, and the temperature difference between them decreased, which indicated that with the addition of bis(2-ethylhexyl) phosphate, the microphase separation of soft segment and hard segment decreased, which was beneficial to the movement of chain segments. When the material was damaged, the ability of molecular chain movement was enhanced, which was conducive to the self-healing of the material.
Analysis of equilibrium swelling rate and gel content
The polyurethane sample was swollen in DMF solution, and the mass was recorded every 10 min. Table 2 and Fig. 4 were calculated using formula (3).
Table 2 and Fig. 5 show that each polyurethane sample swells when immersed in DMF solution at room temperature, and the mass increases rapidly during the initial immersion, and tends to increase slowly with the extension of time. In the initial process of swelling, the solvent small molecules permeate into the cross-linked network, which increases the distance between the networks, expands the volume of the material, and the mass increases rapidly, and tends to smooth after a period of time. For PU-0.6, PU-0.8 and PU-1.0 that appear to reach a plateau at about 100 min and then they swell at a higher rate. This is because the addition of bis(2-ethylhexyl) phosphate hinders the crosslinking of polyurethane, reduces the cross-linking degree of the material, weakens the intermolecular force, strengthens the force between solvent and chain segment, and further increases the mass. With the increase of volume expansion, the elastic retraction force of the cross-linked network also increases, and the two forces counteract each other and finally reach the swelling equilibrium. According to the formula (3) to calculate the equilibrium swelling rate, it was found that the addition of bis(2-ethylhexyl) phosphate affected the equilibrium swelling rate of the material. The more bis(2-ethylhexyl) phosphate was added, the more the mass and volume of the sample was increased, and the higher the equilibrium swelling rate was obtained.
The sample which was reached swelling equilibrium was soaked in deionized water to obtain the final weight of the sample after drying. The gel content of the sample is calculated according to formula (4), and the results are shown in Table 3.
In Table 3, the addition of bis(2-ethylhexyl) phosphate changes the gel content of polyurethane materials. The more bis(2-ethylhexyl) phosphate is added, the lower the gel content is. The gel content of all polyurethane materials is more than 50%, indicating that the synthesized polyurethane is still a cross-linked network structure. The equilibrium swelling ratio and gel content data of each self-repairing polyurethane were compared to PU-0 and integrated to obtain Fig. 6.
According to Fig. 6, the more bis(2-ethylhexyl) phosphate is added, the higher the equilibrium swelling ratio of the material is, and the lower the gel content is. This is because the addition of bis(2-ethylhexyl) phosphate hinders the cross-linking of polyurethane and reduces the cross-linking degree of the material, so the more liquid is absorbed when soaking DMF solution, the higher the equilibrium swelling ratio is, and the uncross linked linear molecules will be dissolved after swelling, resulting in lower gel content of the material.
Themo-gravimetric analysis (TGA) of the samples
It can be seen from Fig. 7 that a series of self-healing polyurethanes began to lose weight at around 100 °C and no longer continued to lose weight at around 550 °C. As shown in Table 4, at 200 ℃, the weight loss rates of PU-0, PU-0.2, PU-0.4, PI-0.6, PU-0.8 and PU-1 are 2.92%, 3.56%, 5.27%, 5.38%, 5.76% and 7.08% respectively, and the weight loss rate of the material increases with the increase of the amount of bis(2-ethylhexyl) phosphate, and the char yields of PU-0, PU-0.2, PU-0.4, PI-0.6, PU-0.8 and PU-1 are 13.39%, 13.28%, 13.15%, 12.78%, 12.70%, 12.48%, respectively at 700 ℃. This shows the addition of bis(2-ethylhexyl) phosphate hinders the cross-linking of polyurethane, which reduces the degree of cross-linking of the material, and the internal molecular chains and segments of the material were easier to move, resulting in a reduction in the heat resistance of the material. However, when each self-repairing polyurethane material is heated to 200 ℃. The mass loss rate was less than 8%, indicating that each self-repairing polyurethane material still had good thermal stability and could maintain the basic performance of the material at this time.
Test and analysis of tensile properties
In order to characterize whether the addition of different contents of bis(2-ethylhexyl) phosphate has an effect on the mechanical properties of polyurethane, the mechanical properties of PU-(0/0.2/0.4/0.6/0.8/1.0) are tested. The tensile curve and tensile data are shown in Fig. 8 and Table 5.
According to Fig. 8 and Table 5, it could be seen that with the increase of bis(2-ethylhexyl) phosphate, the tensile strength of the polyurethane material was decreasing and the elongation at break was increasing. The polyurethane materials gradually become soft and elastic, indicating the degree of cross-linking of the polyurethane material decreases with the addition of bis(2-ethylhexyl) phosphate. It was more conducive to segment movement and made the molecular chain of materials have more space to move, which was conducive to self-repair. It provided the possibility of self-repair without external stimulation at room temperature.
Self-healing performance analysis
Firstly, the repair ability of acyl hydrazone bond was verified, and the PU-0 spline was cut off in the middle to simulate the damage of the material in nature, and then the damage healing was promoted by dripping glacial acetic acid. Figure 9 is the schematic illustration of the self-healing mechanism of PU-0 materials. Figure 10a is the self-repair process diagram of PU-0, b is the tensile curve of PU-0 repair at different time, and Table 6 shows the healing efficiency of PU-0 repair at different time, which is calculated by formulas (1) and (2).
In Fig. 10a, the PU-0 is cut off and repaired with glacial acetic acid for 48 h, and the spline can be stretched from 7 to 13 cm without breaking, indicating that the acyl Hydrazone bond of the material can be dynamically reversible and can be repaired under acid stimulation. According to Fig. 10b and Table 6, the polyurethane synthesized by acyl hydrazone bond can be self-repaired under the stimulation of glacial acetic acid, and the self-repair efficiency increases gradually with the extension of time, and the highest self-repair efficiency is 86.21% for tensile strength.
Then, the self-repairing efficiency of each polyurethane material without external stimulation was verified, and each self-repairing polyurethane spline was cut off at 25 ℃ for 72 h without any stimulation, and then the repair spline was tensile tested. Tensile curve and data see Fig. 10 and Table 7.
As can be seen from Fig. 11 and Table 7, after cutting off the synthesized self-repairing polyurethane and repairing without external stimulation for 72 h, the repair efficiency of PU-0, PU-0.2 and PU-0.4 is too low to be measured by tension machine. After 72 h of repair with PU-0.6, PU-0.8 and PU-1.0, the self-repair efficiency characterized by elongation at break and tensile strength gradually increased, up to 9.86%. This shows that with the addition of bis(2-ethylhexyl) phosphate, the reversible bond of acyl Hydrazone can be promoted, and the material can still have the ability of self-repair without external stimulation, and can carry out self-repair to a certain extent.
Conclusions
In summary, a self-repairing polyurethane based on acyl hydrazone bond was successfully synthesized in this paper. Self-repairing polyurethane (PU-0) has a high self-repairing efficiency (86.21% for tensile strength) under the stimulation of acid. Self-repairing polyurethane (PU-X, X = 0.2, 0.4, 0.6, 0.8, 1) without acid stimulation was prepared by adding a certain amount of bis(2-ethylhexyl) phosphate to PU-0. According to the experimental results, PU-X can still repair itself to a certain extent without external stimulation, which makes up for the deficiency that acyl Hydrazone bond can be repaired only under certain external stimulation, and provides a new route for the preparation of completely independent repair materials.
References
Lin CH, Sheng DK, Liu XD et al (2018) NIR induced self-healing electrical conductivity polyurethane/graphene nanocomposites based on Diels-Alder reaction. Polymer 140:150–157
Yoshie N, Watanabe M, Araki H et al (2010) Thermo-responsive mending of polymers crosslinked by thermally reversible covalent bond: Polymers from bisfuranic terminated poly(ethylene adipate)and tris-maleimide. Polym Degrad Stab 95:826–829
Turkenburg DH, Bracht HV, Funke B, Schmider M, Janke D, Fischer HR (2017) Polyurethane adhesives containing Diels-Alder based thermo-reversible bonds. Appl Polym Sci 134:44972
Galhenage TP, Hoffman D, Silbert SD et al (2016) Fouling-release performance of silicone oil-modified siloxane-polyurethane coatings. ACS Appl Mater Interfaces 8:29025–29036
Cornille A, Guillet C, Benyahya S et al (2016) Room temperature flexible isocyanate-free polyurethane foams. Eur Polym J 84:873–888
Burattini S, Greenland BW, Chappell D et al (2010) Healable polymeric materials: a tutorial review. Chem Soc Rev 39:1973–1985
Garrido MA, Gerecke AC, Heeb N et al (2017) Isocyanate emissions from pyrolysis of mattresses containing polyurethane foam. Chemosphere 168:667–675
Martin P (1997) Wound healing-aiming for perfect skin regeneration. Science 276:75–81
Wilson GO, Moore JS, White SR, Andersson HM et al (2008) Autonomic healing of epoxy vinyl esters via ring opening metathesis polymerization. Adv Funct Mater 18:44–52
Bleay SM, Loader CB, Hawyes VJ et al (2001) A smart repair system for polymer matrix composites. Compos PartA-Appl S 32:1767–1776
Toohey KS, Sottos NR, Lewis JA et al (2007) Self-healing materials with microvascular networks. Nat Mater 6:581–585
Williams HR, Trask RS, Bond IP (2008) Self-healing sandwich panels: Restoration of compressive strength after impact. Compos Sci Technol 68:3171–3177
Watanabe M, Yoshie N (2006) Synthesis and properties of readily recyclable polymers from bisfuranic terminated poly(ethylene adipate) and multi-maleimide linkers. Polymer 47:4946–4952
Deng G, Tang C, Li F et al (2010) Covalent cross-linked polymer gels with reversible sol-gel transition and self-healing properties. Macromolecules 43:1191–1194
Yuan C, Rong MZ, Zhang MQ (2014) Self-healing polyurethane elastomer with thermally reversible alkoxyamines ascrosslinkages. Polymer 55(7):1782–1791
Li JH, Zhang GP, Sun R et al (2018) Self-healing and shape memory linear polyurethane based on disulfide linkages with excellent mechanical property. Macromol Res 26:365–373
Marschner DE, Frisch H, Offenloch JT et al (2018) Visible light [2 + 2] cycloadditions for reversible polymer ligation. Macromolecules 51:3802–3807
Xiang Z, Zhang L, Yuan T et al (2018) Healability demonstrates enhanced shape-recovery of graphene-oxide-reinforced shape-memory polymeric films. ACS Appl Mater Interfaces 10:2897–2906
Yang L, Lu XL, Wang ZH et al (2018) Diels-Alder dynamic crosslinked polyurethane/ polydopamine composites with NIR triggeredself-healing function. Polym Chem 9:2166–2172
Duarah R, Karak N (2018) High performing smart hyperbranched polyurethane nanocomposites with efficient self-healing, self-cleaning and photocatalytic attributes. New J Chem 42:2167–2179
Li JH, Liu Q, Ho D et al (2018) Three-dimensional graphene structure for healable flexible electronics based on Diels−Alder chemistry. ACS Appl Mater Interfaces 10:9727–9735
Yang YL, Lu X, Wang WW (2017) A tough polyurethane elastomer with self-healing ability. Mater Des 127:30–36
Feng LB, Yu ZY, Bian YH et al (2017) Self-healing behavior of polyurethanes based on dual actions of thermo-reversible Diels-Alder reaction and thermal movement of molecular chains. Polymer 124:48–59
Jian XX, Hu YW, Zhou WL et al (2018) Self-healing polyurethane based on disulfide bond and hydrogen bond. Polym Adv Technol 29:463–469
Cheng CJ, Li J, Yang FH, Wang JL et al (2018) Renewable eugenol-based functional polymers with self-healing and high temperature resistance properties. J Polym Res 25:57
Wu XX, Li JH, Li G et al (2018) Heat-triggered poly(siloxane-urethane)s based on disulfide bonds for self-healing application. J Appl Polym Sci 135:46532
Ma XY, Shi CY, Huang XW et al (2019) Effect of natural melanin nanoparticles on a self-healing cross-linked polyurethane. Polym J 51:547–558
Chang R, Wang X, Li X et al (2016) Self-activated healable hydrogels with reversible temperature responsiveness. ACS Appl Mater Interfaces 8:25544–25551
Wang L, Deng F, Wang W et al (2018) Construction of injectable self-healing Macroporous hydrogels via a template-free method for tissue engineering and drug delivery. ACS Appl Mater Interfaces 10:36721–36732
Ruff Y, Lehn JM (2008) Glycodynamers: fluorescent dynamic analogues of polysaccharides. Angew Chem Int Ed 47(19):3556–3559
He L, Jiang Y, Tu C et al (2010) Self-assembled encapsulation systems with pH tunable release property based on reversible covalent bond. Chem Commun 46(40):7569–7571
Deng G, Li F, Yu H et al (2012) Dynamic hydrogels with an environmental adaptive self-healing ability and dual responsive sol−gel transitions. ACS Macro Lett 1:275–279
Zhang P, Deng F, Peng Y et al (2014) Redox- and pH-responsive polymer gels with reversible sol–gel transitions and self-healing properties. RSC Adv 4:47361–47367
Lu S, Bai X, Liu H et al (2017) An injectable and self-healing hydrogel with covalent cross-linking in vivo for cranial bone repair. J Mater Chem B 5:3739–3748
Wang Y, Yu H, Yang H et al (2017) an injectable interpenetrating polymer network hydrogel with tunable mechanical properties and self-healing abilities. Macromol Chem Phys 218:1700348
Huang X, Wang X, Shi C, Liu Y, Wei Y (2021) Research on synthesis and self-healing properties of interpenetrating network hydrogels based on reversible covalent and reversible non-covalent bonds. J Polym Res 28(1):1–13
Ma X, Shi C, Huang X, Liu Y, Wei Y (2019) Effect of natural melanin nanoparticles on a self-healing cross-linked polyurethane. Polym J 51(6):547–558
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51103078).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Shi, C., Zhang, Z. et al. Preparation and properties of self-healing polyurethane without external stimulation. Polym. Bull. 79, 10723–10739 (2022). https://doi.org/10.1007/s00289-022-04075-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-022-04075-8