Abstract
Both pyrogallol-furfurylamine-based and pyrogallol-aniline-based di-benzoxazines (PG-FA and PG-A) possess a free hydroxyl and latent catalytic characteristics. To decrease the ring-opening polymerization (ROP) temperature of benzoxazine based on phenol and 4,4′-diaminodiphenylmethane (P-DDM) and increase simultaneously thermal property of its polymer, PG-FA and PG-A as catalysts were introduced into P-DDM. DSC and FTIR tests were used to investigate the effect of PG-FA and PG-A on the ROP of P-DDM; DMA, FTIR and TGA were performed to study their effects on the hydrogen bonds and thermal property of the cured P-DDM (PP-DDM). The results indicate that the ROP temperature of P-DDM remarkably decreased with the incorporation of PG-FA and PG-A. FTIR and DMA results illustrate that the total amounts of hydrogen bonds in PP-DDM increased with the addition of PG-FA or PG-A; however, its fraction of –OH⋯N (intra) and O−⋯+HN (intra) hydrogen bonds decreased. The decrease in –OH⋯N hydrogen bonds made the cross-linked density of polybenzoxazine increase, resulting in that the thermal stability of PP-DDM enhanced with the introduction of PG-FA and PG-A.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polybenzoxazine has been one of the rare new polymers of successful commercialization in the past few decades, owing to its unusual properties, such as good thermal and mechanical properties, low surface energy and water uptake, near-zero shrinkage upon polymerization process. Polybenzoxazine can be obtained through the ring-opening polymerization (ROP) of benzoxazine, synthesized by Mannich reaction of formaldehyde, phenolic and amine compounds. Diversity of amine and phenol makes benzoxazine show the abundant molecular design flexibility, which endows polybenzoxazine tailored properties and extensive applications [1,2,3,4,5]. However, there are still some deficiencies in benzoxazine and polybenzoxazine, such as the higher ROP temperature (usually ≥ 180 °C) [3, 6] and relative weaker thermal properties, which restrict its applications in many fields. Thereby, how to decrease the ROP temperature and further improve thermal properties is always the research focus. There are many methods to reduce ROP temperature, such as introducing additional catalysts into benzoxazine [7,8,9,10,11,12,13,14], synthesizing benzoxazines with special structures [15,16,17,18]. Thereinto, the incorporation of catalyst, such as phenolic compound, amine and so on, into benzoxazine is a kind of more convenient methods. The studies show that ROP temperature of benzoxazine decreased evidently with the addition of phenolic compound and the ROP could proceed even at ambient temperature [11, 12]. Moreover, polybenzoxazine with a higher molecular weight [14] or good thermal properties [12, 13] could be obtained in the presence of bi- and trifunctional phenolic compounds, and the incorporation of pyrogallol made polybenzoxazine exhibit better thermal stability compared to bifunctional phenols assigned to its higher nucleophilicity and additional hydrogen [14]. However, ring-opening addition reaction of benzoxazine with phenols at room temperature could make their preservation become difficult. Besides, the obtained polybenzoxazine in the presence of phenolics showed an early decomposition (with a weight loss of 6.9–9.5%) at a low temperature (about 150–250 °C) due to degradation of phenolic terminal groups, which made its initial weight loss temperature (Td5 and Td20) shift to lower one compared to polybenzoxazine without phenolics [13]. In addition, other catalysts also usually show the disadvantage of deteriorating the thermal properties of polybenzoxazine. Thereby, seeking a way of decreasing ROP temperature without compromising thermal properties of polybenzoxazine is a meaningful work.
Our study shows that pyrogallol-furfurylamine-based and pyrogallol-aniline-based di-benzoxazines (PG-FA and PG-A) containing a free hydroxyl possessed lower ROP temperature and latent catalytic characteristics; in addition, their char yield at 800 °C in N2 was also higher (53% and 47%, respectively) [19]. Thus, the introduction of PG-FA or PG-A as catalyst into other benzoxazines could not only reduce ROP temperature but also increase hydrogens and thermal properties of polybenzoxazines, and the addition of PG-FA or PG-A could not result in the early decomposition of polybenzoxazine at a low temperature similar to that of phenolics, due to the absence of phenolic terminal groups. Besides, the latent catalytic characteristics of PG-FA and PG-A could not affect the shelf-life of benzoxazines.
As a kind of ordinary industrial products, benzoxazine based on phenol and 4,4′-diaminodiphenylmethane (P-DDM) has been used in many fields due to excellently comprehensive performance. But it also has the defects of higher ROP temperature and longer cured time; thus, how to further reduce its ROP temperature and time is very valuable. At the present work, PG-FA and PG-A as catalysts were added into P-DDM to promote its ROP and thermal property. There is an additional free phenolic hydroxyl in PG-FA or PG-A compared with other benzoxazines; thus, the incorporation of PG-FA and PG-A into benzoxazine could change its hydrogen bonds. During ROP process, hydrogen bonds would influence chain propagation [20, 21] and then alter the cross-linking density of polybenzoxazine. Thus, the strength, modulus, thermal stability and so on of polybenzoxazine would change with the introduction of PG-FA and PG-A. Consequently, DSC, FTIR and DMA tests were performed to investigate the effect of PG-FA and PG-A on the polymerization behaviors and hydrogen bonding of P-DDM; TGA and DMA were used to study thermal properties of polybenzoxazine in this work.
Experimental
Materials
Pyrogallol, paraformaldehyde, 4,4′-diaminodiphenylmethane, dichloromethane and dioxane were purchased from Sinopharm Chemical Reagent Co., Ltd. Furfurylamine was obtained from Aladdin. Aniline and sodium hydroxide were received from Tianjin Damao Chemical Reagent Factory. All reagents were used as received.
Pyrogallol-furfurylamine-based benzoxazine, pyrogallol-aniline-based benzoxazine and Phenol-4,4-diaminodiphenylmethane-based benzoxazine (PG-FA, PG-A and P-DDM) were prepared according to previous methods [19, 22]. The structure of PG-FA, PG-A and P-DDM is illustrated in Scheme 1. The NMR, FTIR spectra and DSC thermograms of PG-FA and PG-A are displayed in Figures S1-S4 of Supporting Information.
Preparation of benzoxazine blends
Preparation of PG-FA/P-DDM blends: PG-FA, P-DDM and dichloromethane were added into bottle and stirred for 30 min. Then dichloromethane was removed in vacuum to obtain PG-FA/P-DDM.
The preparation of PG-A/P-DDM blends was identical with that of PG-FA/P-DDM. PG-FA/P-DDM and PG-A/P-DDM blends are named as nPG-FA and nPG-A, respectively, in which n represents the weight ratio of PG-FA (or PG-A) and P-DDM. The value of n is 0.05, 0.1, 0.15, 0.2 and 0.25, respectively.
Preparation of polybenzoxazine
Benzoxazines were degassed at 120 °C for 1 h in a vacuum oven and then cured in an air-circulating one as the procedures in Table 1. The cured P-DDM, nPG-FA and nPG-A were abbreviated as PP-DDM, PnPG-FA and PnPG-A, respectively. The final curing temperature and time were determined based on the DSC test.
Characterization
Fourier transform infrared (FTIR) spectrum was recorded using a Nicolet IS 10 Fourier transform spectrometer with a resolution of 4 cm−1.
Differential scanning calorimetry (DSC) analysis was performed by a TA Instruments Q 20 at a heating rate of 10 °C/min and a N2 flow rate of 50 mL/min. Testing temperature was in the range of 40 to 320 °C.
Thermogravimetric analysis (TGA) was performed on a TA Instruments TGA Discovery at a heating rate of 10 °C/min and a flow rate of 25 mL/min from 40 to 810 °C in N2.
Dynamic Mechanical Analysis (DMA) was performed on a TA Instruments DMA Q800 at 1 Hz in stretching Mode. Specimens with a dimension of 80 × 10 × 2 mm3 were measured from 35 to 260 °C at a heating rate of 5 °C/min.
Results and discussion
Effect of PG-FA and PG-A on the ROP of P-DDM
DSC and gelation time tests
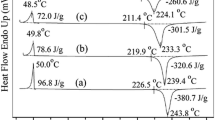
To investigate the influence of PG-FA and PG-A on the ROP of P-DDM, DSC tests were performed and the results are displayed in Figs. 1, 2 and Table 2. As shown in Fig. 1, the initial and peak temperatures of ROP (Ti and Tp) of P-DDM were shifted to lower ones with the incorporation of PG-FA and PG-A. PG-FA and PG-A possess the lower ROP temperatures, whose Ti and Tp are 158 and 177 °C, 172 and 189 °C, respectively [19], and the hydroxyl from PG-FA and PG-A could also catalyze the opening of oxazine ring in P-DDM, resulting in that Ti and Tp of PG-FA/P-DDM and PG-A/P-DDM blends were remarkably lower than those of P-DDM. Moreover, the introduction of PG-FA and PG-A also made the exothermic peak of P-DDM broaden, which would be benefit to the processing and application of benzoxazine.
Furthermore, the gelation time of P-DDM evidently shortened with the addition of PG-FA and PG-A (shown in Table 3), further demonstrating that PG-FA and PG-A promoted the ROP of P-DDM.
The curing procedure of benzoxazine is usually designed according to its structure characteristics, and a shorter curing time or lower curing temperature is expected. Especially, the lower curing temperature is more welcomed due to thermal degradation possibly occurring at higher one. Considering the catalysis of PG-FA and PG-A, most of PG-FA/P-DDM and PG-A/P-DDM blends were cured at a reduced temperature and time as described in Table 1. The DSC results of the cured samples are shown in Fig. 2 and Table 4. In Table 4, the residual enthalpy in the range of 100–200 °C and 200–320 °C, resulting from polymerization and simultaneously occurring polymerization and degradation of the cured samples, respectively, is very little except P0.2PG-FA. These phenomena indicate that the cured degree of all samples was enough, implying that PG-FA and PG-A not only lowered the ROP temperature of P-DDM but also accelerated its polymerization rate.
FTIR test
To further investigate the catalysis of PG-FA and PG-A on ROP of P-DDM, FTIR test of the cured samples was also performed and the results are shown in Fig. 3. In Fig. 3a, the bands at 1227 and 1034 cm−1 assigned to C–O–C and that at 943 cm−1 due to oxazine ring could not be observed in PP-DDM and the cured PG-FA/P-DDM blends, indicating that the oxazine ring in those cured samples had almost completely opened. However, there was a weak shoulder peak at about 1660 cm−1 assigned to imines ion intermediates in all PPG-FA/P-DDM. The ring-opening polymerization of benzoxazine is a two-step process; the existence of imines ion shows that the polymerization degree of PPG-FA/P-DDM did not reach 100%, which were also demonstrated by the occurring of a little polymerization heat at higher temperature in the above DSC tests (Fig. 2 and Table 4). It should be owing to that gelation process of PG-FA/P-DDM happened at lower temperature compared with P-DDM and the early formed cross-linking structure restrained the motion of chain, leading to that some imines ions could not polymerize at lower temperature. Considering the very little residual enthalpy of the cured samples, thus, the curing temperature and time were not further elevated to avoid the degradation of polybenzoxazine.
As to PG-A/P-DDM, Fig. 3b shows that the peaks at 1227, 1034 and 943 cm−1 in P-DDM had disappeared but the shoulder peak at 1660 cm−1 could also be detected in all PG-A/P-DDM. Those suggest that the effect of PG-A on the ROP of P-DDM was identical with that of PG-FA. That is to say, the introduction of PG-FA and PG-A not only decreased the curing temperature of P-DDM but also promoted its curing rate.
Effect of PG-FA and PG-A on thermal properties of PP-DDM
TGA test
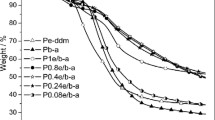
TGA results of PPG-FA/P-DDM and PPG-A/P-DDM are shown in Figs. 4 and 5. As can be seen from Fig. 4a, the char yield at 800 °C of P-DDM significantly increased with the incorporation of PG-FA except P0.05PG-FA. Figure 4b indicates that there was a three-stage weight loss process in all PPG-FA/P-DDM, similar to PP-DDM. Namely, the addition of PG-FA only increased the thermal stability of PP-DDM but did not change its thermal degradation mechanism. Moreover, the weight loss rate between 330 and 650 °C of PPG-FA/P-DDM was evidently lower than that of PP-DDM except P0.05PG-FA. It should mainly result from that the cross-linking density of PPG-FA/P-DDM was more than that of PP-DDM, which would be demonstrated by the following FTIR test (Fig. 7), leading to that the volatilization of degradation products was inhibited.
Figure 5 is the TGA results of PPG-A/P-DDM; it could be seen that the introduction of PG-A could also improve the char yield at 800 °C of PP-DDM except P0.05PG-A. Figure 5b indicates that all PPG-A/P-DDM presented a three-stage weight loss similar to PP-DDM, suggesting that the addition of PG-A also did not change the thermal degradation mechanism of PP-DDM. Moreover, the incorporation of PG-A significantly reduced the weight loss rate of PP-DDM between 320 and 630 °C. Those results indicate that the effect of PG-A on the thermal stability of PP-DDM was same to that of PG-FA. However, the first weight loss rate of PPG-FA/P-DDM and PPG-A/P-DDM in the range of 200–320 °C was slightly higher than that of PP-DDM. But the weight loss of PPG-FA/P-DDM and PPG-A/P-DDM before 250 °C was between 2.6 and 4.3% (shown in Table S1 of Supporting Information), which was obviously less than that of polybenzoxazine in the presence of pyrogallol as catalyst (6.9% weight loss) [13]. It implies that the addition of pyrogallol-based benzoxazine as catalyst was better than that of pyrogallol considering thermal stability of polybenzoxazine.
It can be concluded that both PG-FA and PG-A could enhance the thermal stability of PP-DDM except P0.05PG-FA and P0.05PG-A. The thermal stability of P0.05PG-FA and P0.05PG-A was weaker than that of PP-DDM, possibly resulting from that the cross-linking density of P0.05PG-FA and P0.05PG-A was lower than that of PP-DDM. The following DMA and FTIR tests would demonstrate that the hydrogen bonds of P0.05PG-FA and P0.05PG-A were most in all polybenzoxazines. The hydrogen bonds could obstruct the chain propagation during ROP process of benzoxazine [20, 21], which made the cross-linking density of P0.05PG-FA and P0.05PG-A decrease.
DMA test
Though the lower cross-linked density, extensive inter- and intra-molecular hydrogen bonds render polybenzoxazine high strength and modulus [23]. There is an additional hydroxyl and oxygen atom (hydrogen-bond donor and acceptor) in PG-FA and PG-A compared with P-DDM; thus, the incorporation of PG-FA and PG-A could influence the formation of hydrogen bonding and properties of PP-DDM. At the present, DMA test of PPG-FA/P-DDM as representatives was performed to exploit the effect of pyrogallol-based benzoxazine. Figure 6a and Table 5 show that the introduction of PG-FA enhanced storage modulus (E′) at room temperature of PP-DDM besides P0.2PG-FA and that of P0.05PG-FA reached maximum value. However, the reduction of E′ of P0.05PG-FA with the elevating temperature was also fastest in all cured samples. E′ of polybenzoxazine in the glass region heavily depends on its hydrogen bonds, which were broken gradually with increased temperature and thus made E′ decrease. All these suggest that hydrogen bonds of P0.05PG-FA were the most in all the cured samples. The large numbers of hydrogen bonds restrained chain propagation [20, 21] and reduced cross-linked density of polybenzoxazine, leading to that the thermal stability of P0.05 PG-FA lessened (shown in Fig. 4).
Figure 6b indicates that all samples exhibited a single glass transition temperature (Tg), suggesting that the mixing of PG-FA and P-DDM was well or they could take place co-polymerization. (The possible reaction process of PPG-FA and P-DDM is shown in Scheme 2.) Moreover, Tg of polybenzoxazine decreased with the incorporation of PG-FA. This phenomenon might be attributed to the plasticization effect of flexible methylene of furfurylamine in PG-FA, similar to the effect of PCL on the Tg of polybenzoxazine though the increased cross-linking density [24].
Effect of PG-FA and PG-A on hydrogen bonds
FTIR spectroscopy is a powerful tool for studying hydrogen bonding. In this work, FTIR test of PP-DDM and PPG-FA/P-DDM were carried out to understand the effect of pyrogallol-based benzoxazine on the hydrogen bonds, E′ and thermal stability of polybenzoxazine. Because there are different types of hydrogen bonds in polybenzoxazine (Scheme 3) and they were overlapped in FTIR spectrum, a Gaussian function was used to separate these peaks [21, 25, 26] to gain the fraction of different hydrogen bonds and the results are shown in Fig. 7 and Table 6.
In Fig. 7, the absorbing peak of free hydroxyl was in the range of 3700–3600 cm−1, the band at 3600–3500 cm−1 was due to –OH⋯π hydrogen bond, the bands between 3500 and 3150 cm−1 were assigned to –OH⋯O (intra) and –OH⋯O(inter) hydrogen bonds, and the absorbing peaks of –OH⋯N (intra-6) and O−⋯+HN (intra-6) hydrogen bonds were located at the range of 3150–2500 cm−1 [25]. In general, the free hydroxyl and –OH⋯π intra-molecular hydrogen bonds hardly affect E′ of polybenzoxazine; besides, the effect of –OH⋯O intra-molecular one (intra-5) was limited to benzene ring and could not significantly suppress chain motion. That is to say, only –OH⋯N(intra), O−⋯+HN(intra), –OH⋯O(intra) and –OH⋯O(inter) hydrogen bonds could obviously influence E′ of polybenzoxazine, due to their inhibition of chain motion. The order of integrated peak area of those four types of hydrogen bonds is shown in Table 6, which is consistent with that of the above E′.
Moreover, Table 6 also shows that the relative content of –OH⋯N (intra) and O−⋯+HN (intra) hydrogen bonds (6 and 7) in PP-DDM was most, and they gradually decrease with the incorporation of PG-FA. The hydrogen bonds, especially –OH⋯N ones, obstructed polymerization of benzoxazine and thus made its cross-linking density decrease [21]. All those further illustrate that the incorporation of pyrogallol-based benzoxazine increased the cross-linked density and thermal stability of polybenzoxazine due to the decreasing of –OH⋯N hydrogen bonds, except P0.05PG-FA/P-DDM and P0.05PG-FA/P-DDM.
Conclusions
Pyrogallol-based di-benzoxazines (PG-FA and PG-A) could promote the ROP of P-DDM, which not only decreased its ring-opening temperature but also accelerated its polymerization rate. The introduction of PG-FA and PG-A into P-DDM made –OH⋯N (intra) and O−⋯+HN (intra) hydrogen bonds decrease, resulting in that the cross-linked density of polybenzoxazines increased. Therefore, the thermal stability of PP-DDM increased with the addition of PG-FA and PG-A. However, the total hydrogen bonds increased with the incorporation of PG-FA and PG-A, which enhanced the modulus of polybenzoxazines at room temperature.
References
Lyu Y, Ishida H (2019) Natural-sourced benzoxazine resins, homopolymers, blends and composites: a review of their synthesis, manufacturing and applications. Prog Polym Sci 99:101168
Kaya G, Kiskan B, Yagci Y (2018) Phenolic naphthoxazines as curing promoters for benzoxazines. Macromolecules 51:1688–1695
Teng N, Yang S, Dai J, Wang S, Zhao J, Zhu J, Liu X (2019) Making benzoxazine greener and stronger: renewable resource, microwave irradiation, green solvent, and excellent thermal properties. ACS Sustain Chem Eng 7:8715–8723
Li X, Zhao S, Hu W, Zhang X, Pei L, Wang Z (2019) Robust superhydrophobic surface with excellent adhesive properties based on benzoxazine/epoxy/mesoporous SiO2. Appl Surf Sci 481:374–378
Li P, Dai J, Xu Y, Ran Q, Gu Y (2019) A conjugated alkyne functional bicyclic polybenzoxazine with superior heat resistance. J Polym Sci Part A Polym Chem 57:1587–1592
He J, Li X, Fu Y, Zhu H, Zhao G, Wang Z (2018) Curing reaction mechanism and heat resistance properties of hexa-(4-carboxyl-phenoxy)-cyclotriphosphazene/bisphenol A aniline benzoxazine blends. J Appl Polym Sci 135:46389
Kocaarslan A, Kiskan B, Yagci Y (2017) Ammonium salt catalyzed ring-opening polymerization of 1,3-benzoxazines. Polymer 122:340–346
Sun J, Wei W, Xu Y, Qu J, Liu X, Endo T (2015) A curing system of benzoxazine with amine: reactivity, reaction mechanism and material properties. RSC Adv 5:19048–19057
Zong J, Ran Q (2019) Ring opening reaction of 3,4-dihydro-2H-1,3-benzoxazine with amines at room temperature. Chem Sel 22:6687–6696
Sudo A, Yamashita H, Endo T (2011) Ring-opening polymerization of 1,3-benzoxazines by p-toluenesulfonates as thermally latent initiators. J Polym Sci Part A Polym Chem 49:3631–3636
Oie H, Mori A, Sudo A, Endo T (2012) Synthesis of networked polymer based on ring-opening addition reaction of 1,3-benzoxazine with resorcinol. J Polym Sci Part A Polym Chem 50:4756–4761
Oie H, Mori A, Sudo A, Endo T (2013) Polyaddition of bifunctional 1,3-benzoxazine and 2-methylresorcinol. J Polym Sci Part A Polym Chem 51:3867–3872
Kolanadiyil SN, Azechi M, Endo T (2016) Synthesis of novel tri-benzoxazine and effect of phenolic nucleophiles on its ring-opening polymerization. J Polym Sci Part A Polym Chem 54:2811–2819
Deliballi Z, Kiskan B, Yagci Y (2020) Advanced polymers from simple benzoxazines and phenols by ring-opening addition reactions. Macromolecules 53:2354–2361
He Y, Gao S, Lu Z (2018) A mussel-inspired polybenzoxazine containing catechol groups. Polymer 158:53–58
Zhang K, Tan X, Wang Y, Ishida H (2019) Unique self-catalyzed cationic ring-opening polymerization of a high performance deoxybenzoin-based 1,3-benzoxazine monomer. Polymer 168:8–15
Arslan M (2019) Synthesis and characterization of novel bio-based benzoxazines from gallic acid with latent catalytic characteristics. React Funct Polym 139:9–16
Monisha M, Yadav N, Lochab B (2019) Sustainable framework of chitosan-benzoxazine with mutual benefits: low curing temperature and improved thermal and mechanical properties. ACS Sustain Chem Eng 7:4473–4485
Lin R, Zhu Y, Zhang Y, Wang L, Yu S (2018) Pyrogallol-based benzoxazines with latent catalytic characteristics: The temperature-dependent effect of hydrogen bonds on ring-opening polymerization. Eur Polym J 102:141–150
Chirachanchai S, Laobuthee A, Phongtamrug S (2009) Self termination of ring opening reaction of p-substituted phenol-based benzoxazines: an obstructive effect via intramolecular hydrogen bond. J Heterocyclic Chem 46:714–721
Luan X, Wang B, Yang P, Gu Y (2019) Enhancing the performances of polybenzoxazines by modulating hydrogen bonds. J Polym Res 26:85
Men W, Lu Z (2007) Synthesis and characterization of 4,4′-diaminodiphenyl methane-based benzoxazines and their polymers. J Appl Polym Sci 106:2769–2774
Yang P, Wang X, Fan H, Gu Y (2013) Effect of hydrogen bonds on the modulus of bulk polybenzoxazines in the glassy state. Phys Chem Chem Phys 15:15333–15338
Ishida H, Lee YH (2001) Synergism observed in polybenzoxazine and poly(ε-caprolactone) blends by dynamic mechanical and thermogravimetric analysis. Polymer 42:6971–6979
Kim H, Ishida H (2002) A Study on hydrogen-bonded network structure of polybenzoxazines. J Phys Chem A 106:3271–3280
Bai Y, Yang P, Wang T, Gu Y (2017) Hydrogen bonds in the blends of polybenzoxazines and N, Nʹ- (pyridine-2,6-diyl)diacetamide: Inter- or intra-molecular hydrogen bonds? J Mol Struct 1147:26–32
Acknowledgements
This work was supported by the Natural Science Foundation of Guangxi Autonomous Region (2018GXNSFAA138057).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, Y., Li, P., Lin, R. et al. Promoting ring-opening polymerization of benzoxazine and its thermal property through incorporation of pyrogallol-based benzoxazines. Polym. Bull. 78, 4403–4417 (2021). https://doi.org/10.1007/s00289-020-03327-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03327-9