Abstract
Sago and yucca commercial starches were used in order to evaluate their best possible response adhesive properties. The application of response surface methodology in the optimization of properties was used. A central composite design to evaluate the effect of four independent variables (starch percentage, NaOH percentage, temperature and cooking time) with respect to one response variable (peel strength) was also used. The properties of sago and yucca starches were compared with the properties of corn starch as reference. DSC showed that sago and yucca starches possess similar gelatinization temperatures when compared to corn starch (68.4 °C, 65.7 °C and 69.7 °C, respectively). Adhesion tests indicated that sago and yucca starches presented maximum peel strengths of 125 N/m and 115 N/m, respectively. In this work, the coefficient of determination R2 remained between 0.84 and 0.90; consequently, the quality and effectiveness of the statistical models are considered adequate. These results suggested that sago and yucca starches have potential to be competitive in the global starch market for adhesive applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, modern society is extremely dependent on a constant supply of natural or synthetic adhesives. These are used by many industries such as wood industry [1], footwear industry [2], aerospace industry [3] and the automotive sector [4]. For many years, these adhesives were manufactured based on oil products [5], which contain chemical products such as formaldehyde, phenols and methylene diisocyanate. Moreover, these chemical products are volatile and toxic for living beings. Therefore, they can be transferred to the atmosphere of our planet contributing to air contamination and the reduction in the ozone layer. However, it is also possible to obtain eco-friendly adhesives from renewable, eco-efficient raw materials (efficient in both ecological and economic aspects), for example from proteins, natural rubber, starch or cellulose [6]. Starch is a natural polymer, available in large quantities and at low cost [7]. Starch is one of the most abundant and inexpensive polysaccharides [8]. Starches are obtained from many botanical sources, including corn [9], wheat [10], tapioca [11], potato [12], Ramón [13], etc., and thus, they have been employed as raw material in multiple applications. In the Peninsula of Yucatan, Mexico, a diversity of roots and tubers are cultivated in the milpas of the farmers, such as makal, sweet potato, yucca, jicama, sago and potato, all of them having potential for the extraction of starch. Yucca (Manihot esculenta crantz) and sago (Maranta arundinacea) are unusual or nonconventional sources, presenting a significant prospective for production in the region. Sago starch is obtained from the pith of nonbranching sago palm trees. On the other hand, yucca starch is extracted from a tuber [14]. An adhesive can be defined as a substance, generally of polymeric nature, that holds materials together in a functional manner by surface attachment that resists separation [15]. It binds materials together by mechanical, adsorption or electrostatic forces. Resistance to separation or adhesive effectiveness can be determined by means of a large variety of analytical techniques [16]. Each one of these techniques investigates different parameters of adhesion due to the mechanisms involved in their procurement. The materials to be joined are called substrates; however, after joining, the term adherent is generally used [17]. One of the most important techniques used for obtaining a better estimation of the true efficiency of an adhesive union (adherent/adhesive/adherent) is the technique known as “peeling.”
The effectiveness of the adhesive bond depends on several factors, which are intermolecular forces of the adhesive (van der Waals forces), wettability of the adhesive in the adherent, types of chemical links, functional groups, type of interface between the adhesive and the adherent, type of substrate and surface tension generated [18]. It is pertinent to note also that the use of mathematical models to predict the behavior of experimental adhesive analyses has increased considerably [19].
The response surface methodology (RSM) is one of the most popular methods of optimization used in recent years [20]. It is a statistical and mathematical technique used to develop empirical models with the application of Design of Experiments with the purpose of optimizing the response variable, “output variable,” which is influenced by diverse factors or independent variables, “input variables.” In other words, the method consists in conducting a series of experiments (running experiments) in which changes are provided in the input variables in order to identify the reasons for the changes in the output variable. Both starches have been commonly studied and characterized in order to identify their different properties such as granule structure, pasting properties and functional properties (swelling power and solubility). However, very few analyses have been conducted for studying and optimizing the adhesive capabilities of these starches.
Therefore, the purpose of this work was to optimize temperature, cooking time, starch and NaOH concentration of sago and yucca starches. The objective of optimizing the independent variables (X1, X2, X3 and X4) lies in knowing under what conditions the best or greatest mechanical resistance to peeling (N/m) is obtained when bond paper (scribe) is used as substrate or adherent. This can be observed in the results during the contour graphs (circle with a smaller diameter) in this work. In other words, this optimization determines the influence that these independent factors or variables have on the identified response or response variable. Therefore, with the escalation of these variables, it is expected to contribute to the knowledge of the use of these factors in the preparation of adhesives for the nonfood industry. A response surface method was used in order to evaluate the best possible response and performance of the starch adhesives system.
Materials and methods
Materials
Starch
Sago (Maranta arundinacea L.) and yucca starch (Manihot esculenta C.) were acquired in local stores. Corn starch (Zea Mays) was used as reference starch. NaOH was obtained from the company Sigma-Aldrich®. Sheets of Scribe® duplicator papers, having 98% whiteness, a thickness of 0.1 mm and a weight of 75 g/m2, were used as adherent. We strongly believe that it is important to remark that starches are locally acquired meaning that natural sources from our environment (South Mexico) were used because, normally, both sago and yucca starches can be obtained from plants that are found around the world. It is also very well known that depending on the growing condition (type of soil, climate, nutrients, etc.) of such plants, starch efficiency and chemical components percentage could vary. Likewise, it is important to comment that they are not reactive-grade starches (100% pure); the method of extraction of such starches is also unknown, which is an important factor since the starch can undergo modifications. The amylose–amylopectin ratio may be affected by the extraction method. Estrada-León et al. [21] reported that the extraction process of a starch affects the physicochemical, functional properties, etc., of the starch. The amylose content affects the gelatinization and retrograde properties, the swelling power and the enzymatic susceptibility of starches, hence the importance of their characterization. For all this, its physicochemical characterization was necessary. Most of the references studying, for example, Maranta arundinacea L explain in the title or in the abstract the origin of the used plant [22,23,24].
Experimental methodology
Thermal analysis
Starches gelatinization temperature was determined employing a DSC-6 (Perkin–Elmer®). For this analysis, 1 mg of starch was weighed and placed in an aluminum capsule. Subsequently, 3 µl of deionized water was added to the sample using a micro-syringe, obtaining a starch–water ratio of 1:3 w/w. Finally, the aluminum capsule was sealed. The capsules were heated in an interval of 50–100 °C with a heating ramp of 10 °C/min, under a nitrogen atmosphere, in order to prevent oxidization. One empty aluminum capsule was used as reference. The gelatinization temperature was determined (initiation of the peak, To, the maximum peak, Tgel and the final point of the peak, Tc), and the enthalpy variation in the thermal transition (ΔHgel) was estimated by integration of the area below the curve of the peak according to a base line taken between To and Tc, and expressed in J/g, depending on the content of dry base starch.
Evaluation of amylose and amylopectin content
The quantification of apparent amylose content was carried out in accordance with the methodology described by Ratnayake et al. [25]. Such analysis consists of solubilizing the starch (20 mg) in dimethyl sulfoxide (8 mL, 90% in distilled water) at 85 °C for 15 min; after that, it is exposed to an iodine solution, and the absorbance of the solution was read at 600 nm with a UV–Vis spectrophotometer PerkinElmer Lambda 11 against a reagent blank. The apparent amylose content was determined from a standard curve using amylose and amylopectin solutions and expressed as percentage. The sum of amylose and amylopectin corresponds to 100% of starch. The quantification of amylopectin was calculated based on the difference with the 100% of the amylose content, using the colorimetric method described by Morrison and Laignelet [26].
Morphological analysis
The morphological analysis and granule size of the commercial starches were determined by means of a JEOL®, scanning electron microscope model JSM-6360, with a voltage of 6 kV at high vacuum and an amplification 2500×. Before the analysis, the samples were dried in a convection oven at 40 °C for 24 h; after this process, they were placed in an aluminum sample carrier using a double-sided tape of adhesive carbon. Lastly, the starches were coated with gold using an electro-depositor to facilitate their observation.
Central composite design (CCD)
A rotatable, orthogonal CCD was used to randomize the experimental variables. Four independent variables were used: starch concentration (% w/v), NaOH concentration (% w/w), cooking temperature (°C) and cooking time (min). The intervals used for each independent variable were determined based on the preliminary studies (DSC). The optimal values of the experimental conditions were calculated with the application of a second-order regression model, according to Eq. (1):
where Y denotes the predicted response variable (peel strength); k denotes the number of independent variables (factors studied); xi and xj denote the codified variables; β0 denotes the constant of the coefficient; βi, βii and βij denote the coefficients of first order, quadratic and the effects of the interactions, respectively; i and j denote the indexes of each variable or factor; and ε denotes the residual error. For statistical calculations, the variables are coded as xi (dimensional values of the independent variables) and were calculated with Eq. (2):
where xi denotes the codified value of the variable i; xi is the real value of the independent variable without codifying; x0 is the value of xi in the central point; and δx is the difference between levels 0 and 1. The values of the coded levels with respect to the different independent variables are shown in Table 1. The central composite design, data analysis and the graphics of response surface and contour were obtained using the statistical program Design-Expert® 7.0.0. The base of the CCD consisted in a factorial 24 + star combinations of the rotatable and orthogonal type, with four independent variables and one response variable. A total of 36 runs were performed, including 12 central points, with an estimated error degree of 21 and with an axial distance between codes of α ± 2 and randomized.
Preparation of the adhesives
The adhesives were prepared based on the CCD. All starches were dissolved in 50 ml of distilled water under mechanical agitation at 650 rpm for a certain period of time. Once all the starch had been dispersed, the corresponding quantity of NaOH was incorporated maintaining the agitation for another period of time. Subsequently, the content was completed to 100 ml. The dissolution was placed in a 250-ml beaker and subjected to a water bath at the corresponding temperature and for the period of time defined in the CCD under constant manual agitation. At the end of the cooking period, the sample was removed from the water bath and allowed to cool at room temperature. The samples were placed in plastic containers and stored in refrigeration at 6 °C until required for their application on the adherent (duplicator paper Scribe®).
Mechanical characterization of the adhesives
The test pieces were prepared according to the standard ASTM D1876-08 (Type-T test). Before applying the adhesive to the adherent, the samples were removed from the refrigerator and were maintained on the work table until they reached room temperature (~ 25 °C). Once this was achieved, the adhesive was applied with the aid of a spatula, taking care to apply a considerable quantity (~ a thickness of 1 mm). The test pieces were kept at room temperature for approximately 3 h, after which they were stored in hermetically sealed bags to avoid the absorption of humidity. A universal testing machine (China), Shimadzu® model AGS-X, equipped with a load cell of 500 N was utilized for mechanical characterization. A head speed of 20 mm/min was employed. The software used was Trapezium®.
Validation of statistical model
Several researches have applied the validation of statistical models to evaluate the adhesive properties of starches [27]. CCD validation is carried out by an appropriate variance analysis (ANOVA). The values of the response variable, the estimation of the coefficients of the model and the statistical significance of the independent variables in the model were estimated using the method of minimum quadrants and an analysis of variance, with the aid of the software Design-Expert® 7.0.0. Goodness of fit or quality of the polynomial model is expressed by the coefficient of determination (R2) and adjusted R2. The independent variables or factors which have a P < 0.05 are considered to be statistically significant. A regression model exhibits lack of fit regarding a “not-significant” type, when it can adequately describe the functional relationship between the experimental factors and the response variable. A “significant” lack of fit can occur if important terms of the model are not included, such as terms of interaction or quadratic effects. It can also occur if the adjustment of the model produces various, strangely large residues. The response variables that present a lack of fit of the “not-significant” type are considered adequate prediction models.
Results and discussion
Thermal analysis
The temperature of gelatinization and enthalpy of gelatinization are two of the most important physicochemical properties in the characterization of starches. In a gelatinization process, To is defined as the temperature of the initiation of gelatinization, Tgel is the temperature of gelatinization and Tc is the end of gelatinization temperature [28]. Starch thermograms obtained by DSC are presented in Fig. 1. An endothermic peak in a temperature interval between 60 °C and 80 °C can be observed, which is associated with starches gelatinization process. Similar results were observed by Han et al. [29]. The precise position of the peak depends on various factors such as the botanical source and the relationship between amylose and amylopectin [30]. Table 2 presents the thermal properties of the starches, regarding the gelatinization temperature (initiation, To; peak, Tgel; and conclusion, Tc) and the enthalpy of gelatinization (ΔHgel), measured by DSC, respectively. A slight difference can be observed in the thermal properties of the starches. Corn starch exhibited a greater Tgel (69.7 °C) in comparison with sago (68.4 °C) and yucca starch (65.7 °C). The area below the curve in the thermogram represents the enthalpy of gelatinization, which is related to the concentration of the amorphous phase of the starch (concentration of amylose, %). The values obtained concerning the enthalpies for corn, sago and yucca starches were 12.35 J/g, 11.87 J/g and 10.49 J/g, respectively, indicating that the starch, which requires greater thermal energy for gelatinization, is that of corn, while the yucca starch requires the least. Corn starch presented the higher enthalpy of gelatinization despite having lower crystallinity, but possibly its crystals are smaller, indicating that this starch requires much greater energy for its transition. Considering the percentage crystallinity values reported by Moo-Huchin et al. [31], Klein et al. [32] and Polnaya et al. [33], yucca starch is characterized by a higher crystallinity value (32.1%) compared to corn and sago starches (between 23.09 and 27%); then, yucca starch was expected to have a higher gelatinization enthalpy, as reported by Fujita et al. [34], who observed that, as the percentage of crystallinity increases, the gelatinization enthalpy in wheat starch also increases. However, of the three starches evaluated, corn starch had the highest gelatinization enthalpy and was probably due to differences in amylose content, crystal size and internal arrangement of starch fractions within the granule. This event was somehow expected, given that corn starch displays a lower percentage of amylopectin content in comparison with the sago and yucca starches (Table 3). Amylose influences the packing of amylopectin into crystallites and the organization of crystalline lamella within starch granules. This is important for properties related to water uptake as swelling and gelatinization [35].
Amylose and amylopectin content
As with the gelatinization temperature, the amylose /amylopectin relationship depends mainly on the botanical source. In general, the amylopectin constitutes between 70–85% of most starches [36]. In nature, amylose and amylopectin exist as semicrystalline aggregates with disordered and orderly regions, respectively [37]. However, the concentrations can vary according to the process of starch extraction. Table 3 presents the results obtained regarding amylose and amylopectin content for starches. It can be noted that the highest amylose content was found in corn starch, with 25.3%, followed by sago starch with 22.7% and lastly yucca starch with 16.9%. High amylopectin concentration reduces the ability to hold water and decreases the capacity of wettability on the surface of substrate. Therefore, the adhesive properties of corn starch are expected to be superior to sago and yucca starches.
Morphological analysis
Starches' commercial applications depend on their availability and the physical characteristics of the starch granule such as size, form and structure [38]. The SEM images of starches are shown in Fig. 2. All starches presented diverse morphologies and sizes. Corn and sago starches present irregular forms with polyhedral faces and relatively sharp edges (Fig. 2a and b, respectively). In contrast, yucca starch presented a spherical geometry (Fig. 2c). The starch granules are classified as A, B and C types according to their dimensions: the A (> 15 μm), B (5–15 μm) and C types (< 5 μm) [39] and A type for corn and sago starches (15–20 μm) and B-type (10–15 μm) for yucca starch. These values are similar to those reported by other authors [40]. The morphology and size of the starch granules are commonly attributed to the botanical origin, the degree of maturity and the plant physiology. A number of authors have reported that the size of the starch granule has a significant influence on the functional properties. The smaller they are, more digestible they become. In addition, they are considered to be most resistant to processes with high temperatures, such as sterilization [41].
The size of the starch granules has a direct relationship with the proportion of amylose/amylopectin content in the granule [42]. Other authors [43] correlated that the size of the granule is a function of the length of the α-glycosides chains and the degree of cross-linking of the amylopectin chains. Thus, the adhesive properties of starches are influenced by the size and shape of the granule. The starch granule morphology, the length distribution and proportion of each polymer affect the interaction between them and with other components, such as water, which interacts with the starch granules [44]. In other words, the physical and chemical characteristics of the granule starches are directly involved in their properties and functionality.
Optimization and validation of the adhesive properties
Table 4 shows the factorial design 24 + star combinations, constructing 36 experiments, including 12 central points. The main objective focuses on obtaining the mathematical models that are representative of the data generated experimentally and explain the effects of factors on response variable (variance analysis, Table 5), the generation of the diagnostic graphics (actual vs. predicted values and the normal plot of residuals) and the verification of the models by means of the term “lack of fit” of the model, R2 and adjusted R2. Central composite design, experimental design and the results obtained for the response variable (Y: peel strength, N/m) are also presented.
A statistical analysis of variance was performed to evaluate which process parameters are statistically important. The values presented by the quadratic regression model for the mechanical properties of the adhesives are shown in Table 5.
Based on the experimental results, a statistical analysis was carried out using Fisher’s test (F value). Fisher’s value predicts the relationship between two variances; in other words, the variance is a measurement of data dispersion; how far are the data with respect to the average. By using this model, experimental mechanical tests and statistical analysis can be correlated to understand such mechanical behavior (cushion or adhesive strength). In Table 4, results of response variable are shown by exhibiting the peel strength according to the experimental parameters and the statistical design (central composite design) to evaluate the effect of four independent variables (starch percentage, NaOH percentage, temperature and cooking time) with respect to one response variable (peel strength). Therefore, a consistent information is established in such parameters. Finally, conclusions determine the correlation found between the starches analyzed and its comparison with corn starch from the adhesive properties. High F value of the model represents a great dispersion of the data. As can be seen in Table 5, the F value of model remained small (8.7 for corn, 10.18 for sago and 14.08 for yucca) and regression models are statistically significant (P value < 0.0001). The “lack of fit” test is used to assess whether a relationship between independent variables and the response variable fits better in a curvilinear (quadratic) way than a linear model. Therefore, it is important that nonadjustment values (residual) are minimal to be considered nonsignificant. In this context, the F values remained small (0.78 for corn, 1.15 for sago and 0.66 for yucca), thereby representing a “nonsignificance” statistically. On the other hand, the P values < 0.05 indicate that terms of the model have statistical significance. Such information designates that the independent variables, in relation to the variable response, have a statistical relationship above 95% of confidence. P values > 0.05 are usually considered as “nonsignificant.” Regarding the data obtained in the ANOVA table, all adhesives presented statistically significant data (P < 0.0001), specifying that the model is adequate. All independent variables (x1, x2, x3 and x4) are important factors individually for the preparation of adhesives since they influence their main properties, since their "P" values are < 0.05.
The interactions among the principal effects behaved differently depending on the type of starch. In the case of corn starch, results showed that the only interactions with values of P < 0.05 were x1x2, x1x3, x12, x22, x32 and x42. Sago starch displayed significant interactions for x1x2, x1x4, x2x4, x12, x22 and x42. On the other hand, yucca starch exhibited values for x1x2, x1x4, x12, x22 and x42. The virtue of the fit model, essential to assess whether the statistical model obtained is adequate, was also corroborated by means of the determination coefficients (R2) and adjusted R2. The R2 is the proportion of the variance in the peel strength experimental data (response variable) that is explained from the factors or independent variables in the model. The adjusted R2 is a parameter which provides similar information, but after ignoring the terms or factors of the model which are not significant (P > 0.05). In this work's analysis, the coefficient R2 remained between 0.8481 and 0.9037, implying that the model presents a high goodness of fit. In addition, the values of adjusted R2 remained between 0.6469 and 0.8396.
Discrimination analysis of the model
The adequate control of the model is an important element in every experiment design, since an erroneous model could produce deficient or misleading results. A normal probability analysis of residuals is one way to measure the validity of the experimental design by assuming that the data follow a normal distribution and the probabilities of the values follow an approximately straight line [45]. The graphs of normal plot of residuals estimated by the model for all adhesives are presented in Fig. 3a–c. In all the graphs, it is possible to observe that the residues present a normal distribution, with the data following an approximately straight line, and thus, confirming the normality and independence of the residues. Another way to evaluate the validity or benefits of the model is by comparing the actual experimental values with the values estimated or predicted by the model. The actual values are the data of measured response for a particular execution, while the predicted values are evaluated based on the model and are generated with the use of the functions of approximation. The graphs of actual current values and those estimated or predicted by the model for all adhesives are presented in Fig. 4a–c. It is possible to perceive that in all cases, there is a good correlation between the experimental data and the estimated data by the model, obtaining points very close to the straight line. Therefore, the results obtained validate the experimental design.
Effect of starch concentration vs. temperature
Figure 5d–f shows the contour plots for peel strength in relation to starch concentration (x1) and temperature (x3) for all adhesives. It is noticed that the contour lines form a family of hyperbolas that corresponds to the “saddle-type” response surface. A “saddle type” has a canonical equation where coefficients have different signs. Hyperbolas are spread along the axis, to which the smallest coefficient corresponds. The response value in that case grows as it moves away from the surface center–saddle along one axis and falls along the other axis. In all adhesives, the maximum value of peel strength can be observed when starch concentration is between 10 and 20%w/v and temperature is between 75 and 80 °C. Figure 6d–f shows the response surface plots concerning the values obtained for the response variable (Y) for all starches, as a function of x1 and x3. These properties are achieved when x2 = + 2 y x4 = 0. A saddle-like behavior can be observed in all adhesives tested. This kind of behavior suggests a complex relationship between the variables involved and exhibits pairs of level curves found with the same value, giving as a result two maximum values for all adhesive. Corn adhesive exhibited the first at a temperature superior to its gelatinization temperature (~ 80 °C) and the other at values close to 60 °C. On the other hand, sago and yucca adhesives displayed it only at a temperature superior to 80 °C.
Effect to starch concentration vs. NaOH content
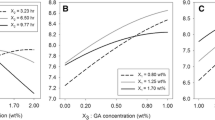
One particularly recommended technique to support the observation and analysis of a 3D response surface consists in representing the graph contour of the surface on which the contour lines are drawn. These curves correspond to constant values of the response variable (Y) on the xixj plane whose coordinate axes are given by the levels xi and xj of the principal factors. The contour graph is useful to study the levels of the factors in which a change in the form or height of the response surface occurs. Figure 5a–c shows the contour plots for peel strength in correlation with starch and NaOH concentration for all adhesives. Clearly, a maximum value in the response variable, Y (peeling strength), can be observed for all adhesives. Regarding the adhesive based on corn starch 5(a), values close to 151 N/m were obtained, while the starches from sago 5(b) and yucca 5(c) values were found to be close to 125 N/m and 115 N/m, respectively. This indicates that corn adhesive properties are 17% and 23% more effective than sago and yucca starch, respectively. In relation to corn adhesive, the maximum peel strength values were found when the x1 variable was between 10 and 20% w/v and the x2 between 1.8 and 2.4% w/w. Sago adhesive exhibited a maximum reached when x1 was between 10 and 20% w/v and x2 between 2.4 and 3.0% w/w, and for yucca adhesive when x1 was reached between 20 and 30% w/v and x2 between 1.8 and 2.4% w/w. Thus, yucca starch needs more concentration of starch than corn and sago starches. However, sago starch needs more concentration of NaOH to reach the maximum peel strength properties. These properties are achieved when x3 = + 2 y x4 = 0. The peel strength diminishes in all adhesives when starch concentrations are below 20% w/v and higher than 40% w/v; this behavior can be attributed to increase or decrease in the viscosity of the solution [46].
On the other hand, with NaOH concentration values below 1.8% w/w and higher than 3% w/w, the adhesive properties decrease in practically all the starches. This could be attributed to the fact that high concentrations of NaOH increase the viscosity. The high viscosity of the solution results in large shear and elongation stresses, which may break down the starch, especially the large content of amylopectin molecules. Increases in the viscosity of the adhesive result in insufficient humidity in the adhesive to allow penetration in the adherent. The behavior observed in all adhesives, when peel strength diminished at NaOH concentrations below than 1.8%w/w, could be attributed to the fact that, at low concentrations of alkali, the concentration of NaOH is insufficient to allow an adequate gelation of the starch, and consequently, its properties decrease.
The visualization of the equation for the estimated model can be obtained by means of a response surface plot. The first goal when applying response surface method is to find the optimum response and secondly to understand how the response changes in a given direction by adjusting the design variables. The graph is helpful to see the shape of a response surface: hills, valleys and ridge lines. Figure 6a–c shows the response surface plots according to the values obtained for the response variable (Y) for all starches, as a function of x1 and x2. In this graph, each value of x1 and x2 generates a y value. In all adhesives, a maximum value can be observed in the response variable, indicating that significant quadratic factors exist for the statistical model. The starch granule is a water-insoluble compound that can be hydrated at high temperatures. When the starch granules are hydrated and submitted to high temperatures, the hydrogen bonds are broken and replaced with water. In this regard, the capacity of starch granules to hydrate and swell depends on the capacity of starch molecules to hold water via hydrogen bonding. High amylose starches induce very low swelling power and low viscosity even at high temperatures, as can be seen for yucca starch.
Mathematical equations estimated by the model
The response surface models are a collection of mathematical techniques used in the treatment for problems in which the response of interest is affected by various quantitative factors. The initial objective of these techniques is to design an experiment, which provides reasonable values of the response variable and, subsequently, determine the mathematical model which best adjusts to the data obtained. The final mathematical model (Table 6) predicts, in terms of the coded factors, the mechanical properties at peeling of the corn, sago and yucca adhesives.
Conclusions
In this work, it was found that the starches obtained from sago and yucca exhibit similar mechanical and thermal properties compared to corn starch. The four independent variables, namely the starch concentration (x1), the NaOH concentration (x2), the cooking temperature (x3) and the cooking time (x4), had a significant effect on the preparation of the adhesives. The size and shape of the starch granules affect the adhesive properties of the starches, varying between 10 and 20 µm for corn and sago, as well as between 10 and 15 µm for yucca; the size of the granule presented polyhedral shapes for corn and sago and spherical for yucca. Amylose–amylopectin relationship in starches is a vital factor that is directly reflected in the adhesive properties of starches. It was identified that, at lower concentrations of amylopectin, better mechanical properties are obtained. The CCD models used for factors optimization to predict a greater peeling force presented a good goodness of fit with coefficients of determination R2 between 0.848 and 0.904 and R2 adjusted between 0.6469 and 0.8396. Optimal mechanical properties for adhesives are obtained when using starch concentrations (~ 30% w/v), NaOH concentrations (~ 1.8% w/w), cooking temperatures above the gelling temperature of the starches (~ 80 °C) and cooking times (~ 15 min). The corn adhesive showed the highest peel strength properties of 135 N/m; however, the sago and yucca adhesives exhibited peel strengths of 127 and 109 N/m, respectively, close to corn values and suitable for a biodegradable adhesive, so they present potential mechanical peeling properties to be used in the global adhesive market. Finally, it is noteworthy to establish that the highest adhesive properties were obtained when the conditions of the independent variables x1, x2 and x4 are at the central point (α = 0) and x3 when its level is at the highest point (α = + 2).
References
Zhou X, Du G (2019) Applications of tannin resin adhesives in the wood industry. In: Tannins-Structural Properties, Biological Properties and Current Knowledge. IntechOpen,
Paiva RM, Marques EA, da Silva LF, Antonio CA, Arán-Ais F (2016) Adhesives in the footwear industry. Proc Ins Mech Eng Part L J Mater Des Appl 230(2):357–374
Imam SH, Bilbao-Sainz C, Chiou B-S, Glenn GM, Orts WJ (2013) Biobased adhesives, gums, emulsions, and binders: current trends and future prospects. J Adhes Sci Technol 27(18–19):1972–1997. https://doi.org/10.1080/01694243.2012.696892
Schneberger G (1990) Adhesives in the Automobile Industry. In: Skeist I (ed) Handbook of adhesives. Springer, Boston, pp 729–735
Fiorelli J, Curtolo DD, Barrero NG, Savastano H Jr, Pallone EMdJA, Johnson R (2012) Particulate composite based on coconut fiber and castor oil polyurethane adhesive: an eco-efficient product. Ind Crop Prod 40:69–75. https://doi.org/10.1016/j.indcrop.2012.02.033
Yu L, Dean K, Li L (2006) Polymer blends and composites from renewable resources. Prog Polym Sci 31(6):576–602. https://doi.org/10.1016/j.progpolymsci.2006.03.002
Baumann MG, Conner AH (1994) Carbohydrate polymers as adhesives. Marcel Dekker, New York
Pan Y, Farmahini-Farahani M, O’Hearn P, Xiao H, Ocampo H (2016) An overview of bio-based polymers for packaging materials. J Bioresour Bioprod 1(3):106–113
Samaraweera S, De Silva AB, Samaranayake MD, Gunawardhane KV, Herath HMT (2018) Potential application of locally grown Sri Lankan corn varieties to utilize in the food industry; Corn Starch and Corn Syrup. Int J Innov Res Technol Sci 4:17-22
Shevkani K, Singh N, Bajaj R, Kaur A (2017) Wheat starch production, structure, functionality and applications—a review. Int J Food Sci Tech 52(1):38–58. https://doi.org/10.1111/ijfs.13266
Hernández-Carmona F, Morales-Matos Y, Lambis-Miranda H, Pasqualino J (2017) Starch extraction potential from plantain peel wastes. J Environ Chem Eng 5(5):4980–4985. https://doi.org/10.1016/j.jece.2017.09.034
Noda T, Matsuura-Endo C, Ishiguro K (2019) Physicochemical properties of potato starches manufactured in Hokkaido factories. J Food Sci Technol 56(5):2501–2507. https://doi.org/10.1007/s13197-019-03727-4
Pérez-Pacheco E, Moo-Huchin V, Estrada-León R, Ortiz-Fernández A, May-Hernández L, Ríos-Soberanis C, Betancur-Ancona D (2014) Isolation and characterization of starch obtained from Brosimum alicastrum Swarts Seeds. Carbohydr Polym 101:920–927. https://doi.org/10.1016/j.carbpol.2013.10.012
Fakir M, Jannat M, Mostafa M, Seal H (2012) Starch and flour extraction and nutrient composition of tuber in seven cassava accessions. J Bangladesh Agric Univ 10(2):217–222. https://doi.org/10.3329/jbau.v10i2.14698
Kinloch AJ (2012) Adhesion and adhesives: science and technology. Springer, Berlin
Wang Z, Zhu H, Huang J, Ge Z, Guo J, Feng X, Xu Q (2019) Improvement of the bonding properties of cassava starch-based wood adhesives by using different types of acrylic ester. Int J Biol Macromol 126:603–611. https://doi.org/10.1016/j.ijbiomac.2018.12.113
Silva LF, Öchsner A, Adams RD (2011) Introduction to adhesive bonding technology. In: Lucas FM (ed) Handbook of adhesion technology. Springer, Berlin
Lyons JS, Ahmed MR (2005) Factors affecting the bond between polymer composites and wood. J Reinf Plast Compos 24(4):405–412. https://doi.org/10.1177/0731684405044898
Tang ZS, Lim YY, Smith ST, Izadgoshasb I (2019) Development of analytical and numerical models for predicting the mechanical properties of structural adhesives under curing using the PZT-based wave propagation technique. MSSP 128:172–190. https://doi.org/10.1016/j.ymssp.2019.03.030
Ayoola A, Fayomi O, Akande I, Adeeyo O, Obanla O, Abatan O, Babatunde D, Olawepo V, Fagbiele O, Olomo V Production of Adhesive from Cassava Starch. In: Journal of Physics: Conference Series, 2019. vol 3. IOP Publishing, p 032079
Estrada-León R, Moo-Huchin V, Ríos-Soberanis C, Betancur-Ancona D, May-Hernández L, Carrillo-Sánchez F, Cervantes-Uc J, Pérez-Pacheco E (2016) The effect of isolation method on properties of parota (Enterolobium cyclocarpum) starch. Food Hydrocolloid 57:1–9. https://doi.org/10.1016/j.foodhyd.2016.01.008
Erdman M (1986) Starch from arrowroot (Maranta arundinacea) grown at Tifton. Georgia Cereal Chem 63(3):277–279
Ezell KC, Pearsall DM, Zeidler JA (2006) Root and tuber phytoliths and starch grains document manioc (Manihot esculenta) arrowroot (Maranta arundinacea) and llerén (Calathea sp.) at the real alto site Ecuador. Econ Bot 60 (2):103–120. https://doi.org/10.1663/0013–0001(2006)60[103:RATPAS]2.0.CO;2
Kumalasari ID, Harmayani E, Lestari LA, Raharjo S, Asmara W, Nishi K, Sugahara T (2012) Evaluation of immunostimulatory effect of the arrowroot (Maranta arundinacea. L) in vitro and in vivo. Cytotechnology 64(2):131–137. https://doi.org/10.1007/s10616-011-9403-4
Ratnayake W, Hoover R, Shahidi F, Perera C, Jane J (2001) Composition, molecular structure, and physicochemical properties of starches from four field pea (Pisum sativum L.) cultivars. Food Chem 74(2):189–202. https://doi.org/10.1016/S0308-8146(01)00124-8
Morrison WR, Laignelet B (1983) An improved colorimetric procedure for determining apparent and total amylose in cereal and other starches. J Cereal Sci 1(1):9–20. https://doi.org/10.1016/S0733-5210(83)80004-6
Ojewumi M, Kayode G, Omoleye J, Oyekunle D (2019) Statistical optimization and sensitivity analysis of rheological models using cassava starch. Int J Civil Eng Technol (IJCIET) 10(1):623–639
Cai C, Cai J, Zhao L, Wei C (2014) In situ gelatinization of starch using hot stage microscopy. Food Sci Biotechnol 23(1):15–22. https://doi.org/10.1007/s10068-014-0003-x
Han H, Hou J, Yang N, Zhang Y, Chen H, Zhang Z, Shen Y, Huang S, Guo S (2019) Insight on the changes of cassava and potato starch granules during gelatinization. Int J Biol Macromol 126:37–43. https://doi.org/10.1016/j.ijbiomac.2018.12.201
Szwengiel A, Lewandowicz G, Górecki AR, Błaszczak W (2018) The effect of high hydrostatic pressure treatment on the molecular structure of starches with different amylose content. Food Chem 240:51–58. https://doi.org/10.1016/j.foodchem.2017.07.082
Moo-Huchin V, Cabrera-Sierra M, Estrada-León R, Ríos-Soberanis C, Betancur-Ancona D, Chel-Guerrero L, Ortiz-Fernández A, Estrada-Mota I, Pérez-Pacheco E (2015) Determination of some physicochemical and rheological characteristics of starch obtained from Brosimum alicastrum Swartz seeds. Food Hydrocolloid 45:48–54. https://doi.org/10.1016/j.foodhyd.2014.11.009
Klein B, Vanier NL, Moomand K, Pinto VZ, Colussi R, da Rosa ZE, Dias ARG (2014) Ozone oxidation of cassava starch in aqueous solution at different pH. Food Chem 155:167–173. https://doi.org/10.1016/j.foodchem.2014.01.058
Polnaya F, Marseno D, Cahyanto M (2013) Effects of phosphorylation and cross-linking on the pasting properties and molecular structure of sago starch. Int Food Res J 20:1609–1615
Fujita S, Yamamoto H, Sugimoto Y, Morita N, Yamamori M (1998) Thermal and crystalline properties of waxy wheat (Triticum aestivumL.) starch. J Cereal Sci 27(1):1–5. https://doi.org/10.1006/jcrs.1997.0152
Cornejo-Ramírez YI, Martínez-Cruz O, Del Toro-Sánchez CL, Wong-Corral FJ, Borboa-Flores J, Cinco-Moroyoqui FJ (2018) The structural characteristics of starches and their functional properties. CyTA-J-Food 16(1):1003–1017. https://doi.org/10.1080/19476337.2018.1518343
Koski C, Bose S (2019) Effects of amylose content on the mechanical properties of starch-hydroxyapatite 3D printed bone scaffolds. Addit Manuf 30:100817. https://doi.org/10.1016/j.addma.2019.100817
Ma Z, Boye JI (2018) Research advances on structural characterization of resistant starch and its structure-physiological function relationship: a review. Crit Rev Food Sci Nutr 58(7):1059–1083. https://doi.org/10.1080/10408398.2016.1230537
Alcázar-Alay SC, Meireles MAA (2015) Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci Tech-Brazil 35(2):215–236. https://doi.org/10.1590/1678-457X.6749
Singh S, Singh N, Isono N, Noda T (2010) Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J Agric Food Chem 58(2):1180–1188
Li L, Yuan TZ, Setia R, Raja RB, Zhang B, Ai Y (2019) Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem 276:599–607. https://doi.org/10.1016/j.foodchem.2018.10.064
Chiotelli E, Le Meste M (2002) Effect of small and large wheat starch granules on thermomechanical behavior of starch. Cereal Chem 79(2):286–293. https://doi.org/10.1094/CCHEM.2002.79.2.286
Bhat FM, Riar CS (2016) Effect of amylose, particle size & morphology on the functionality of starches of traditional rice cultivars. Int J Biol Macromol 92:637–644. https://doi.org/10.1016/j.ijbiomac.2016.07.078
Jane J, Chen Y, Lee L, McPherson A, Wong K, Radosavljevic M, Kasemsuwan T (1999) Effects of amylopectin branch chain length and amylose content on the gelatinization and pasting properties of starch. Cereal Chem 76(5):629–637. https://doi.org/10.1094/CCHEM.1999.76.5.629
Lindeboom N, Chang PR, Tyler RT (2004) Analytical, biochemical and physicochemical aspects of starch granule size, with emphasis on small granule starches: a review. Starch-Stärke 56(3–4):89–99. https://doi.org/10.1002/star.200300218
Ali M, Shahid M, Lodhi HAK, Naseer D, Sadiq I (2019) Interface adhesion strength of adhesive-bonded materials using ultrasonic technique. J Multidiscip Approaches Sci 11(1):8–17
Emengo F, Chukwu S, Mozie J (2002) Tack and bonding strength of carbohydrate-based adhesives from different botanical sources. Int J Adhes Adhes 22(2):93–100. https://doi.org/10.1016/S0143-7496(01)00025-2
Acknowledgements
The authors would like to express their gratitude to the Tecnológico Nacional de México for the financial support for the project: 536.18-PD. The authors thank Dr. Wilberth Antonio Herrera-Kao for his technical assistance on SEM and DSC experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ortiz-Fernández, A., Ríos-Soberanis, C.R., Chim-Chi, Y.A. et al. Optimization of biodegradable starch adhesives using response surface methodology. Polym. Bull. 78, 3729–3749 (2021). https://doi.org/10.1007/s00289-020-03297-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03297-y