Abstract
In this study, the free radical copolymerization of glycidyl methacrylate (GMA) and methyl methacrylate (MMA) was investigated for the first time by solution free radical copolymerization in toluene at 80 °C using azobisisobutyronitrile as an initiator. The 1H-NMR spectroscopy has been used for determining the copolymer composition. Monomer reactivity ratios (r values) were calculated by various linear least-square methods. According to the results, using the Kelen–Tüdös (KT) and extended Kelen–Tüdös (Ex KT) methods the r values were obtained as \( r_{\text{G}} = 1.528 \pm 0.168 \), \( r_{\text{M}} = 0.789 \pm 0.121 \), and \( r_{\text{G}} = 1.577 \pm 0.186 \), \( r_{\text{M}} = 0.783 \pm 0.129 \), respectively. The calculated monomer reactivity ratios showed the higher reactivity for GMA (rG) compared to MMA (rM). Furthermore, the findings demonstrated random or ideal behavior (\( r_{\text{G}} \cdot r_{\text{M}} \simeq 1 \)) for these copolymers. The monomers sequence distribution as probability of finding the multiple sequence distribution of the GMA and MMA units in copolymers was calculated and showed higher probabilities for GMA sequences. Thermogravimetric analysis of the copolymers had three degradation stages, and the main degradation occurred at third stage (340–456 °C) with 56% weight loss. Also, with regard to initial temperature of degradation and T50, the thermal stability was improved 62% and 2.3%, respectively, by increasing MMA content in copolymer. These studies could uncover the underlying GMA–MMA composition in copolymer, shedding light on the future design of top-performing applications such as UV printing ink and resin industry.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The copolymerization similar to polymer blending is a versatile and diverse procedure for preparation of new polymeric materials. Many types of the copolymers with a wide range of interesting properties can be synthesized by simultaneous polymerization of two or more different monomers [1, 2]. In fact, the synthesized copolymers by the free radical method mainly have statistical or random structure. The monomer reactivity ratios, also known as copolymer constants, determine the copolymer structure and mole fraction of each monomer in the final product. So the acknowledgment of these parameters is necessary for preparation of copolymers with the specific and arbitrary composition [3]. Therefore, many studies have been carried out on determining the monomer reactivity ratios. The existing methods for calculating the copolymer constants can be divided into theoretical and experimental methods. In theoretical methods by using the results of some experimental studies, the equations and parameters are suggested to predict the monomer reactivity ratios without the requirement to doing of any copolymerization reaction. Although these methods provide initial estimates for monomer reactivity ratios, in some cases they have a considerable difference with obtained actual amounts from experimental measurements [4].
In the experimentally based methods, several reactions with different molar ratios of two monomers are performed and copolymer composition is determined experimentally. Then, by using these data and copolymerization differential or integral equations, the monomer reactivity ratios are calculated [5]. Also, these methods can be divided into two linear least-square (LLS) and nonlinear least-square (NLLS) methods. The LLS methods are suitable for determining the monomer reactivity ratios at low conversion [6].
The methacrylate-based polymers and copolymers have many applications in various industrial areas [7,8,9]. Many commercial products have been prepared from their homopolymers or copolymers with other monomers, so research on their properties and copolymerization of them with other monomers has always been one of the topics of interest to researchers [7, 10, 11]. The glycidyl methacrylate (GMA) monomer due to the presence of the epoxy group in its structure has high potential for using at click chemistry and post-polymerization processes [12, 13]. For example, the UV-curable compounds recently have been synthesized by copolymerization of GMA and MMA that can be used in UV printing ink and resin industry. Actually, the GMA mole fraction in copolymer is the key factor in determining the curing time [14]. Therefore, the importance of the precise calculation of the monomer reactivity ratios and monomer sequence distributions becomes more and more evident.
Paul and Ranby [15] have studied the copolymerization of GMA–MMA by bulk free radical copolymerization at 60 °C and calculated the monomer reactivity ratios using infrared technique and calculated the data just by one method (Fineman–Ross). They found out that the both monomers have the same reactivity and monomer reactivity ratio of the GMA is close to the MMA ones. However, measuring monomer reactivity ratio with infrared technique in comparison with 1H-NMR technique, even by using Fineman–Ross method, does not lead into precise data [16, 17].
Besides, researches have shown that reaction conditions (e.g., temperature) and polymerization technique (e.g., bulk polymerization, solution polymerization, etc.) have a major impact on the amount of monomer reactivity ratios [16,17,18]. Neugebauer et al. [18] investigated the copolymerization of GMA–MMA by atom transfer radical polymerization (ATRP) at 70 °C, with ethyl 2-bromoisobutyrate as an initiator and 4,4′-dinonyl-2,2′-bipyridyne (dNbpy)/CuBr as a catalyst system in anisole. They calculated the monomer reactivity ratios by the application of the conventional linearization Fineman–Ross and Mayo–Lewis methods. Obtained results show the similar values for reactivity ratio of the GMA and MMA, and nonlinear dependence of the copolymer composition versus initial comonomer concentration led to the conclusion of a statistical composition in the resulting copolymers [18].
In the current study, the monomer reactivity ratios of GMA and MMA in binary solution free radical copolymerization in toluene at 80 °C have been determined by various LLS methods (i.e., Fineman–Ross (FR), inverted Fineman–Ross (IFR), Kelen–Tüdös (KT), extended Kelen–Tüdös (Ex KT), Mayo–Lewis (ML), Joshi–Joshi (JJ), Yezrielev, Brokhina and Roskin (YBR), and Braun, Brendlein and Mott (BBM)) using 1H-NMR data at low conversion. The statistical evaluations such as standard deviation and regression coefficient are also provided to measure the validity and accuracy of each method. Furthermore, the monomer sequence distribution in copolymer chain length and thermal properties of the copolymers have been discussed in detail.
Experimental
Materials
The glycidyl methacrylate (GMA, ≥ 97%, Merck, Germany) and methyl methacrylate (MMA, ≥ 99%, Merck, Germany) monomers were passed from active alumina column for several times to eliminate their inhibitors. The purity of the azobisisobutyronitrile (AIBN, 98%, Sigma-Aldrich, Switzerland) was enhanced by recrystallization in ethanol. Toluene (99%, Alfa Aesar, the USA) was distilled under reduced pressure before usage. The methanol and chloroform were acquired from Merck and were used as received.
Copolymerization
The copolymerization was performed in a 50-ml round-bottom glass reactor under magnetic stirring. First, the predetermined amounts of monomers were dissolved in 20 ml toluene. The total weight percent of the monomers is equal to 10%. Then, AIBN was added as 2.5% by weight relative to the total weight of monomers and its amount was constant in all of the experiments. The copolymerization conditions and calculated copolymer composition by 1H-NMR analysis are given in Table 1. The fG and FG denote mole fraction of the GMA in feed and copolymer, respectively.
After adding the AIBN to the reaction mixture, it was purged with nitrogen for 15 min in order to remove oxygen from the reaction media. Then, the reactor was sealed and transferred into an adjusted silicone oil bath at 80 °C. After progress of the reaction to a definite time, the reaction mixture was poured into the methanol and a white precipitate was obtained. To increase the product purity, the copolymer was redissolved in chloroform and precipitated in methanol again. Eventually, the purified copolymer was filtered and dried in vacuum oven at 50 °C for overnight. After complete drying of the copolymer, the reaction conversion was determined as gravimetrically. A schematic of the copolymerization is shown in Fig. 1.
Characterization
The 1H-NMR spectrum of the samples was recorded by using 250-MHz FT NMR spectrometer (Bruker, Germany) in CDCl3 at room temperature. The Fourier transform infrared (FTIR) analyses were done by FTIR spectrometer (Nicolet iS10, Thermo Fisher Scientific, Germany) in the ranges of 400–4000 cm−1 at a resolution of 0.5 cm−1. Thermogravimetric analysis (TGA) was carried out using a TGA laboratory instrument (TGA-PL/PL 1500, England) from 25 to 600 °C under the N2 atmosphere at a heating rate of 20 °C/min.
Result and discussion
In this work, the free radical copolymerization of GMA and MMA as two methacrylate-based monomers have been investigated in toluene as solvent by using AIBN as thermal initiator at 80 °C. For this purpose, the samples were prepared by varying the monomer molar ratios in feed and their compositions were determined by 1H-NMR spectroscopy. The monomer reactivity ratios for conversions lower than 20% were estimated by various methods. Besides, the FTIR and TGA analyses were performed for more characterization.
FTIR characterization
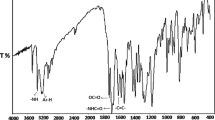
The FTIR spectra of the homopolymers and their corresponding copolymer are shown in Fig. 2. The peak appeared at 912 cm−1 are related to symmetric stretching vibration of epoxy groups in GMA unit. The asymmetric stretching vibration of epoxy groups shows a sharp peak in 750 cm−1 [7, 19]. The stretching vibrations of carbonyl groups and C–O bonds, which exist in both of monomers, appear in 1724 and 1148 cm−1, respectively. The stretching vibrations of C–H bonds in methyl and methylene groups show adsorption peaks in 2952 and 2996 cm−1. Also, the peaks that appeared in 1389 and 1487 cm−1 are related to bending vibration of the C–H bonds. The simultaneous presence of peaks corresponding to both of monomers and epoxy ring in spectra of the copolymer confirms the successful synthesis of the poly(GMA-co-MMA) and stable preservation of the epoxy groups during the synthesis process, respectively.
Copolymer composition analysis
Some of the most important experimental methods for determining the copolymer composition include gravimetry, conductometric titration [20], potentiometry [21], elemental analysis [22] and methods based on NMR spectroscopy [23]. Among them, the NMR spectroscopy is one of the most powerful and simplest methods. The accurate determination of the copolymer composition as well as mole fraction of incorporated monomers into the copolymer structure has a significant role in the validity of the calculated monomer reactivity ratios. In the other words, using a precise experimental procedure in computing the copolymer composition leads to an increased accuracy and reduced error in the calculation of the monomer reactivity ratios. Therefore, the 1H-NMR spectroscopy was used for this purpose.
A schematic illustration of the copolymerization reaction for investigated system in this work is presented in Fig. 3. The 1H-NMR spectra of the homopolymers and one of the copolymers (G6 sample) are also shown in Fig. 3a. Besides, the copolymer structure and the position of each proton are present in this figure. Two similar peaks with the approximately same intensity that appear in 4.28 and 4.31 ppm, are related to germinal protons of methylene groups in the epoxy ring. A single peak that appears in 3.6 ppm is related to –OCH3 protons in the MMA segment. The methylene protons of the copolymer main chains appear in the 0.5–2 ppm [24]. The various peaks that are observed in this region are related to different microstructures due to various multiple sequences of monomers in the copolymer structure. The peaks that have been singed as b and d were used to determine the copolymer composition. Moreover, as shown in Fig. 3b, by increasing the GMA content in feed (from G1 to G9), the intensity of the GMA peaks increased.
Copolymer composition equation
Copolymerization is one of the most versatile and widely used methods for preparation of new polymeric compounds. The precise knowledge of the monomer reactivity ratios is the key to synthesis of copolymers with desired composition and predict the monomer sequence distribution. On this point, the monomer reactivity ratios of MMA and GMA were calculated by several conventional methods. All of the used methods in estimating the copolymerization constants in this work are based on copolymerization deferential equation (Eq. 1), so they are called as linear least-squares (LLS) methods [1]:
The GMA and MMA were chosen as M1 and M2 monomers. \( \left[ {M_{1} } \right] \), \( \left[ {M_{2} } \right] \) and \( \left[ {m_{1} } \right] \), \( \left[ {m_{2} } \right] \) are the molar concentrations of the monomers in the feedstock and copolymer, respectively, and \( r_{1} \), \( r_{2} \) are reactivity ratios of the GMA and MMA monomers, respectively. In fact, the approximations were considered to simplify these methods [6]. However, these approaches lead to an unavoidable error in computations. To increase the accuracy of calculations and minimize the error of approximations, the copolymerization conversion must be as low as possible (usually lower than 20%).
Monomer reactivity ratios
The Fineman–Ross (FR), inverted Fineman–Ross (IFR) and Kelen–Tüdös (KT) equations are given in the following, respectively [1], and their corresponding plots are shown in Fig. 4. In all graphs, the linear regression coefficient is very close to 1 and it shows good data compliance with the above methods. The subscripts G and M denote GMA and MMA, respectively:
The distribution of data in the F–R and IF–R methods is asymmetric and more toward lower H and 1/H values. This is one of the disadvantages of these two methods that their results are influenced by arbitrary factors. Selecting each monomer as M1 or M2 usually results in different r1 and r2 values.
However, the presence of \( \alpha \) parameter in KT method (Eq. 4) caused more uniformly distribution of \( \xi \) in the (0, 1) interval. The effect of conversion was considered in the extended Kelen–Tüdös (Ex KT) method. The partial molar conversion of MMA and GMA is defined as:
where W is the weight conversion of copolymerization and \( \mu \) is the ratio of the molecular weight of MMA to that of GMA. Then, Z as a conversion-dependent parameter is defined as:
The pervious parameters are redefined as: \( H = F/Z^{2} \); \( G = \left( {F - 1} \right)/Z \); \( \eta = G/\left( {\alpha + H} \right) \) and \( \xi = H/\left( {\alpha + H} \right) \) [1]. Plot related to the Ex KT method is also shown in Fig. 4.
As a result, KT and Ex KT methods, unlike F–R and IF–R, result in unique data for r1 and r2 that are not affected by arbitrary factors.
The molar fraction of the GMA in the copolymer versus its molar fraction in the feedstock is shown in Fig. 5.
As shown in Fig. 5, there is no azeotropic point. These data are based on the reactivity ratios calculated from Ex KT method. In all of the experiments, the GMA consumption rate is higher than to MMA that indicates the higher tendency of growing radicals to react with GMA. As shown in Table 2, from the Ex KT method, the r values were obtained as \( r_{\text{G}} = 1.577 \pm 0.186 \) and \( r_{\text{M}} = 0.783 \pm 0.129 \). The calculated monomer reactivity ratios show the higher reactivity for GMA compared to MMA. Furthermore, the findings demonstrate random or ideal behavior (\( r_{\text{G}} \cdot r_{\text{M}} \simeq 1 \)) for these copolymers [25], while Paul and Ranby [15] have found out that the both monomers have the same reactivity and monomer reactivity ratio of the GMA (\( r_{\text{G}} \) = 0.7) is close to the MMA ones (\( r_{\text{M}} \) = 0.8). Besides, the plot of the molar fraction of the GMA in the copolymer versus its molar fraction in the feedstock shows an azeotropic point [15]. These result demonstrate alternating behavior (\( r_{\text{G}} < 1\,{\text{and}}\,r_{\text{M}} < 1 \)) for GMA–MMA copolymer. As a result, bulk copolymerization of the GMA and MMA leads to alternating copolymer, whereas solution copolymerization leads to random or ideal copolymer. Elsewhere, Neugebauer et al. [18] investigated the copolymerization of GMA–MMA by atom transfer radical polymerization (ATRP) in anisole. Their results show the similar values for reactivity ratio of the GMA and MMA (\( r_{\text{G}} \ge r_{\text{M}} \sim 1 \)), and nonlinear dependence of the copolymer composition versus initial comonomer concentration led to the conclusion of a statistical composition in the resulting copolymers [18].
Mayo–Lewis method
The Mayo–Lewis (ML) method is another valuable method for determining the monomer reactivity ratios. In this method for each experiment, a line based on Eq. 8 is plotted in \( r_{\text{M}} \), \( r_{\text{G}} \) plane:
Now if we consider Eq. 8 as (ax + by + c = 0), x and y are rG and rM, respectively. In the absence of experimental errors, these lines intersect each other at a certain point. But due to the presence of systematic errors, the lines intersect each other at several points. Therefore, the point that has minimum distance with all lines is considered as the best answer [16] and the square of the distance of this optimal point (x0, y0) from the lines can be calculated from Eq. 9. The ML plot and the obtained results for monomer reactivity ratios by this method are shown in Fig. 6:
The summation of the distance of the desired point with all the lines, which is a function of x0 and y0, is obtained from Eq. 10:
We want to minimize the square of a point with all lines. So by deriving the above equation to x0 and y0 and setting them to zero, the following equations can be obtained. Solving the following equations can get the coordinates of the desired point (x0, y0) or the optimal rM and rG:
Joshi–Joshi and YBR method
Joshi–Joshi (JJ) method is another useful method for determining the monomer reactivity ratios based on the following equations [26]:
The Yezrielev, Brokhina and Roskin (YBR) method is one of the numerically invaluable methods that is not affected by arbitrary factors and results in a unique solution for \( r_{\text{G}} \) and \( r_{\text{M}} \). The YBR least-square procedure includes the following equations [7]:
where \( m = f^{2} /F \), \( c = f\left( {1/F - 1} \right) \), and N denotes the number of experiments.
Braun, Brendlein and Mott (BBM) method
The BBM method has proposed a computerized program based on curve-fitting method for determining copolymerization reactivity ratios [27]. In this method, the first estimated values for \( r_{\text{G}} \) and \( r_{\text{M}} \) are obtained as follows:
-
\( r_{\text{M}} \approx f_{\text{G}} /F_{\text{G}} \) from the first measured values.
-
\( r_{\text{G}} \approx 100 - f_{\text{G}} /100 - F_{\text{G}} \) from the last measured values.
Then, the average differences between measured and calculated values for the two parts of the diagram (part A and part B: measured value below and above the 50% mole GMA, respectively) were calculated and used for new estimations of \( r_{\text{G}} \) and \( r_{\text{M}} \). This procedure repeated until the fowling termination criteria are fulfilled:
\( r_{\text{G}}^{k} - r_{\text{G}}^{k - 1} \le 0.001 \) and \( r_{\text{M}}^{k} - r_{\text{M}}^{k - 1} \le 0.001 \); k here refers to the number of steps of iteration. Table 2 represents the iteration results by this method.
As can be seen in Table 3 after eight iteration steps, the \( r_{\text{G}} \) and \( r_{\text{M}} \) are approximately fixed. In BBM method unlike the conventional curve-fitting methods, the obtained amounts for \( r_{\text{G}} \) and \( r_{\text{M}} \) are not affected by personal judgment [28]. The obtained amounts for \( r_{\text{G}} \) and \( r_{\text{M}} \) by different methods are summarized in Table 4.
Also, the monomer reactivity ratios can be calculated theoretically by using of revised pattern based on the following equation:
where the subscripts 1 and 2 are attributed to radical and monomer, respectively. The \( r_{12} \) is the reactivity ratio of the monomer 1 in copolymerization with monomer 2, \( r_{1S} \) is the general reactivity of the radical of monomer 1 that polymerized with styrene, \( u_{2} \) is the polarity of the monomer 2, \( \pi_{1} \) is the polarity of the radical of monomer 1, and \( \nu_{2} \) is the general reactivity of the monomer 2 [29]. The calculated monomer reactivity ratios by theoretical data, which are reported in Table 4, have a considerable difference with experimental ones. According to Table 4, the r values were obtained as \( r_{\text{G}} = 0.712 \) and \( r_{\text{M}} = 0.589 \). These values suggest that the copolymer is alternating \( (r_{\text{G}} < 1\,{\text{and}}\,r_{\text{M}} < 1) \), whereas experimental results indicate that the copolymer is ideal \( (r_{\text{G}} \cdot r_{\text{M}} \simeq 1) \) [25]. This theoretically obtained result is approximately the same as the bulk copolymerization result in Reference 15. The main reason for this deviation can be related to solubility effects which are not considered in revised patterns. However, theoretical data also predict higher reactivity for GMA to MMA which is consistent with experimental data.
The regression coefficient (R2) is a statistical criterion for evaluating the validity of the used methods in determining the monomer reactivity ratios. It can be calculated for each method by using the following equation:
where \( F_{\exp } \) is the molar ratio of the monomers in the copolymer obtained experimentally, \( \bar{F} \) is the molar ratio of the monomers in the copolymer obtained by curve fitting of \( F_{\exp } \) versus \( f \) plot, and \( F_{\text{model}} \) is monomers molar ratio in the copolymer from Eq. 8 and using our obtained reactivity ratios for each LLS method. In fact, a value of 1.0 for \( R^{2} \) indicates the perfect match of the regression line with experimental data. As can be seen from Table 4, all of the used methods for determining the monomer reactivity ratios are reasonable, but the IFR and YBR compared to other methods are slightly better.
The sequence distribution of monomers
The monomers sequence distribution in copolymer chain is one of the most important factors in determining the copolymer properties. To calculate them, terminal model and first-order Markovian model were considered for copolymerization and description of the copolymer chain growth process, respectively. The multiple sequence distributions of the GMA and MMA units were calculated by using following equations [3]:
where \( P_{\text{GG}} \) and \( P_{\text{MM}} \) are the probability of addition of a growing chain to the same monomer that are calculable by following equations:
The obtained amounts from FR method were selected for these calculations. The calculated \( N_{\text{GMA}} \left( n \right) \) and \( N_{\text{MMA}} \left( n \right) \) for three samples are shown in Fig. 7. By increasing GMA mole fraction in initial feed (from G4 to G6), the probability of finding the greater sequence of GMA increases compared with MMA. In all of samples, the probability of finding sequence of GMA units with n > 3 is considerably greater than sequence of MMA units. This phenomenon is due to higher reactivity of GMA. In the other words, the growing radicals show higher tendency to react with GMA monomer.
The copolymer composition in this study was determined by two approaches: experimentally by 1H-NMR data and calculations by different LLS methods. From rG and rM obtained by each of the described LLS methods, the initial feed composition and the copolymerization equation (Eq. 1), the molar fraction of GMA in copolymer has been calculated and the results are summarized in Table 5. These results were compared by the obtained GMA molar fraction through experimental results (1H-NMR). For evaluating the validity of each LLS method, their standard error amounts were determined. The small standard error for all of the methods represents the good fitting with experimental data and the adequate ability of these methods for determining the monomer reactivity ratios.
Thermal properties
The thermogravimetric analysis (TGA) and differential thermogravimetry (DTG) thermograms for G5 and G8 samples are shown in Fig. 8.
Both samples show three distinct stages in their thermal degradation process. The first stage is from 100 to 255 °C, the second stage is from 255 to 340 °C, while the last stage begins at 340 °C and continues to 456 °C and the maximum weight loss occurs at this stage. The weight loss in these stages is about 14, 28 and 56%, respectively.
The initial temperature of degradation (63.4 vs. 102.9 °C), the T50, the temperature at which 50% degradation occurred (349 vs. 341 °C) and char yield amount (0.72 vs. 0.49%), all of them indicate slightly higher thermal stability for G5 compared to G8. On the other hand, thermal stability was improved by increasing MMA content in the copolymer.
In the second and third stages of the thermal degradation, the copolymer backbone was degraded. poly(methyl methacrylate) (PMMA) degrades thermally in a radical process to give quantitative yields of monomer. The random main-chain scission mechanism is the fundamental mechanism in degradation of PMMA parts in copolymer. By formation of isobutyryl macro-radicals, PMMA parts in copolymer have been depolymerized to give MMA [30]. In poly(glycidyl methacrylate) (PGMA), thermal degradation was taken by ester decomposition and depolymerization mechanism. The thermal degradation products for GMA units in copolymer include CO2, dimethyl ketene, propene, isobutene, acrolein, glycidol and glycidyl methacrylate [31].
Conclusion
The free radical copolymerization of MMA and GMA monomers in toluene at 80 °C was investigated in this study. The copolymer composition was determined by 1H-NMR spectroscopy. The monomer reactivity ratios were determined by some known LLS methods. In all cases, the GMA comonomer had higher reactivity ratios compared to MMA. The theoretically calculated amounts for \( r_{\text{G}} \) and \( r_{\text{M}} \) were equal to 0.712 and 0.589, respectively, while \( r_{\text{G}} = 1.577 \pm 0.186 \) and \( r_{\text{M}} = 0.783 \pm 0.129 \) were estimated by the extended Kelen–Tüdös method. Therefore, the theoretical method is not able to predict the monomer reactivity ratios correctly. The accuracy of the methods was evaluated by the regression coefficients that were satisfactory for all of them. The monomers sequence distribution was studied based on the terminal model and first-order Markovian model and showed higher probabilities for GMA sequences. In the other words, the growing radicals showed higher tendency to react with GMA monomer due to higher reactivity of GMA. The TGA thermograms indicate three degradation steps for these copolymers. Generally, the sample was completely degraded up to 500 °C, but the thermal stability was slightly improved by increasing the MMA content in the copolymer. By determining the monomer reactivity ratios, the curing time at UV-curable coatings based on MMA/GMA copolymers simply can be controlled through the GMA content in the copolymer.
References
Abdollahi H, Najafi V, Ziaee F et al (2014) Radical copolymerization of acrylic acid and OEGMA475: monomer reactivity ratios and structural parameters of the copolymer. Macromol Res 22:1330–1336. https://doi.org/10.1007/s13233-014-2190-y
Amiri F, Kabiri K, Bouhendi H et al (2018) High gel-strength hybrid hydrogels based on modified starch through surface cross-linking technique. Polym Bull. https://doi.org/10.1007/s00289-018-2593-6
Odian G (2004) Principles of polymerization, 4th edn. Academic Press, New York
Ko KY, Baek SS, Hwang SH (2018) Synthesis of imide-based methacrylic monomers and their copolymerization with methyl methacrylate: monomer reactivity ratios and heat resistance properties. Polym Int 67:957–963. https://doi.org/10.1002/pi.5594
Gabriel VA, Dubé MA (2018) Bulk free-radical co- and terpolymerization of n-butyl acrylate/2-ethylhexyl acrylate/methyl methacrylate. Macromol React Eng 1800057:1–8. https://doi.org/10.1002/mren.201800057
Mao R, Huglin MB (1994) A new linear method to calculate monomer reactivity ratios by using high-conversion copolymerization data: penultimate model with r2 = 0. Polymer (Guildf) 35:3525–3529. https://doi.org/10.1016/0032-3861(94)90918-0
Darvishi A, Zohuriaan Mehr MJ, Marandi GB et al (2013) Copolymers of glycidyl methacrylate and octadecyl acrylate: synthesis, characterization, swelling properties, and reactivity ratios. Des Monomers Polym 16:79–88. https://doi.org/10.1080/15685551.2012.705493
Najafi V, Ziaee F, Kabiri K et al (2012) Aqueous free-radical polymerization of PEGMEMA macromer: kinetic studies via an on-line 1H NMR technique. Iran Polym J (English Ed) 21:683–688. https://doi.org/10.1007/s13726-012-0072-8
Jalilian SM, Farhadnejad H, Ziaee F et al (2016) Poly(n-octyl methacrylate) viscosity index improver: kinetic study via on-line 1H-NMR technique. Polym Sci Ser B 58:675–680. https://doi.org/10.1134/S1560090416060087
Debnath D, Baughman JA, Datta S et al (2018) Determination of the radical reactivity ratios of 2-(N-ethylperfluorooctanesulfonamido)ethyl acrylate and methacrylate in copolymerizations with N,N-dimethylacrylamide by in situ 1H NMR analysis as established for styrene-methyl methacrylate copolymerization. Macromolecules 51:7951–7963. https://doi.org/10.1021/acs.macromol.8b01526
Feng J, Oyeneye O, Xu WZ, Charpentier P (2018) In-situ NMR measurement of reactivity ratios for copolymerization of methyl methacrylate and diallyl dimethylammonium chloride. Ind Eng Chem Res 57:15654–15662. https://doi.org/10.1021/acs.iecr.8b04033
Barsbay M, Güven O, Kodama Y (2016) Amine functionalization of cellulose surface grafted with glycidyl methacrylate by γ-initiated RAFT polymerization. Radiat Phys Chem 124:140–144. https://doi.org/10.1016/j.radphyschem.2015.12.015
Muzammil EM, Khan A, Stuparu MC (2017) Post-polymerization modification reactions of poly(glycidyl methacrylate)s. RSC Adv 7:55874–55884. https://doi.org/10.1039/c7ra11093f
Hong S, Kim J, Kim MS, Kim BW (2012) Radical polymerization of acrylate copolymer-based GMA for use as a UV-curable layer via thin coating. Adv Polym Technol 31:271–279. https://doi.org/10.1002/adv.20250
Paul S, Ranby B (1976) Studies of methyl methacrylate-glycidyl methacrylate copolymers: copolymerization to low molecular weights and modification by ring-opening reaction of epoxy side groups. J Polym Sci 14:2449–2461. https://doi.org/10.1002/pol.1976.170141012
Ziaee F, Nekoomanesh M (1998) Monomer reactivity ratios of styrene-butyl acrylate copolymers at low and high conversions. Polymer 39:203–207. https://doi.org/10.1016/S0032-3861(97)00249-8
Bradbury JH, Melville HW (1954) The co-polymerization of styrene and butyl acrylate in benzene solution. Proc R Soc Lond Ser A Math Phys Sci 222:456–470. https://doi.org/10.1098/rspa.1954.0088
Neugebauer D, Bury K, Wlazło M (2012) Atom transfer radical copolymerization of glycidyl methacrylate and methyl methacrylate. J Appl Polym Sci 124:2209–2215. https://doi.org/10.1002/app.35234
Abdollahi H, Salimi A, Barikani M et al (2019) Systematic investigation of mechanical properties and fracture toughness of epoxy networks: role of the polyetheramine structural parameters. J Appl Polym Sci. https://doi.org/10.1002/app.47121
Erbil C, Özdemir S, Uyanik N (2000) Determination of the monomer reactivity ratios for copolymerization of itaconic acid and acrylamide by conductometric titration method. Polymer 41:1391–1394. https://doi.org/10.1016/S0032-3861(99)00291-8
Uyanik N, Erbil C (2000) Monomer reactivity ratios of itaconic acid and acrylamide copolymers determined by using potentiometric titration method. Eur Polym J 36:2651–2654. https://doi.org/10.1016/S0014-3057(00)00045-8
Thirumoolan D, Anver Basha K, Kanai T et al (2016) Synthesis, characterization and reactivity ratios of poly N-(p-bromophenyl)-2-methacrylamide-Co-N-vinyl-2-pyrrolidone. J Saudi Chem Soc 20:195–200. https://doi.org/10.1016/j.jscs.2013.09.003
Hasanzadeh R, Moghadam PN, Bahri-Laleh N, Ziaee F (2016) A reactive copolymer based on glycidylmethacrylate and maleic anhydride: 1-synthesis, characterization and monomer reactivity ratios. J Polym Res. https://doi.org/10.1007/s10965-016-1048-8
Bakhshi H, Zohuriaan-Mehr MJ, Bouhendi H, Kabiri K (2009) Spectral and chemical determination of copolymer composition of poly(butyl acrylate-co-glycidyl methacrylate) from emulsion polymerization. Polym Test 28:730–736. https://doi.org/10.1016/j.polymertesting.2009.06.003
Chanda M (2006) Introduction to polymer science and chemistry. CRC Press, Boca Raton
Erol I, Devrim DN, Ciftci H et al (2017) Novel functional copolymers based on glycidyl methacrylate: synthesis, characterization, and polymerization kinetics. J Macromol Sci Part A Pure Appl Chem 54:434–445. https://doi.org/10.1080/10601325.2017.1320747
Bauduin G, Boutevin B, Belbachir M, Meghabar R (1995) Determination of reactivity ratios in radical copolymerization: a comparison of methods for a methacrylate/N-vinylpyrrolidone system. Macromolecules 28:1750–1753. https://doi.org/10.1021/ma00110a004
Braun D, Brendlein W, Mott G (1973) A simple method of determining copolymerization reactivity ratios by means of a computer. Eur Polym J 9:1007–1012. https://doi.org/10.1016/0014-3057(73)90077-3
Jenkins AD, Hatada K, Kitayama T, Nishiura T (2000) Revised patterns of reactivity scheme. VII. Revised patterns scheme and its relationship to carbon-13 NMR spectra. J Polym Sci Part A Polym Chem 38:4336–4342. https://doi.org/10.1002/1099-0518(20001215)
Manring LE (1991) Thermal degradation of poly(methyl methacrylate): random side-group scission. Macromolecules 24:3304–3309. https://doi.org/10.1021/ma00011a040
Zulfiqar S, Zulfiqar M, Nawaz M et al (1990) Thermal degradation of poly(glycidyl methacrylate). Polym Degrad Stab 30(2):195–203
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdollahi, H., Najafi, V. & Amiri, F. Determination of monomer reactivity ratios and thermal properties of poly(GMA-co-MMA) copolymers. Polym. Bull. 78, 493–511 (2021). https://doi.org/10.1007/s00289-020-03123-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-020-03123-5