Abstract
The effect of ultrasonic (US) irradiation on solutions of low-density polyethylene (LDPE) was studied. Different irradiation times and intensities were examined. It was found that gel content increased very little as a result of US irradiation. However, this increase showed no variation with either the US irradiation time or intensity. The IR spectra of irradiated LDPE showed new absorption bands, indicating the presence of C–O groups, assumed to be the result of the US irradiation. GPC showed that the LDPE average molecular weight (Mw) decreases with an increase in either the US irradiation time or intensity. But these MWD curves, however, do not say if the “observed” modifications in Mw are due to chain scission or chain branching, which was inferred from the chain scission distribution function (CSDF) curves. From the GPC curves, it appears that chain scission is the dominant reaction at all US irradiation times and intensities. On the contrary, using the CSDF methodology, it appears that chain scission is the dominant reaction up to the intermediate irradiation times and intensities, but chain branching becomes dominant at the US higher times and intensities. On the contrary, using the proposed methodology, it appears that chain scission is the dominant reaction up to the intermediate irradiation times and power intensities, but chain branching becomes dominant at higher times and power intensities.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyethylene is among the most widely used polymers because of its low cost, low weight, low temperature toughness, low density, low moisture absorption, good optical properties, and ease of processing and recycling. In recent years [1,2,3,4,5,6,7,8,9,10,11,12,13], interest in using high-intensity ultrasonic radiation, as an alternative method for the generation of free radicals in polymers, has arisen. Some studies [12,13,14,15,16,17,18,19,20,21,22,23,24,25] have reported on the effect of ultrasonic energy on polymer degradation in which temperature has a significant effect. The time and intensity of incidence of the ultrasonic energy play an important role in the formation and rate of formation of free radicals, which ultimately will be related to the degree of chain degradation and/or cross-linking and therefore will have a direct influence on the performance and properties of the polymer [26].
Knowledge of the effect of different operating parameters of ultrasonic energy, such as time and intensity of irradiation, on the polymer properties is very important in order to recommend the most suitable operating conditions for large-scale operation [14, 17].
The chemical effects on the polymers caused by the ultrasonic irradiation (sonochemistry) are commonly related to the phenomena of acoustic cavitations which are the formation of bubbles with the negative pressure. These bubbles grow to a certain size, become unstable, and collapse [6, 7]. These implosions produced during the bubble’s collapse generate high temperatures (> 5000 K), high pressures (> 20 MPa: > 200 kg/cm2), and high rates of cooling (> 107 K/s) which are large enough to break chemical bonds [4, 23, 26]. Polymer chains sufficiently close to the collapsing bubble will experience very high stresses along the chain that can cause various levels of uncoiling, bond deformation, or scission [2]. Bond scission tends to occur more readily in high molecular weight polymers, as has been reported by Wu et al. [7] and Kaan and Isayev [27], and this scission is more probable near the middle point of the chain [2, 6, 21, 25, 28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57].
Considering the above-mentioned facts, it is evident that if a polymer macromolecule is subjected to ultrasonic irradiation, its degradation is inevitable. Shifting of the GPC curves to lower molecular weights is commonly observed during studies of polymer degradation due to the action of ultrasonic irradiation [7, 11, 21, 39, 40]. However, it is still difficult to discriminate which is the governing mechanism between chain scission or chain branching, or both. In addition, it is difficult to establish the effect of the polymer initial molecular weight on the preferred occurrence of each mechanism.
In order to differentiate these subtle changes due to the different initial molecular weights (when subjecting a polymer molecule to ultrasonic irradiation), David et al. [26] proposed the use of the “chain scission average number” (ChSc), which can quantify the magnitude of the degradation process. This is obtained from the ratio of the “number average molecular weight” of the non-degraded sample (Mn0) to that of the degraded sample (Mnf). This ChSc parameter, however, represents just one point in the GPC curve, does not cover the entire MWD curve.
In this sense, a study by Canevarolo [44], of the degradation during multiple extrusion of PP, proposed a method that estimates the average number of chains that participate in the degradation processes, both, by chain scission and chain branching, as a function of the initial Mw. This is known as the chain scission distribution function (CSDF), and this does considers the entire MWD curve. This methodology has been used by Martini et al. [45] to study the degradation process of PP, in solution, when subjected to high temperature and high pressure. Otaguro et al. [46] studied the effect of gamma radiation on the PP chain scission and branching. Penheiro et al. [47] studied the HDPE degradation during processing in an internal mixer and observed that Phillips-type HDPE produces a higher level of chain branching than the Zieglere Natta’s type at the same processing conditions. Cáceresa et al. [48] studied the thermomechanical degradation of PP with and without thermal and UV stabilizers in a twin-screw extruder using the CSDF methodology, finding a predominant chain scission at low molecular weights and a preferential chain scission at higher molecular weights. When the stabilizers were incorporated the cPP Mw is kept constant, even after four extrusions, independently of the stabilizers concentration used. Its chain scission is greatly reduced, only being noteworthy at high values of molecular weight, presenting in this case a preferential chain scission process. Cosate et al. [49] studied the PLA degradation during the extrusion in a single-screw extruder. During the PLA recycling in a single-screw extruder using a chain extender and applying the CSDF methodology, they found that the chain extender was able to recover the molecular weight although it caused an increase in the polydispersity, showing a change in the chain structure. In all these cases, the form of the CSDF curve is clearly related to the type of degradation process chain scission or chain branching. The interesting thing about this methodology is that it provides information on what type of long or short chains the type of polymer degradation process is carried out.
The goal of his work is to study the effect of high-energy and time ultrasonic radiation on molecular structure and molecular weight of polyethylene in solution, using the methodology proposed by Canevarolo [44], as well as a modification to this methodology, proposed in this study. These results will help to understand the structural changes and degradation processes of polyethylene when subjected to high-energy and time ultrasonic irradiation.
Experimental
Materials
The polyethylene used in this study was: low-density polyethylene (LDPE) of Mw = 194,500, Mn = 16,200, Mw/Mn = 12, and MFI = 25 g/10 min, from Sigma-Aldrich (USA). Xylene and acetone were from JT Baker (USA).
Methods
Ultrasonic irradiation of polyethylene solution
LDPE solutions in xylene were subjected to different times and amplitudes of ultrasonic irradiation, as shown in Table 1. The ultrasonic irradiation was produced using a 12.5-mm-diameter disruptor horn probe at amplitudes of 21, 76, and 146 μm, which correspond to power intensities of 50, 100, and 150 W, respectively. The instrument was a Model 250, from Branson Ultrasonics, Danbury, USA, with a maximum power output of 250 W at 20 kHz. All ultrasonic irradiations were carried out in a Branson reactor (Fig. 1), at 60 °C.
The polymer solutions were prepared by dissolving 1 g of polymer in 60 ml of xylene at about 120 °C and magnetically stirring it until completely dissolved. The solution was cooled down to about 60 °C, at which temperature the polymer still remained in solution. In this reactor, the solutions were sonicated in the presence of air, for different periods of time, at different intensities, as shown in Table 1. After the ultrasonic treatment, the polymer was precipitated with acetone at room temperature and separated by vacuum filtration. The polymer was then washed with acetone ten times to remove the xylene. The product was dried overnight in a vacuum oven at 40 °C.
Characterization
Gel content of samples was measured according to ASTM D2765, putting ca. 0.5 g samples under xylene reflux, at 120 °C for 12 h. Gel content was determined by differences in weight. Films of samples for FTIR were prepared by compression molding at 180 °C, under a pressure of 130 kg/cm2, for 5 min. The carbonyl and C–O index of the different samples were conducted using an FTIR spectrometer (Nicolet Mod 710) with 32 scans. A high-temperature Waters GPC was used to determine the molecular weight distribution (MWD) of all polymer samples, using 1,2,4-trichlorobenzene (TCB), at 140 °C. In this case, each CSDF curve was obtained, using a proprietary Excel software, by comparing the MWD curve at a given time and intensity of irradiation, with the MWD curve of the original untreated LDPE. The chain scission distribution function (CSDF) curves were calculated using an Excel running software, called CSDF4.1, that is, comparing the sample treated for 15 min at 50 W, with the untreated one, and the sample treated for 20 min at 50 W, with the untreated one.
In the other case, each CSDF curve is obtained, using the same proprietary excel software, but comparing now the MWD curve at a given time and intensity of irradiation, with the MWD curve of the sample treated for the previous shorter period of time and the same intensity, that is, comparing the sample treated for 15 min at 50 W with that treated for 10 min at 50 W, and the sample treated for 20 min at 50 W with that treated for 15 min at 50 W.
According to Canevarolo et al. [44,45,46,47], the type of the CSDF curve indicates the type of reaction that occurs in the polymer being subjected to a degradation/modification process. CSDF values above zero (positive values) indicate that chain scission is happening, whereas CSDF values below zero (negative values) indicate that chain branching is happening. CSDF values above zero and constant (horizontal in a CSDF vs Log(Mw) graph) indicate random chain scission, irrespective of molecular weight, whereas CSDF values above zero with positive slope indicate random and preferential chain scission (preferential with respect to molecular weight, i.e., preferential degradation of the longest chains). CSDF values below zero and constant (horizontal in a CSDF vs Log(Mw) graph) indicate random chain branching, irrespective of molecular weight, whereas CSDF values below zero with positive slope indicate random and preferential chain branching (preferential degradation of the longest chains). CSDF curves with a slope ≠ zero (usually positive slope) indicate preferential chain branching (preferential degradation of the longest chains). If both processes (random and preferential chain scission) happen at the same time the curve will start with a constant positive value (slope equal to zero) that increases continuously (slope continuously increasing). The effect of ultrasonic irradiation on the crystallization and fusion behavior of the polyethylene samples was studied using a TA Instruments differential scanning calorimetry (DSC). The samples were first heated to 160 °C, at 5 °C/min, and let there for 3 min to eliminate any thermal history. Thereafter, the samples were cooled down at − 5 °C/min down to 25 °C to obtain the crystallization temperatures Tc and finally heated to 160 °C at 5 °C/min to obtain the fusion temperature.
Results and discussion
Gel content
Gel content increased very little as a result of ultrasonic irradiation. However, this increase showed no variation with either irradiation time or intensity. All ultrasonic irradiation-treated samples presented a gel content of 0.0050 ± 0.0006%. These results are presented in Table 2; however, this represents an increase of ca. 35% over the gel content of the untreated LDPE (0.0037% ± 0.0003). These results indicate that the cross-linking produced in the LDPE by this ultrasonic irradiation treatment is still negligible.
Infrared analysis
Figure 2 shows the FTIR spectra of samples 0, 1, 5, and 9 at increasing ultrasonic irradiation energies, as shown in Table 1. The spectra of irradiated LDPE showed new absorption bands (narrow) at 1250 and 1020 cm−1. The significant increase in these bands indicates the presence of C–O groups. The C–O index, determined from the 1250 cm−1 band, for ultrasonic-treated LDPE sample 9 (time and power intensity of irradiation equal to: 20 min and 150 W), had a value of 0.074, while the LDPE without treatment did not show any peak at this wave number. These C–O groups are evidence of the occurrence of polymer oxidation due to ultrasonic irradiation. In addition, when polyethylene is degraded under ambient air and ultrasonic irradiation, oxidized structures like alcohols, aldehydes, ketones, esters are produced [42, 43], most of them showing the carbonyl group.
FTIR of LDPE samples 0, 1, 5, and 9 of Table 1, which, respectively, correspond to samples treated with irradiation times and intensities of: 0–0; 10–50; 15–100; 20–150
In our case, this carbonyl band around 1720 cm−1 was present in all cases, in the untreated as well as in the ultrasonic-treated samples, maintaining always a very similar weak intensity. That is, the ultrasonic treatment does not show any apparent effect on the carbonyl signal at 1720 cm−1. This indicates that the oxidation mechanism of polyethylene is different. With high-energy ultrasonic irradiation and long exposure times, as in this study, the oxidative degradation process of polyethylene results in the formation of C–O groups only. Nonetheless, these results are different from those reported by Li et al. [15]. They found both C = O and C–O groups when subjecting HDPE to high-power intensity ultrasonic irradiation for 10 min.
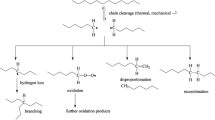
According to these results, we suggest that the macro-radicals produced by ultrasonic irradiation are formed by chain scission. Then, the oxygen reacts with other macro-radicals to form the COOH groups. According to this, ultrasonic degradation of LDPE in solution can be symbolized as shown in Scheme 1. Hydroperoxides formed by the oxygen in the air can be decomposed to produce hydroxyl macro-radical that can attack other chains, forming –C–O–C– bonds and promote chain cross-linking by oxygen linkage. The –*CH–CH2– macro-radical may lead to chains scission and/or chain cross-linking by oxygen linkage (Scheme 2).
Comparing the obtained results in US degradation mechanism with other reported degradation mechanisms of polymers applied high energy, an important difference in the LDPE oxidation can be identified. For instance, in weathering degradation, the UV irradiation can interact with the polymer to induce the formation of macro-radicals along the polymer chain and subsequently introduce oxygen O2 [50]. This same process is observed in thermal [51] and gamma radiation [52] degradation. However, in US degradation, the macro-radical is generally formed during chain scission with the subsequent introduction of oxygen O2 at the end of the chain. On the other hand, the amount of US energy considered in this study varies from low to high energy, which increases the oxidation magnitude to a certain extent. This simple difference in the polymer degradation by US radiation, which induces the formation of macro-radicals during the chain scission, could be considered as a great scientific and technological advantage over the other types of degradation by the application of high energy. The specific knowledge of defining where hydroperoxide groups (COOH) will be formed in the chain, opens up the possibilities to control and/or modify the polymer functionalization. This is because this type of oxidized groups (COOH) is highly reactive according to previous studies of LDPE [53].
The effect of time and power intensity of ultrasonic irradiation on LDPE, in solution, was also determined by changes in molecular weight, molecular weight distribution, and intrinsic viscosity. The results are listed in Tables 3 and 4.
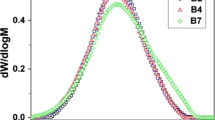
The average molecular weight (Mw) of LDPE decreases with an increase in either the time or the power intensity of ultrasonic irradiation. The GPC curves in Fig. 3 show a bimodal distribution for both, the untreated and the ultrasonic irradiated samples. Coinciding with Tables 3 and 4, the GPC curves in Fig. 3 shift to lower molecular weight as the time and/or power intensity of irradiation increases. Additionally, the intrinsic viscosity showed a significant change with the application of ultrasonic irradiation, decreasing with either power intensity and/or time of ultrasonic treatment, and this effect was also observed by Desai et al. [19, 20]. These changes clearly indicate a decrease in the molecular weight of LDPE due to ultrasonic radiation.
The thing here is that with these curves, it is difficult to establish if the “observed” modifications in molecular weight are due to chain scission or chain branching, or if these two different reactions occur at random or preferentially. Additionally, the GPC curves do not say if the initial polymer molecular weight has an influence on the reaction route or in the extent of the reaction.
In order to elucidate the questions presented in the last paragraph, the molecular weight distribution curves obtained from the GPC studies were used to obtain the “chain scission distribution function” (CSDF).
CSDF curves
In the Canevarolo methodology, each CSDF curve is obtained, using a proprietary Excel software, by comparing the MWD curve at a given time and intensity of irradiation, with the MWD curve of the original untreated LDPE, that is, comparing the sample treated for 15 min at 50 W with the untreated one, and the sample treated for 20 min at 50 W with the untreated one. In this case, the CSDF curves are presented in Figs. 4a, 5a, and 6a, as a function of time and intensity of ultrasonic irradiation.
In the proposed modified methodology, each CSDF curve is obtained, using a proprietary Excel software, but comparing now the MWD curve at a given time and intensity of irradiation, with the MWD curve of the sample treated for the previous shorter period of time and the same intensity, that is, comparing the sample treated for 15 min at 50 W with that treated for 10 min at 50 W, and the sample treated for 20 min at 50 W with that treated for 15 min at 50 W. In this case, the CSDF curves are presented in Figs. 4b, 5b, and 6b, as a function of time and intensity of irradiation.
For an irradiation intensity of 50 W, Fig. 4a shows that, along the three irradiation intervals, between 0 and 10 min, 0 and 15 min, and 0 and 20 min, the CSDF curves indicate that the ultrasonic irradiation produces mostly random chain scission, independently of the Mw. Figure 4b, on the other hand, shows that, along the two irradiation intervals, between 0 and 10 min, and 10 and 15 min, the CSDF curves indicate that the ultrasonic irradiation produces mostly random chain scission, independently of the Mw, whereas the third irradiation interval, between 15 and 20 min, indicates chain scission as well as chain branching.
For an irradiation intensity of 100 W, Fig. 5a shows that, along the three irradiation intervals, between 0 and 10 min, 0 and 15 min, and 0 and 20 min, the CSDF curves indicate that the ultrasonic irradiation produces mostly random chain scission, independently of the Mw. Figure 5b, on the other hand, shows that, along the first irradiation interval, between 0 and 10 min, the CSDF curve indicates that the ultrasonic irradiation produces mostly random chain scission, independently of the Mw, whereas the second irradiation interval, between 10 and 15 min, indicates chain scission in the low Mw part, as well as chain branching in the high Mw part, but finally, the third irradiation interval, between 15 and 20 min, produces apparently, only chain branching.
For an irradiation intensity of 150 W, Fig. 6a shows that, along the three irradiation intervals, between 0 and 10 min, 0 and 15 min, and 0 and 20 min, the CSDF curves indicate that the ultrasonic irradiation produces mostly random chain scission, independently of the Mw. Figure 6b, on the other hand, shows that, along the first irradiation interval, between 0 and 10 min, the CSDF curve indicates that the ultrasonic irradiation produces mostly random chain scission, independently of the Mw, whereas along the following two irradiation intervals, between 10 and 15 min and between 15 and 20 min, the CSDF curves indicate mostly chain branching, along the molecular weights being examined.
The occurrence of scattered data at very low and very high molecular weights (below 2000 and above 500,000 in our case) should be taken with care because the low concentration of chains in these regions greatly increases the uncertainty of the calculus. The discussion that follows, therefore, considers only the molecular weight data between 2000 and 500,000.
That is, if the complete interval is considered as a whole (the Canevarolo methodology), the CSDF curves tend to show the “average” or the “most dominant reactions”, as the ones that occur during the induced polymer modification/degradation. If this interval is sectioned (the proposed methodology), the CSDF curves will tend to show, again, the “average” or the “most dominant reactions”, but, of that section, as the ones that occur during the induced polymer modification/degradation. And the differences clearly stand out. According to the first methodology, all combinations of time and intensity of ultrasonic irradiation (Figs. 4a, 5a, 6a) indicate random chain scission reactions. But, according to the proposed modified methodology (Figs. 4b, 5b, and 6b), it seems that as the time and/or intensity of ultrasonic irradiation increases, the chain scission reactions give way to the chain branching reactions.
It appears then that, in general, at low ultrasonic irradiation intensities and/or times, polymer chain scission is the dominant reaction, but as intensity and/or time of irradiation increases, polymer chain branching tends to occur. These results show that through the use of ultrasonic irradiation, it is possible to mechanically activate the polymer chains, in order to produce chain alterations. Also, these results show that, depending on the time and intensity of irradiation, these chain alterations can be either chain scission or chain branching. This knowledge is very useful in the sense that, by selecting the intensity and/or time of ultrasonic irradiation, it is possible to produce certain desired alterations in the polymer chain.
Además comparando los resultados de los espectros FTIR de la Fig. 2 y los mecanismos de degradación propuestos en los esquemas 1 y 2 con las curvas de CSDF obtenidas, se puede destacar que con esta última técnica de análisis del peso molecular proporciona una perspectiva más clara de la degradación del LDPE a baja y alta energía ultrasonica, sobre todo proporciona el conocimiento de en qué tipo de cadenas (bajo, mediano o alto peso molecular) se está llevando a cabo la reacción de sciccion de cadenas y por lo tanto la oxidación del polímero con grupos hidroperóxidos (COOH) los cuales son altamente reactivos [53]. Este precedente abre la posibilidad de controlar con radiación US que tipo de cadenas se desean fracturar, oxidar y/o funcionalizar, con lo cual se pueden generar diferentes tipos de LDPE con MWD modificadas y estructuras ramificadas, así como la funcionalización de tipos de cadenas.
13C RMN spectroscopy
Figure 7 shows the 13C NMR spectra of untreated as well as ultrasonic irradiation-treated LDPE at increasing ultrasonic irradiation energies, as shown in Table 1. New bands around 29.9 ppm, indicating the presence of (CH2)n, can be observed, which in time suggest the appearance of additional chain branches in the ultrasonic-treated LDPE [54,55,56,57]. A slight increase in the intensity of band at 33.8 ppm (which would correspond to CH; br), with respect to that at 29.9 ppm, corresponds to α of the treated LDPE. The branching scheme below represents localization of the C atoms in the branching of the LDPE structure [54, 55].

Figure 2 shows that, as time and power intensity of ultrasonic irradiation increased, the magnitude of the band at 780 cm−1 of the FTIR spectra also increased. This can be related to ethylene branches, as reported by Blitz and McFaddin [58] These FTIR results, in conjunction with those of the 13C NMR, strongly indicate an increase in the number of branches in the LDPE molecule, as a result of the ultrasonic treatment.
Differential scattering calorimetry
Crystallization and melting results are presented in Table 5. The crystallization temperature (Tc) increased with the ultrasonic irradiation, from 98.4 for the untreated LDPE to 100.4 ± 0.6 °C for all the treated LDPE samples, irrespective of the time and intensity of irradiation. That is, the effect of ultrasonic irradiation on Tc is very little.
The melting temperature (Tm), as well, increased with the ultrasonic irradiation, from 111.3 for the untreated LDPE to 111.9 ± 0.3 °C for all the treated LDPE samples, also, irrespective of the time and intensity of irradiation. That is, the effect of ultrasonic irradiation on Tm is negligible.
The heat of fusion and the corresponding crystallinity percent, on the other hand, do change with an increase in time and intensity of irradiation. First, they decrease to the lowest values at 10 min and 50 W of ultrasonic irradiation, but then, they increase, though a little, with an increase in time and intensity of ultrasonic irradiation, up to 120.6 (J/g) and 41.4 (%), respectively, at 20 min and 150 W.
Flexural modulus
Table 6 shows the flexural modulus results. It is noticeable a continuous reduction in modulus for all the ultrasonic-treated samples, indicating an eminent LDPE degradation by the ultrasonic radiation. This has relation with the reduction in Mw and viscosity results discussed before in Tables 3 and 4. At a power intensity of 100 and 150 w and 20 min of irradiation, the reduction in modulus is less drastic, which can be related to the prevalence of branching (longer and medium Mw chains) over chain scission reactions (higher Mw chains) that are occurring at these conditions. This is in accordance with the methodology proposed as discussed in Figs. 5b and 6b in which these branching reactions prevent the Mw to be reduced. The chain scission reactions (lower Mw chains) observed in the treated samples at lower irradiation times and power intensities (Figs. 4, 5, and 6) promoted a significant reduction, near 12%, on modulus. This indicates that the stiffness of LDPE strongly depends on the longer Mw chains. As was observed in Fig. 2, the LDPE oxidation was very low and had no significant effect in flexural modulus as is observed in Table 6 (samples 1, 5, and 9). The observed chain branching at high US energy and sonication times is directly proportional to the decrease in the chain ends which results in a less drastic reduction in flexural modulus, which can be seen in the results presented in Table 6 (samples 6 and 9). These results show that the LDPE mechanical properties can also be modified and controlled based on the US intensity and sonication time. This type of ultrasonic treatment can be an alternative for the control and modification of polyethylene properties.
Conclusion
Gel content increased very little as a result of ultrasonic irradiation. However, this increase showed no variation with either time or intensity of irradiation. All ultrasonic irradiation-treated samples presented a gel content of 0.0050 ± 0.0006%. These results indicate that the cross-linking produced in the LDPE by this ultrasonic irradiation treatment is still negligible. The IR spectra of irradiated LDPE showed new absorption bands (narrow) at 1250, 1020, and 780 cm−1. The significant increase in these bands indicates the presence of C–O groups. We concluded that the radicals produced by ultrasonic irradiation are formed by scission at or near the middle of the chain. GPC showed that the average molecular weight (Mw) of LDPE decreases with an increase in either the time or the intensity of ultrasonic irradiation. But these curves do not say if the “observed” modifications in molecular weight are due to chain scission or chain branching, or if these two occur at random or preferentially. According to chain scission distribution function (CSDF) curves obtained via the proposed modified methodology, at low ultrasonic irradiation times and/or intensities, polymer chain scission is the dominant reaction, but as time and/or intensity of irradiation increase, polymer chain branching tends to occur. In addition, the amplitude of each interval or “section” of the whole degradation/modification process can be taken as smaller as desired. These results show that through the use of ultrasonic irradiation (sonochemistry), it is possible to mechanically activate macromolecular structures like polymer chains, in order to produce desired chemical modifications in different types of chains of higher or lower molecular weight. The structural changes induced on the LDPE by the ultrasonic irradiation have no significant effect on Tc, Tm, and crystallinity, but these changes promoted a reduction in flexural modulus. On the other hand, it also provides the possibility of not only fracturing certain chain sizes, but of activating those chains by the selective grafting of COOH groups in the PE modification and functionalization in the new chain ends created by the action of US radiation. This also would allow to control and modify the PE mechanical properties.
References
Price GJ, West PJ (1996) Ultrasonic production of block copolymers as in situ compatibilizers for polymer mixtures. Polymer 37:3975–3978
Chen G, Guo S, Li H (2002) Ultrasonic improvement of the compatibility and rheological behavior of high-density polyethylene/polystyrene blends. J Appl Polym Sci 86:23–32
McKenzie TG, Karimi F, Ashokkumar M, Qiao GG (2019) Ultrasound and sonochemistry for radical polymerization: sound synthesis. Chem Eur J 25:5372–5388
Chen G, Guo S, Li Y (2004) Dynamic rheological properties of high-density polyethylene/polystyrene blends extruded in the presence of ultrasonic oscillations. J Appl Polym Sci 92:3153–3158
Chen Y, Li H (2004) Effect of ultrasound on extrusion of PP/EPDM blends: structure and mechanical properties. Polym Eng Sci 44:1509–1513
Feng W, Isayev AI (2004) In-situ ultrasonic compatibilization of unvulcanized and dynamically vulcanized PP/EPDM blends. Polym Eng Sci 44:2019–2028
Wu H, Guo S, Li Z (2005) Molecular structure development of metallocene-catalyzed linear low density polyethylene under ultrasonic irradiation. J Polym Sci Part B Polym Phys 43:2121–2129
Li Y, Chen G, Guo S, Li H (2006) Studies on rheological behavior and structure development of high-density polyethylene in the presence of ultrasonic oscillations during extrusion. J Macromol Sci Part B Phys 45:39–52
Poddar MK, Arjmand M, Sundararaj U, Moholkar VS (2018) Ultrasound-assisted synthesis and characterization of magnetite nanoparticles and poly (methyl methacrylate)/magnetite nanocomposites. Ultrason Sonochem 43:38–51
Azarpour A, Zendehboudi S, Yusup S, Khalid A, Zhang Y (2019) Effects of ultrasonic cavitation on neutralization process of low molecular weight polyethylene glycol. Can J Chem Eng 97:395–405
Chen Y, Li H (2005) Effect of ultrasound on the morphology and properties of polypropylene/inorganic filler composites. J Appl Polym Sci 97:1553–1560
Kumar RV, Koltypin Y, Palchik O, Gedanken A (2002) Preparation and characterization of nickel–polystyrene nanocomposite by ultrasound irradiation. J Appl Polym Sci 86:60–165
Gedanken A (2004) Using sonochemistry for the fabrication of nanomaterials. Ultrason Sonochem 11:47–55
Vijayalakshmi SP, Madras G (2005) Effect of initial molecular weight and solvents on the ultrasonic degradation of poly (ethylene oxide). Polym Degrad Stab 90:116–122
Li Y, Li J, Guo S, Li H (2005) Mechanochemical degradation kinetics of high-density polyethylene melt and its mechanism in the presence of ultrasonic irradiation. Ultrason Sonochem 12:183–189
Price GJ, Garland L, Comina J, Davis M, Snell DJ, West PJ (2004) Investigation of radical intermediates in polymer sonochemistry. Res Chem Intermed 30:807–827. https://doi.org/10.1163/1568567041856972
Peng B, Wu H, Guo S, Lai SY, Jow J (2007) Static ultrasonic oscillations induced degradation and its effect on the linear rheological behavior of novel propylene based plastomer melts. Polym Degrad Stab 92:1632–1639
Mehrdad A, Rostami MR (2007) Effect of temperature and solution concentration on the ultrasonic degradation of the aqueous solutions of polyethylene oxide. Iran Polym J 16:795–801
Desai V, Shenoy MA, Gogate PR (2008) Ultrasonic degradation of low-density polyethylene. Chem Eng Process 47:1451–1455
Desai V, Shenoy MA, Gogate PR (2008) Degradation of polypropylene using ultrasound-induced acoustic cavitation. Chem Eng J 140:483–487
Zachary SK, Stephen LC (2012) Mechanochemical remodeling of synthetic polymers. Polymer 53:1035–1048
Peng P, Hong W, Wenting B, Shaoyun G, Yong C, Hua H, Shih-Yaw L, Jinder J (2012) Ultrasound initiated maleic anhydride grafted onto a novel polypropylene copolymer. Polym Eng Sci 52:518–524
Chen D, He Z, Weavers LK, Chin Y-P, Walker HW, Hatcher PG (2004) Sonochemical reactions of dissolved organic matter. Res Chem Intermed 30:735–753
Nasir BRB, Rajender SV (2012) Alternative energy input: mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem Soc Rev 41:1559–1584
Li J, Liang M, Guo S, Lin Y (2004) Studies on chain scission and extension of polyamide 6 melt in the presence of ultrasonic irradiation. Polym Degrad Stab 86:323–329
David C, Trojan M, Daro A (1992) Photodegradation of polyethylene: comparison of various photoinitiators in natural weathering conditions. Polym Degrad Stab 37:233–245
Kaan G, Isayev AI (2011) In situ compatibilization of PEN/LCP blends by ultrasonic extrusion. J Appl Polym Sci 122:354–365
Jun Q, Zhang H, Yongshen X (2010) Grafting of maleic anhydride onto polyethylene wax by melt ultrasound and solid co-irradiation. Plasma Sci Plasma Technol 165:834–844
Ali A, Catalgil GH, Ahmet G (2009) Effect of solvent characteristics on the ultrasonic degradation of poly (vinylpyrrolidone) studied by on-line monitoring. Macromol Chem Phys 210:1331–1338
Taghizadeh T, Rad H, Abdollahi R (2012) A kinetic study of ultrasonic degradation of carboxymethyl cellulose. J Appl Polym Sci 123:1896–1904
Qing SZ, Yuan YP, Su XL, Qing W, Xiao W, Shan Ch (2011) Ultrasonic degradation of dextran in aqueous solution. Adv Mater Res 396–398:1624–1627
Mohod AV, Gogate PR (2011) Ultrasonic degradation of polymers: effect of operating parameters and intensification using additives for carboxymethyl cellulose (CMC) and polyvinyl alcohol (PVA). Ultrason Sonochem 18:727–734
Goodwin DJ, Picou DR, Ross-Murphy SB, Holland SJ, Martini LG, Lawrence MJ (2011) Ultrasonic degradation for molecular weight reduction of pharmaceutical cellulose ethers. Carbohydr Polym 82:843–851
Sathiskumar PS, Madras G (2012) Ultrasonic degradation of butadiene, styrene and their copolymers. Ultrason Sonochem 19:503–508
Rooze J, Groote R, Jakobs RTM, Sijbesma RP, Van-Iersel MM, Rebrov EV, Schouten JC, Keurentjes JTF (2011) Mechanism of ultrasound scission of a silver–carbene coordination polymer. Polym J Phys Chem B 115:11038–11043
Mehrdad A (2011) Ultrasonic degradation of polyvinyl pyrrolidone in mixed water/acetone. J Appl Polym Sci 120:3701–3708
Shinobu K, Kimihiko T, Kazunori F (2011) Effects of frequency and a radical scavenger on ultrasonic degradation of water-soluble polymers. Polymer 18:276–281
Kumar VK, Madras G (2010) Ultrasonic degradation of poly (methyl methacrylate-co-alkyl acrylate) copolymers. Ultrason Sonochem 17:403–408
Tran KVB, Koda S (2010) Frequency dependence of acoustic degradation of polymer in solution. In Proceedings of symposium on ultrasonic electronics, vol 31, pp 433--434
Madras VRG (2011) Kinetics of sono-photooxidative degradation of poly (alkyl methacrylate). Ultrason Sonochem 18:608–616
Price GJ, Smith PF (1993) Ultrasonic degradation of polymer solutions—III. The effect of changing solvent and solution concentration. Eur Polym J 29:419–424
Price GJ, Clifton AA, Keen F (1996) Ultrasonically enhanced persulfate oxidation of polyethylene surfaces. Polymer 37:5825–5829
Price GJ, Keen F, Clifton AA (1996) Sonochemically-assisted modification of polyethylene surfaces. Macromolecules 29:5664–5670
Canevarolo SV (2000) Chain scission distribution function for polypropylene degradation during multiple extrusions. Polym Degrad Stab 70:71–76
Martini ER, Brignole AE, Barbosa ES (2007) Mechanical degradation of polypropylene solutions under large pressure drops. J Polym Sci Part B Polym Phys 45:455–465
Otaguro H, De Lima LFCP, Parra FD, Lugao BA, Chinelatto AM, Canevarolo VS (2010) High-energy radiation forming chain scission and branching in polypropylene. Radiat Phys Chem 79:318–324
Pinheiro AL, Chinelatto AM, Canevarolo VS (2000) Evaluation of Philips and Ziegler–Natta high-density polyethylene degradation during processing in an internal mixer using the chain scission and branching distribution function analysis. Polym Degrad Stab 91:2324–2332
Cáceresa CA, Zborowskia L, Canevarolo SV (2011) Thermo-mechanical degradation and VOC emission of unstabilized and stabilized polypropylene copolymer during multiple extrusions. Mater Res 14:569–575
Cosate MF, Fonseca G, Morales AR, Innocentini LH (2018) Mechanical recycling simulation of polylactide using a chain extender. Adv Polym Technol 37:2053–2060
Rabek JR (1995) Polymer photodegradation mechanisms and experimental methods. Spring, Berlin, pp 73–83
Gardette M, Perthue A, Gardette JL, Janecska T, Földes E, Pukánszky B, Theriasa S (2013) Photo- and thermal oxidation of polyethylene: comparison of mechanisms and influence of unsaturation content. Polym Degrad Stab 98:2383–2390
Sheika S, Chandrashekar KR, Swaroopb K, Somashekarappa HM (2015) Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int Biodeterior Biodegrad 105:21–29
Martinez JG, Benavides R, Guerrero C, Reyes BE (2004) UV sensitisation of polyethylenes for grafting of maleic anhydride. Polym Degrad Stab 86:129–134
Cheng NH (1986) Determination of polyethylene branching through computerized 13C NMR analysis. Polym Bull 16:445–452
Zhu F, Fang Y, Chen H, Lin S (2000) Synthesis of characterization of branched polyethylene by ethylene homopolymerization with monotitanocene and modified methylaluminoxane catalysts. Macromolecules 33:5006–5010
Cudby AEM, Bunn A (1976) Determination of cauin branching in the low density polyethylene by 13C nuclear magnetic resonance and infra-red spectroscopy. Polymer 17:345–347
Bovey AF, Schilling CF, McCracking LF, Wagner LH (1976) Shirt chain and long chain branching in low density polyethylene. Macromolecules 9:76–80
Blitz PJ, McFaddin CD (1994) The characterization of short chain branching in polyethylene using fourier transform infrared spectroscopy. J Appl Polym Sci 51:13–20
Acknowledgements
The authors gratefully acknowledge the financial support of CONACyT through projects Consolidación del Laboratorio Nacional de Materiales Grafenicos 299124 The authors also wish to thank Blanca Huerta, Guadalupe Mendez, Myriam Lozano, Jose Lopez, Ma Concepción González, Francisco Zendejo, Mario Palacios, Rodrigo Cedillo, Jesus Rodríguez, Sergio Zertuche, Adán Herrera, Luis Enrique Reyes, Alejandro Espinosa, Seyma de Leon, Josue Campos, Efraín Alvidrez, Marcelo Ulloa, Rosario Rangel, Anabel Ochoa and Daniel Alvarado, Guadalupe Tellez, Jorge Espinosa for their technical and informatics support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Martinez-Colunga, J.G., Sanchez-Valdes, S., Ramos-deValle, L.F. et al. Effect of ultrasonic irradiation on low-density polyethylene molecular structure. Polym. Bull. 77, 5303–5321 (2020). https://doi.org/10.1007/s00289-019-03006-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-03006-4