Abstract
N-(4-iodo-1,3-diphenylbutyl) acrylamide (NIAM) is a potential hydrophobic monomer for preparation of hydrophobically modified polyacrylamide largely used for enhanced oil recovery. Moreover, it has an iodine group as substitute allowing further modification reactions. This monomer was synthesized via Ritter reaction as described in the literature and characterized by nuclear magnetic resonance (NMR), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). DSC analysis of monomer showed two endothermic peaks referring to the melting points of a mixture of diastereoisomers. Conventional radical homopolymerization of NIAM was investigated, and the polymer was characterized by NMR, TGA, DSC and size-exclusion chromatography. The polymerization was successfully performed only in the presence of large amounts of radical initiator 2,2′-azobis(2-methylpropionitrile) (AIBN) suggesting an iodine transfer polymerization mechanism. The polymer showed low degree of polymerization, dispersity lower than 1.1 and glass transition temperature of 19.5 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyacrylamide is a water-soluble polymer widely used in different applications, such as enhanced oil recovery (EOR), sewage treatment and paper industry [1]. Hydrolyzed hydrophobically modified polyacrylamide (HHMPAM) has overcome the deficiencies of their unmodified or partially hydrolyzed analogs generally used in EOR. They are less sensitive to mechanical degradation, to the presence of electrolytes and high temperature (conditions normally encountered in oil wells) and form hydrophobic aggregates in aqueous solution from a certain polymer concentration [2,3,4,5]. The formation of these aggregates confers an increase in the hydrodynamic volume of the polymer in aqueous solution, providing an increase in mechanical resistance and, consequently, an increase in viscosity, also creating a higher salt tolerance and better shear strength [6].
In most studies found in the literature, the hydrophobic groups in HHMPAM are present in small amounts, so that the solubility coefficient in water does not change [2, 6]. The inclusion of hydrophobic groups in the polymer also improves rheological properties. For example, in EOR, the main effect observed for these polymers is to increase the viscosity of the aqueous phase and consequently improve the sweep efficiency with high molar mass polymers [7].
Few studies have been carried out with respect to polyacrylamides containing aromatic hydrophobic groups as pendant substituents. Branham et al. investigated the effects of the polymer microstructure on the associative behavior of amphiphilic terpolymers of acrylamide, acrylic acid and N-(4-decylphenyl) acrylamide. It observed an increase in the solution viscosity with an increase in the proportion of hydrophobic monomers in the copolymer [8]. Abu-Sharkh et al. showed that copolymers of acrylamide and N-phenylacrylamide prepared from micellar copolymerization technique have technological potential. The hydrophobic moieties of these copolymers promoted intermolecular hydrophobic associations and formation of polymolecular micelles, which exhibited high viscosity. In addition, a relatively high salt tolerance, typical of nonionic polymers, was also exhibited by the copolymers [9]. Other aromatic hydrophobic polyacrylamides employing N-(4-butylphenyl) acrylamide and N-(4-ethylphenyl) acrylamide as comonomers were also studied. These monomers have the advantage of being active in the ultraviolet region, facilitating the determination of the hydrophobic content even at low concentrations [10].
More recently, Abdollahi and Khakpour [11] investigated the heterogeneous and micellar radical copolymerization of acrylamide in the presence of styrene, a vinyl monomer containing an aromatic ring. In the heterogeneous copolymerization method, styrene was incorporated randomly into the copolymer chains, while in micellar copolymerization method, a multiblock distribution of styrene in the copolymer chain was observed. A thickening behavior was observed by increasing copolymer and NaCl concentration, although lower than HHMPAM having other hydrophobic groups as pendant substituents [12].
In 2012 Huang et al. [13] described the synthesis of a new series of N-(4-iodo-1,3-diarylbutyl)acetamide compounds through the Ritter reaction. This methodology presents high efficiency and simplicity to produce secondary amides promoted by the reaction of nitriles with alkenes or alcohols in the presence of acids [14].
N-(4-iodo-1,3-diphenylbutyl) acrylamide compound (Scheme 1) was synthesized by the iodine-mediated and p-toluenesulfonic acid-catalyzed head-to-tail styrene dimerization in the presence of acrylonitrile [13]. The use of this aromatic and hydrophobic acrylamide monomer for the preparation of HHMPAM is very interesting because it contains a potentially polymerizable double bond by radical initiators, besides the fact this monomer presents aromatic pendants groups, which can strongly interact by π–π stacking [15]. Moreover, this compound contains iodine in its molecular structure, which is highly attractive for the synthesis of various organic substances because of the ability of the iodine atom to be a good leaving group, allowing the preparation of several macromonomers through nucleophilic substitution [16].
Besides that, molecules containing iodine are considered efficient transfer agents in controlled radical polymerization [17]. Controlled radical polymerization differs from the conventional process by the lifetime of the propagating radicals caused by the occurrence of bimolecular termination (coupling and/or disproportionation). The controlled radical polymerizations have been achieved by minimizing normal bimolecular termination and extending the lifetime of the radical species by introducing dormant states for the propagating species [18].
The progress in the development of radical polymerization allows the preparation of well-defined polymers with controlled molecular weight, dispersity, composition, chain architecture and site-specific functionality. This process enabled the construction of highly specific materials [19]. Among these developed processes, it is worth noting that iodine transfer polymerization (ITP) as a technique enables polymerization to proceed under air without catalyst and has been accepted as an universal industrial technique of copolymers synthesis [20].
So far, there is no description in the literature about the use of N-(4-iodo-1,3-diphenylbutyl) acrylamide as monomer or comonomer for the synthesis of new materials. Based on that the present study investigated the synthesis and characterization of the monomer and polymers derived from N-(4-iodo-1,3-diphenylbutyl) acrylamide, a hydrophobic aromatic acrylamide containing an iodine functional group. The main aim for this monomer is its use for the preparation of hydrophobically modified polyacrylamide for EOR applications.
Materials and methods
Materials
Iodine molecular (99%), acrylonitrile (99%), 2,2′-azobis(2-methylpropionitrile), benzoyl peroxide, deuterium oxide (D2O) and deuterated chloroform (CDCl3) were purchased from Sigma-Aldrich Chemical Co and used as received. p-toluenesulfonic acid (from Sigma-Aldrich Chemical Co) was purified by recrystallization from ethyl ether. All other reagents and nitrogen gas were purchased from local suppliers and used without any further purification.
Monomer synthesis
N-(4-iodo-1,3-diphenylbutyl)acrylamide (NIAM) was synthesized according to the method described in the literature [13] as shown in Scheme 1.
Briefly, deionized water (2 mL), p-toluenesulfonic acid (19.2 mmol) and molecular iodine (192 mmol) were dissolved in acrylonitrile (150 mL) at 0 °C for 5 min. Styrene (192 mmol) is slowly added to the mixture under magnetic stirring until it reaches room temperature. After 24 h, saturated sodium thiosulfate solution is added. The products are extracted with ethyl acetate and purified by silica gel column chromatography to obtain NIAM.
Homopolymer synthesis
The synthesis of NIAM homopolymer was evaluated under various reaction conditions, as shown in Table 1 and Scheme 2.
The general procedure is described below:
In a 25-mL round-bottom flask, the dried solvent and NIAM were added under nitrogen atmosphere. After heating to the desired temperature, the initiator was transferred to the previous solution, and the reaction was conducted for 24 h using a magnetic stirrer. When the time was reached, the solvent was removed under reduced pressure and the product was characterized by spectroscopic and thermal analyses.
Polymer characterization
Nuclear magnetic resonance spectroscopy (NMR)
The chemical structure of the samples was determined by 1H and/or 13C NMR spectroscopy in deuterated solvent (D2O or CDCl3). All spectra were recorded on a Bruker 400 MHz spectrometer. 1H spectra were obtained at 400 MHz, while 13C at 100 MHz.
Thermogravimetric analysis (TGA)
The thermal stability of monomer and homopolymer was evaluated using a thermogravimetric analyzer TGA Q50 (TA Instruments). TGA scans were carried out at 20 °C min−1 under nitrogen atmosphere (50 mL min−1), from room temperature to 800 °C.
Differential scanning calorimetry (DSC)
The thermal properties of monomer and homopolymer were evaluated using a differential scanning calorimetry analyzer DSC Q2000 (TA Instruments). In order to erase the thermal history, the sample of monomer was heated from room temperature to 128 °C and homopolymer was heated from room temperature to 200 °C, both at a rate of 20 °C min−1. After cooling, the DSC analyses were performed at 20 °C min−1 under nitrogen atmosphere (50 mL min−1), from − 30 to 200 °C.
Size-exclusion chromatography (SEC)
Filtered tetrahydrofuran (HPLC grade) was used as an eluent with a flow rate of 1.0 mL min−1. Samples (1 mg mL−1) were dissolved in tetrahydrofuran and filtered prior to injection using 0.45 μm Teflon filters. The analyses were performed on Viscotek chromatograph with GPCmax module (VE2001) equipped with detector TDA402 and Shodex columns (806M, 805L, 804L and 803L). The molar mass was determined using a calibration curve with polystyrene standards.
Ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry (UHPLC/QTOF-MS)
High-resolution mass spectra were obtained with a QTOF Micro-instrument (Impact II, Bruker) in electrospray ionization positive (ES+) mode. A QTOF system was used to separate the analytes of interest. Shim-pack XR-ODS III column (50 mm × 2 mm × 1.6 µm) was used to separate the analytes in isocratic mode with the mobile phase 40% acetonitrile (0.1% formic acid)/60% water (0.1% formic acid) (LC, Nexera × 2, Shimadzu, Tokyo, Japan). The flow rate was 0.4 mL min−1 and column temperature was 35 °C. The optimal MS parameters were as following: capillary voltage 4500 V, source temperature 200 °C, end plate offset voltage 500 V, mass range (m/z) of 60–800 and calibration with sodium formate.
Results and discussion
Synthesis of N-(4-iodo-1,3-diphenylbutyl)acrylamide
The synthesis of NIAM was performed according to the literature [13]. This molecule has interesting characteristics, as it has a polymerizable acrylamide group and an iodine substituent, which is an excellent leaving group, favoring subsequent nucleophilic substitution reactions. NIAM was obtained with low yield (9%), and its structure was confirmed by NMR spectroscopy.
In the 1H NMR spectrum (Fig. 1), multiplets are seen between 7.40 and 7.15 ppm related to the aromatic hydrogens. The double singlet at 5.92 ppm is attributed to the amide hydrogen of the diastereoisomers, at position C-4. The vinylic hydrogens are observed at 6.25 and 5.64 ppm as two double doublets (J = 1.4 Hz) (C-1) and as a double doublet (J = 10.3 Hz) at 6.05 ppm derived from the hydrogen at position C-2. The remaining signals in the spectrum are referred as a multiplet at 5.00 ppm, to hydrogen at asymmetric carbon C-5; a doublet (J = 7.2 Hz) of 3.41 ppm, corresponding to the hydrogen in the C-8 halogenated carbon; a multiplet at 2.98 ppm, to hydrogen at asymmetric carbon C-7; and two multiplets at 2.47 and 2.18 ppm, relative to the diastereotopic hydrogens at C-6.
In the 13C NMR spectrum (Fig. 2), a signal is observed at 164.8 ppm, referring to carbonyl carbon (C-3). In the region between 143 and 126 ppm, the signals relative to the aromatic carbons and to the vinyl carbons (C-1 and C-2) are observed. At higher field region, a signal is observed at 51.5 ppm, referring to the secondary carbon C-5 bonded to the amide nitrogen and at 45.4 ppm the signal for asymmetric carbon C-7. The signal for the secondary carbon C-6 appears at 42.5 ppm and the signal referring to the primary carbon C-8 at 13.1 ppm, connected to the iodine atom.
Heteronuclear single quantum coherence experiment was used to determine proton–carbon single-bond correlations with the objective of confirming the structure and the assignment of the signals between the carbon and hydrogen spectra (Fig 9, Supplementary material).
DSC thermogram of NIAM is shown in Fig. 3a. Two endothermic transitions between 100 and 150 °C were found, which are associated with the fusion of the diastereoisomers [13]. The monomer also exhibited an exothermic peak at 74.6 °C corresponding to the cold crystallization of NIAM.
The thermal stability of NIAM under nitrogen atmosphere was investigated by TGA (Fig. 3b). A mass loss (~ 3.7%) is observed below 150 °C, typical of the presence of volatile compounds. Thermal decomposition of NIAM takes place in multi-steps (92.1%), and the onset of the thermal decomposition of NIAM occurs at 168 °C.
Radical polymerization of N-(4-iodo-1,3-diphenylbutyl)acrylamide
Thermal radical polymerization reactions of NIAM were investigated at different conditions as shown in Table 1. For the AIBN/NIAM molar ratios of 1:100 and 1:1 (Entries 1 and 2—Table 1) using AIBN as initiator at 90 °C in acetone (closed reactor), no polymer was formed. For Entries 5 and 6 performed in THF, the polymerization was not verified, even at different concentrations. The same result was obtained when benzoyl peroxide was used as initiator (Entry 4). The difficulty of the homopolymerization of NIAM should be related to the presence of the C–I bond in the monomer, which has a low dissociation energy. It is well-known that compounds containing iodine as a substituent can act as inhibitors of free radicals and have been employed in controlled radical polymerization as ITP because they are potential intermediates for macromolecule synthesis [17].
In order to confirm this hypothesis, the reaction products of the Entry 6—Table 1 were analyzed by UHPLC–QTOF-MS and 1H NMR without prior purification. The UHPLC–QTOF-MS technique allows the structural elucidation of different organic compounds. The m/z range established for data acquisition for mass spectrometer was 50 to 1000 Da. Figure 4 shows the chromatogram of the reaction medium, and the compounds associated with the peaks 1–5 (Table 2) could be identified according to the products formed by the reaction of the AIBN free radical and NIAM shown in Fig. 5. The structure was confirmed by comparison with theoretical isotopic profile.
UHPLC–QTOF-MS chromatogram of the reaction from Entry 6—Table 1
The abstraction of iodine by the free radical results in the formation of N-(1,3-diphenylbutyl) acrylamide (2), which can further react with other free radical species in the vinyl group forming 4-cyano-4-methyl-N-(1,3-diphenylbutyl) pentanamide (4) or cyclize originating 4-dihydro-2,4-diphenyl-2H-pyrrole (1). The reaction product of the direct addition of the radical species to the double bond resulting in 4-cyano-N-(4-iodo-1,3-diphenylbutyl)-4-methylpentanamide (5) could also be identified.
The results of the UHPLC–QTOF-MS analysis indicated that the AIBN free radicals can react with the vinyl groups and act on the abstraction of the iodine atom from the NIAM preventing the formation of the polymer. However, a lower amount of NIAM homopolymer was formed in these conditions as confirmed by the 1H NMR spectroscopy and discussed later.
Therefore, a new experiment in the presence of excess of the AIBN initiator to NIAM monomer (4.5:1—Entry 3) was performed resulting in the formation of a homopolymer with conversion higher than 90%. Figure 6 shows the 1H NMR spectrum of the NIAM homopolymer. The signal of the hydrogen linked to the asymmetric carbon vicinal to the amide group is broadened and displaced to high field at 4.76 ppm (H-5), as well as the hydrogen signal of the carbon attached to iodine, shifted to 3.37 ppm (H-8). In the region of 7.55 to 6.75 ppm, the signal of the aromatic hydrogens is observed. Signals associated with the other aliphatic hydrogens of the polymer chain are observed between 3.15 and 0.75 ppm. Through the 1H NMR spectrum of reaction medium, it was possible to estimate a conversion of 92.6%.
1H NMR spectrum of NIAM homopolymer—Entry 3—Table 1 (CDCl3, 400 MHz)
The SEC chromatogram and WF/dLog M vs. log molecular weight of the homopolymer are shown in Fig. 7a, b, respectively. The average molar weight obtained was 2800 g mol−1 and dispersity of 1.1. The narrow molar mass distribution may be due to a degenerative chain transfer process because of the presence of weak C–I bonding. The relative decrease in molar mass depends on the magnitude of the chain transfer rate constant. When the transfer rate constant is much higher than that of propagation, the result is the formation of extremely low molar mass polymer (with degree of polymerization ≈ 1–5) [18]. In this experiment, the degree of polymerization with an average value of 7 was verified.
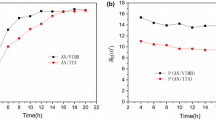
Figure 8a shows the DSC curve (second heating) for the NIAM homopolymer, and a second-order transition is observed at 19.5 °C corresponding to the glass transition of the homopolymer. No endothermic signal was observed indicating the amorphous character of the polymer.
The thermal stability of the NIAM homopolymer was verified by TGA (Fig. 8b). In the thermogram, a mass loss of 84.9% is observed in several stages between 175 and 475 °C. The thermal decomposition temperature range of the homopolymer was different from that presented by the NIAM monomer (Fig. 3b); however, they showed a similar decomposition profile.
Preliminary radical copolymerization studies of NIAM with acrylamide in THF using AIBN as initiator at a molar ratio [AIBN]/[NIAM]/[AM] of 1:5:200 lead to a formation of a copolymer with 98% yield containing 2.2% of NIAM, although with lower number average molar mass (5700 g mol−1) and dispersity of 6.2. Further copolymerization studies will be done in order to increase the molar mass and allow their use as hydrophobically modified polyacrylamide.
Conclusions
In this work, the monomer NIAM was successfully synthesized using the Ritter reaction methodology, despite the low yield. Spectroscopic and thermal analyses confirmed the presence of two diastereoisomers.
The homopolymerization of NIAM was only possible using excess of AIBN initiator in comparison with the monomer due to the abstraction of the iodine of the C–I bond present in the NIAM by the propagating radical species. The hypothesis that NIAM can act as a monomer and also as a transfer chain agent was confirmed by the UHPLC/QTOF-MS technique.
It should be noted that NIAM acts as a monomer and chain transfer agent, and makes it possible to construct polymers with differentiated properties. The inclusion of aromatic and hydrophobic groups in end-functional polyacrylamides presents a potentially wide range of applications.
References
Yuan R, Li Y, Li C, Fang H, Wang W (2013) Study about how the metal cationic ions affect the properties of partially hydrolyzed hydrophobically modified polyacrylamide (HMHPAM) in aqueous solution. Colloid Surf A 434:16–24. https://doi.org/10.1016/j.colsurfa.2013.05.036
Feng Y, Billon L, Grassl B, Khoukh A, Françóis J (2002) Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification. Polymer 43:2055–2064. https://doi.org/10.1016/S0032-3861(01)00774-1
Zhu Z, Jian O, Paillet S, Desbrières J, Grassl B (2007) Hydrophobically modified associating polyacrylamide (HAPAM) synthesized by micellar copolymerization at high monomer concentration. Eur Polym J 43(3):824–834. https://doi.org/10.1016/j.eurpolymj.2006.12.016
Hourdet D, Ducouret G, Varghese S, Badiger MV, Wadgaonkar PP (2013) Thermodynamic behavior of hydrophobically modified polyacrylamide containing random distribution of hydrophobes: experimental and theoretical investigations. Polymer 54:2676–2689. https://doi.org/10.1016/j.polymer.2013.03.039
Viken AL, Skauge T, Svendsen PE, Time PA, Spildo K (2018) Thermothickening and salinity tolerant hydrophobically modified polyacrylamides for polymer flooding. Energy Fuels 32(10):10421–10427. https://doi.org/10.1021/acs.energyfuels.8b02026
Maia AMS, Villetti MA, Vidal RRL, Borsali R, Balaban RC (2011) Solution properties of a hydrophobically associating polyacrylamide and its polyelectrolyte derivatives determined by light scattering, small angle X-ray scattering and viscometry. J Braz Chem Soc 22(3):489–500. https://doi.org/10.1590/S0103-50532011000300012
Ceniceros ACL, Vallejo CR, Regalado EJJ (2007) Synthesis, characterization and rheological properties of three different associative polymers obtained by micellar polymerization. Polym Bull 58:425–433. https://doi.org/10.1007/s00289-006-0675-3
Branham KD, Davis DL (1994) Water-soluble polymers: 59. Investigation of the effects of polymer microstructure on the associative behaviour of amphiphilic terpolymers of acrylamide, acrylic acid and N-[(4-decyl)phenyl]acrylamide. Polymer 35(20):4429–4436. https://doi.org/10.1016/0032-3861(94)90103-1
Abu-Sharkh BF, Yahaya GO, Ali SA, Hamad EZ, Abu-Reesh IM (2003) Viscosity behavior and surface and interfacial activities of hydrophobically modified water-soluble acrylamide/N-phenyl acrylamide block copolymers. J Appl Polym Sci 89:2290–2300. https://doi.org/10.1002/app.12198
Volpert E, Selb J, Candau F (1996) Influence of the hydrophobe structure on composition, microstructure, and rheology in associating polyacrylamides prepared by micellar copolymerization. Macromolecules 29:1452–1463. https://doi.org/10.1021/ma951178m
Khakpour H, Abdollahi M (2016) Synthesis, characterization, rheological properties and hydrophobic nano-association of acrylamide/styrene and acrylamide/sodium styrene sulfonate/styrene co- and terpolymers. J Polym Res 23:168. https://doi.org/10.1007/s10965-016-1064-8
Kang W, Zhua Z, Yang H, Tian S, Wang P, Zhang X, Ali Lashari Z (2019) Study on the association behavior of a hydrophobically modified polyacrylamide in aqueous solution based on host-guest inclusion. J Mol Liq 275:544–553. https://doi.org/10.1016/j.molliq.2018.11.063
Huang J-M, Ye Z-J, Chen D-S, Zhu H (2012) Iodine mediated/Brønsted acid-catalyzed dimerization of vinylarenes: a tandem reaction through Ritter trapping to produce N-(4-iodo-1,3-diarylbutyl) acetamides. Org Biomol Chem 10(18):3610–3612. https://doi.org/10.1039/C2OB25142F
Jiang D, He T, Ma L, Wang Z (2014) Recent developments in Ritter reaction. RSC Adv 4:64936–64946. https://doi.org/10.1039/C4RA10784E
Hunter CA, Sanders JKM (1990) The nature of π–π interactions. J Am Chem Soc 112(14):5525–5534. https://doi.org/10.1021/ja00170a016
Carey FA, Sundberg RJ (2007) Advanced organic chemistry part A: structure and mechanisms. Springer, New York
David G, Boyer C, Tonnar J, Ameduri B, Lacroix-Desmazes P, Boutevin B (2006) Use of iodocompounds in radical polymerization. Chem Rev 106(9):3936–3962. https://doi.org/10.1021/cr0509612
Odian G (2004) Principles of polymerization. Wiley, New York
Azemar F, Rodrigues DG, Robin JJ, Monge S (2016) Synthesis and self-assembly of carbamoylmethylphosphonate acrylamide-based diblock copolymers: new valuable thermosensitive materials. Dalton Trans 45:1881–1885. https://doi.org/10.1039/C5DT03289J
Zhang L, Zhu Z, Azhar U, Ma J, Zhang Y, Zong C, Zhang S (2018) Synthesis of well-defined PVDF-based amphiphilic block copolymer via iodine transfer polymerization for antifouling membrane application. Ind Eng Chem Res 57:8689–8697. https://doi.org/10.1021/acs.iecr.8b00533
Acknowledgements
The authors acknowledge the financial support by PETROBRAS S.A., Dr. Alexsandro Dallegrave for the UHPLC-QTOF-MS measurements, and Chun T-H. thanks the scholarship financial support given by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Huang, C.T., de Oliveira, L.A., de Carvalho Peres, A.C. et al. Studies of radical homopolymerization of N-(4-iodo-1,3-diphenylbutyl) acrylamide. Polym. Bull. 77, 4523–4535 (2020). https://doi.org/10.1007/s00289-019-02982-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02982-x