Abstract
The surface of polyurethane (PU) was modified by the graft polymerization of N,N-dimethylaminoethyl methacrylate (DAMA) and acrylic acid (AA) to enhance its water compatibility. Portions of poly(DAMA) and poly(AA) could be ionized by mutual acid–base neutralization and could notably improve the surface hydrophilicity of PU. The water contact angle, water absorption, and water vapor permeation test results jointly demonstrate the increase in water compatibility resulting from the inclusion of poly(DAMA) and poly(AA) in PU. The melting and glass transition temperatures of PU were not significantly influenced by the grafting of poly(DAMA) and poly(AA) onto PU. Small portions of the grafted poly(DAMA) and poly(AA) were involved in the cross-linking of PU, which sharply increased the shape recovery and the breaking tensile stress while maintaining high shape retention and breaking tensile strain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels have drawn attention for over 50 years due to their immense water-absorbing capability, considering the dry weight, without the collapse of the structure, and their applications include diapers, dish towels, wound dressings, contact lenses, and drug delivery materials [1]. Natural hydrogel materials (chitosan, alginate, etc.) and synthetic hydrogels (polyethylene oxide, polyvinyl alcohol, etc.) were used in the early stage of development, but synthetic cross-linked polymers were predominantly used due to the high water absorption capacity, dimensional stability, and repeated usability of the resulting hydrogels [2]. Polyurethane (PU) hydrogels containing hydrophilic polyol or polyhydroxyl groups have been extensively studied due to their flexibility, chemical and mechanical stability, and water and biocompatibility after surface modification [3]. Various cross-linked PU hydrogels have been reported due to their broad applications in biomedical materials. For example, blended hydrogels of waterborne PU and chitosan were applied in wound dressing material due to the healing capability of chitosan and the biocompatibility of PU [4]. Copolymer hydrogels of PU and itaconic acid were prepared to obtain a hydrophilic PU surface with the mechanical stability needed for biomedical applications [5]. A PU hydrogel containing polyethylene glycol was cross-linked by click chemistry between the azido and alkynyl groups to improve the mechanical strength of the hydrogel [6]. A heparin-mimetic unit was introduced into the cross-linked PU hydrogel frame to develop an anticoagulant drug-delivering material [7]. The end group of PU was graft-polymerized with 2-hydroxyethyl methacrylate (HEMA) to improve the hydrophilicity, cytocompatibility, and hemocompatibility of the PU hydrogel surface [8].
The development of PU hydrogels is similar to PU surface modification for the improvement in biocompatibility, where the hydrophilicity of the PU surface is enhanced using the graft polymerization of AA or poly(ethylene glycol) acrylate [9, 10] onto the PU surface because the initiation step proceeds under mild conditions without requiring a high-energy source, such as γ-ray irradiation [11], electron and ion beams [12], or plasma [13]. We intended to use the graft polymerization technique on the PU surface to develop a water-absorbing hydrogel-like PU using N,N-dimethylaminoethyl methacrylate (DAMA) as the basic monomer and acrylic acid (AA) as the acidic monomer. The grafted poly(DAMA) and poly(AA) could be partially turned into ionic forms by the neutralization equilibrium, which could significantly increase the hydrophilicity of PU. The combined graft polymerization of basic poly(DAMA) and acidic poly(AA) in a water-compatible PU has not been previously attempted and may exhibit superior results due to the ionic charge on the PU surface. 2-Hydroxyethyl acrylate (HEA) was grafted onto PU using the PU functionalization method with a diisocyanate compound and the mild polymerization initiated from the grafted HEA. The functionalization of PU via a diisocyanate compound has been demonstrated in applications to the grafting of poly(ethylene glycol) to improve the water compatibility of PU [10, 14,15,16], of poly(dimethylsiloxane) to improve the low-temperature flexibility of PU [17], a pH indicator to confer pH sensitivity on PU [18], and benzimidazole to confer antifungal activity on PU [19]. This mild grafting method notably enhanced the shape recovery capability and the tensile stress using mild chemical cross-linking and demonstrated the convenient modification of the PU surface.
In this study, poly(DAMA) and poly(AA) were grafted together in the main grafted PU series and were included in PU without chemical bonding in the control PU series. The effects of the grafted poly(DAMA) and poly(AA) in the DA series and the free poly(DAMA) and poly(AA) in the CDA series on the tensile stress and strain, shape recovery and retention, glass transition and melting of soft segments, and the water compatibility of PU will be discussed.
Experimental
Materials
Poly(tetramethylene ether)glycol (PTMG, Mn ~ 2000 g/mol, Sigma-Aldrich, St. Louis, MO, USA), 4,4′-methylenebis(phenyl isocyanate) (MDI, Junsei Chemical, Tokyo, Japan), and 1,4-butanediol (BD, Junsei Chemical) were dried overnight under high vacuum (0.1 Torr). Acrylic acid (AA) and 2-(dimethylamino)ethyl methacrylate (DAMA) were also obtained from Sigma-Aldrich. Azobis(isobutyronitrile) (AIBN) and 2-hydroxyethyl acrylate (HEA) were obtained from Junsei Chemical. Dimethylformamide (DMF, Duksan Chemical, Ansan, Korea) was distilled over CaH2 prior to use.
Polymer synthesis
Solid MDI (MDI-1, 5.00 g, 20.0 mmol) particles were mixed with viscous PTMG (40.0 g, ca. 20.0 mmol) in a 500-mL beaker-type flask equipped with a mechanical stirrer, nitrogen inlet, water-cooled condenser, and heating mantle with a temperature control. The viscous mixture was slowly stirred for 2 h at 50 °C to obtain a prepolymer. A solution of BD (2.7 g, 30.0 mmol) in 10 mL of DMF was quickly added to the prepolymer, and the combined mixture was stirred for 1 h. Then, additional solid MDI (MDI-2, 7.50 g, 30.0 mmol) particles were added to the mixture, and the stirring was continued for 1 h, during which 10 mL of DMF was added dropwise using a dropping funnel to prevent sudden increases in viscosity. Additional MDI (MDI-3, 0.50 g, 2.0 mmol) particles were again added to the reaction mixture, and the stirring was continued for 40 min. Next, a solution of HEA (0.23 g, 2.0 mmol) dissolved in 10 mL of DMF was added to the reaction mixture, and the stirring was continued for 1 h. After separately adding DAMA and AA monomers, as specified in Table 1, and AIBN as an initiator (30 mg) to the reaction mixture, the reactor temperature was raised to 70 °C, and the stirring was continued for 3 h. Finally, the reaction mixture was put into distilled water (1.5 L) to halt the polymerization. The precipitated products were cut into pieces (approximately 4 × 4 mm) and thoroughly washed with distilled water (1.5 L × 2), 0.01 M KCl solution (1 L × 1), and ethanol (1 L × 2) with magnetic stirring for purification. The PU pieces were separated by suction filtration from the washing solution and dried in a convection oven (60 °C) for 3 days. The grafting polymerization of DAMA and AA onto PU is shown in Scheme 1. The synthesized PU samples for the mechanical and shape memory tests were prepared by the solvent casting method: A PU solution in DMF was slowly evaporated at 60 °C for 60 h to obtain a sheet with a thickness of 0.5 ± 0.03 mm. The film thickness was determined using a digital caliper (Mitutoyo CD-15CPX, Tokyo, Japan), and the average was taken of the thickness measurements at five points. Specimens were prepared from the PU sheet according to ASTM D638.
Cross-link density
The cross-link density was determined using a method explained in our previous studies [17,18,19]. Specifically, a specimen (20 × 20 × 1 mm) with a known weight (m1) was swollen in 50 mL of toluene in a closed bottle for 24 h, and the swollen weight of the specimen (m2) was measured after quickly removing the adsorbed toluene on the polymer surface with a tissue. The swollen specimen was dried at room temperature for a week, and the dry weight of the specimen was measured (m3). The solvent volume (Vs) of the swollen specimen was calculated as the average from five swelling experiments using the solvent density (0.8699 g/cm3) and the difference in weight between m2 and m3. The polymer volume (Vp) in the dry state was calculated by dividing m1 by the polymer density. The volume fraction of the swollen polymer (v2) was calculated using the following equation: Vp/(Vs + Vp). The interaction parameter (χ) between toluene and the polymer was calculated from expression (1):
where δ1 and δ2 = solubility parameters of solvent and polymer, V1 = molar volume of solvent, R = gas constant, and T = absolute temperature.
The solubility parameters of toluene (δ1) and PU (δ2) are 18.2 and 20.5 MPa1/2, respectively [20, 21]. The cross-link density is calculated from the Flory–Rehner Eq. (2):
where v2 = volume fraction of polymer in the swollen mass, χ = interaction parameter, and n = cross-link density.
Contact angle, viscosity, and infrared analysis
The contact angle of a water drop (2 µL) on the PU surface was measured using the sessile drop method with a contact shape analyzer (Krűss DSA 100, Hamburg, Germany). The absolute viscosity of the PU dissolved in DMF was measured with a vibrating viscometer (AND SV-10, Tokyo, Japan) at 25 °C and was calculated as the average of five tests at a concentration of 4 wt% (m/v). The carboxyl group content on the PU dissolved in DMF was determined by titration using a 0.05 M NaOH solution in isopropyl alcohol [9]. A Fourier transform infrared (FTIR) spectrophotometer (JASCO 300E, Tokyo, Japan) equipped for attenuated total reflectance measurements was used to collect the infrared spectra. For each sample, 25 scans were performed using a resolution of 4 cm−1 and a scan speed of 2 mm/s.
Thermal analysis
A differential scanning calorimeter (DSC, TA Instruments DSC-Q20, New Castle, DE, USA) was used to obtain calorimetry data from both heating and cooling scans, which were performed at a rate of 10 °C/min between − 50 and 250 °C. After melting at 250 °C for 5 min and cooling quickly to − 50 °C, a 5-mg sample was heated to 250 °C at a rate of 10 °C/min and monitored for phase transitions. The soft segment melting temperature (Tm) and the enthalpy change during the melting (ΔHm) were determined using the Platinum™ software included with the DSC instrument. A dynamic mechanical analyzer (DMA, Triton TTDMA, Lincolnshire, UK) was used to measure the storage and loss moduli in the tension mode between − 150 and 100 °C at 10 Hz.
Tensile and shape memory tests
The tensile mechanical properties were measured according to the ASTM D638 standard at 25 °C using 0.5-mm-thick samples. The measurements were collected on a universal testing machine (UTM, LR10 K, Lloyd Materials Testing, West Sussex, UK) using a 20-mm gauge length, 20 mm/min crosshead speed, and 0.5 kN load cell. A total of seven specimens were tested for each group; the average tensile properties of five specimens, excluding the high and low values, are reported in this work. The same UTM equipped with a temperature-controlled chamber was employed for the shape memory tests. A sample of length L0 was drawn by 100% to 2L0 in the temperature-controlled chamber at 10 °C in 2 min, and the sample was kept at 10 °C for 5 min. The upper grip was released after the specimen was cooled with liquid nitrogen to − 30 °C for 10 min, and then, the shrunken length (L1) of the sample was measured. The shape retention (%) was then calculated using Eq. (3). In the chamber, the specimen was heated to 10 °C for 10 min, and the length (L2) was subsequently measured. The shape recovery (%) was then calculated using Eq. (4).
Water swelling and water vapor permeation of PU
The % swelling of PU in distilled water was determined using Eq. (5) in which the percent increase in the weight (W) after 72 h in distilled water relative to the initial weight (Wo) was used to calculate the % swelling of PU:
The water vapor permeability (WVP) of the PU membrane was determined using the time transfer method [22, 23]: A PU film (0.2 mm thick) was placed over a Petri dish containing distilled water, and the volume of water was adjusted to form an air gap between the water and the PU film. The contact area between the dish and film was tightly sealed. The water vapor that permeated through the PU film was monitored at 60 °C by measuring the weight loss at 1-h intervals over 24 h. The WVP (g/m2 h) per unit area of the PU membrane at 1 h was calculated from the amount of permeated water.
Results and discussion
Synthesis and structure of PU
The grafted poly(DAMA) and poly(AA) on PU were intended to change the hydrophobic PU surface into a hydrophilic one, and the basic dimethylamino groups in the grafted poly(DAMA) and the acidic carboxyl groups in the grafted poly(AA) could partially turn into dimethylammonium and carboxylate ionic forms, respectively, due to acid–base neutralization. The conversion of the grafted basic and acidic groups to ionic forms and the repeating ionic groups on poly(DAMA) and poly(AA) were used to enhance the hydrophilicity of the PU. The radical graft polymerization of DAMA and AA started from the grafted HEA that was linked to PU using MDI-3, as explained in the literature [13,14,15,16] (Scheme 1). The main DA series in Table 1 contained grafted poly(DAMA) and poly(AA), whereas the control CDA series contained free poly(DAMA) and poly(AA) because MDI-3 and HEA were not used. We grafted poly(DAMA) and poly(AA) together onto PU instead of grafting one type of polymer chain because the grafted poly(DAMA) and poly(AA) can simultaneously present cationic and anionic charges on the PU surface after ionization, and this amphoteric character can notably improve the surface hydrophilicity. The DAMA and AA contents of the DA and CDA series gradually increase within each series (Table 1), whereas those of MDI, PTMG, and BD are not changed because this composition has been optimized for the best tensile and shape memory properties.
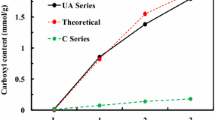
The Flory–Rehner equation was used to determine the cross-link density, in which the degree of cross-linking was inversely related to the polymer swelling in toluene [17,18,19]. The cross-link density of the DA series slightly increased with increasing monomer feed content and that of the CDA series increased to a lesser degree (Fig. 1a). The light chemical linking between the grafted polymer chains in the DA series, as shown in Scheme 1c, slightly increased the cross-link density, whereas the physical cross-linking between PU and the free polymer chains in the CDA series could have increased the cross-link density. A slight increase in the cross-link density will significantly change the tensile and shape memory properties of PU, as explained in the subsequent sections. The solution viscosities of the DA and CDA series in DMF may be different because the friction caused by the grafted polymer chains in the DA series could increase the viscosity of PU, but the unbound polymer chains in the CDA series could not increase the viscosity to a similar degree. The viscosity of the DA series evidently increased with increasing monomer feed content, whereas that of the CDA series did not display a similar behavior compared to the plain PU (Fig. 1b). The viscosity data showed that the grafted polymers made a significant difference in the solution viscosity due to the structural differences from the free polymer chains. The carboxyl group content in the grafted or free poly(AA) was analyzed by titration, and the grafted carboxyl contents of DA series were close to the expected values at a low AA content but did not increase in proportion to the AA feed content (Fig. 1c). The free carboxyl contents in the CDA series likewise did not proportionally increase with increasing AA content. The AA monomers were not fully used for polymerization at a high feed content in the DA and CDA series, and the carboxyl content decreased because the unreacted monomers were washed away during the cleaning step. The water contact angles of the DA and CDA series significantly decreased with the increase in DAMA and AA content compared to that of plain PU (L) (Fig. 1d, e). The contact angle did not further decrease with increasing DAMA and AA content in both the DA and CDA series, because the hydrophilicity of the PU surface was already maximized at lower DAMA and AA contents. Therefore, the grafted poly(DAMA) and poly(AA) in the DA series and the free poly(DAMA) and poly(AA) in the CDA series contributed to the surface hydrophilicity of PU.
IR and thermal analysis
The IR spectra show bands related to N–H stretching at 3310 cm−1, aromatic C–H stretching at 2940 cm−1, alkyl C–H stretching at 2850 cm−1, and carbonyl stretching at 1700 and 1730 cm−1 (Fig. 2a, b). The carbonyl absorbance bands were divided into free carbonyl (1730 cm−1) and bonded carbonyl (1700 cm−1) [24, 25]. The areas of the free and bonded carbonyl bands were used to calculate the degree of phase separation (DPS), which refers to the degree of interactions between hard segments, as explained in previous reports [17, 18]. The separation of the carbonyl bands is shown in Fig. 2c, d, and a small weakly bonded carbonyl band was detected at approximately 1715 cm−1. It is evident that the band area of the bonded carbonyl in DA50 decreases relative to that of L. The DPS of DA and CDA series notably declined as the monomer feed content increased: 63.8% for L declined to 55.7% for DA50 and 44.9% for CDA50. Therefore, the grafted polymers in the DA series and the free polymers in the CDA series considerably affected the attraction between the hard segments.
The melting and crystallization in the soft segments were monitored by DSC, and the results are summarized in Table 2 and displayed in Fig. 3. The Tm was between 19 and 22 °C for both the DA and CDA series in the second heating scans and was slightly increased compared with that of L. However, ΔHm clearly increased with the inclusion of poly(DAMA) and poly(AA) in the DA and CDA series. The poly(DAMA) and poly(AA), whether grafted or not, disturbed the melting of the soft segment and increased ΔHm. The crystallization temperature (Tc) of soft segments slowly increased with increasing monomer content, but the enthalpy change with crystallization (ΔHc) notably increased. The presence of poly(DAMA) and poly(AA) in the DA and CDA series increased Tc and ΔHc compared with those of L due to the restriction of the soft segment crystallization. Therefore, poly(DAMA) and poly(AA), whether grafted or not, hindered the phase transition of the soft segment.
The storage and loss modulus values were scanned by DMA from − 150 to 100 °C to detect the glass transition in the soft segment (Fig. 4). A sudden decline in the storage modulus and the advent of a loss modulus peak at approximately − 65 °C signaled the glass transition. The glass transition temperature (Tg) data in Table 2 are based on the loss modulus results. The Tg of the DA and CDA series slowly increased with increasing monomer feed content: The Tg increased from − 65.8 °C for L to − 63.6 °C for DA30, − 63.2 °C for DA50, and − 63.0 °C for CDA50. The poly(DAMA) and poly(AA) in the DA and CDA series did not influence the Tg because the soft and flexible poly(DAMA) and poly(AA) did not restrict the rotation of PU. However, the cold crystallization peak in the DA series observed at approximately − 23 °C decreased with increasing monomer feed content, whereas that in the CDA series did not decrease. The grafted poly(DAMA) and poly(AA) in the DA series moderately influenced the cold crystallization of PU, but the free poly(DAMA) and poly(AA) in the CDA series did not influence the cold crystallization. Therefore, the grafted poly(DAMA) and poly(AA) in the DA series did not make a significant difference in the glass transition compared with the free poly(DAMA) and poly(AA) chains in the CDA series.
Tensile and shape memory properties
The breaking tensile stress of the DA series steeply increased as the monomer feed content increased and remained above 50 MPa with further increases in monomer content. However, the breaking tensile stress of the CDA series did not increase greatly compared with that of the DA series as the monomer feed content increased (Table 3). The steep increase in the breaking tensile stress of the DA series resulted from the chemical linking between the grafted polymers, whereas the physical cross-linking between the free poly(DAMA) and poly(AA) in the CDA series also raised the breaking tensile stress but not as high as that in the DA series. The breaking tensile strains of the DA and CDA series did not notably decline with increasing monomer feed content because the soft segment responsible for the tensile strain was not hindered by the grafted or free poly(DAMA) and poly(AA). Therefore, the breaking tensile stress of the DA series was clearly enhanced by the grafting of poly(DAMA) and poly(AA) onto PU while maintaining the high breaking tensile strain. The shape recovery of the DA series at 10 °C increased steeply compared with that of L as the monomer feed content was increased and stayed above 95% with further increases in the monomer feed content (Table 3): The shape recovery increased from 46.9% for L to 95.4% for DA30 and 95.4% for DA50. The shape recovery of the CDA series also increased but to a lesser degree. The shape retention of the DA series at − 25 °C decreased slightly as the monomer feed content increased: 95.2% for L decreased to 94.8% for DA30 and 92.3% for DA50. However, the shape retention of the CDA series did not decrease with increasing monomer feed content. The chemical linking between the grafted poly(DAMA) and poly(AA) increased the shape recovery of the DA series, and the physical cross-linking in the CDA series also improved the shape recovery. The shape retention of the DA series decreased slightly due to the chemical linking, but that of the CDA series was not influenced by the weak physical cross-linking. Therefore, the chemical linking in the DA series influenced both the shape recovery and the shape retention, in contrast to the CDA series.
Water swelling and WVP
The comparison of the water swelling of the DA and CDA series for 72 h is shown in Fig. 5. The percent water swelling of the DA series slowly increased with time, as shown in Fig. 5a, but DA50 surpassed the other DA series samples in water swelling capability because of its higher contents of poly(DAMA) and poly(AA). The water swelling results of the CDA series in Fig. 5b show that CDA50 likewise outperformed the other CDA series samples due to its higher poly(DAMA) and poly(AA) contents. The comparison of the appearances of PU samples (L, DA50 and CDA50) after the swelling tests is shown in Fig. 5c and clearly shows that DA50 and CDA50 became opaque and white compared with the plain PU (L) due to the excessive water absorption. The water swelling capability of CDA50 was almost twice that of DA50, suggesting that the free poly(DAMA) and poly(AA) in the CDA series performed better than the grafted ones in absorbing moisture. DA50 and CDA50 are comparable to the previous hydrogel PU in water swelling capability but are far superior with respect to the breaking tensile stress and strain [8]. Therefore, it was revealed that the water swelling capability of PU could be drastically improved by the combined grafting of poly(DAMA) and poly(AA).
The comparison of the WVPs of the DA and CDA series is shown in Fig. 6 to determine the effect of surface hydrophilicity on the WVP. The temperature was raised to 60 °C to obtain enough water vapor pressure for the passage across the PU membrane. The WVP of DA50 was higher than those of DA30 and L throughout the test period due to the higher poly(DAMA) and poly(AA) contents of DA50 (Fig. 6a). The WVPs of the CDA series were higher than those of the DA series, and CDA50 displayed a higher value than CDA30 and L due to its higher poly(DAMA) and poly(AA) contents (Fig. 6b). The WVPs of the DA and CDA series were higher than that of L because the PU surface was made water-compatible by the inclusion of poly(DAMA) and poly(AA), and the water vapor could pass across the PU membrane with ease. The WVPs of the CDA series were higher than those of the DA series, which agrees with the water swelling results. Therefore, the free poly(DAMA) and poly(AA) in the CDA series could enhance the hydrophilicity of PU slightly more than the grafted poly(DAMA) and poly(AA) in the DA series. Overall, the grafted poly(DAMA) and poly(AA) in the DA series clearly enhanced the tensile strength and shape recovery but did not affect the soft segment melting, and the CDA series improved the hydrophilicity of PU more effectively than the DA series did due to the free poly(DAMA) and poly(AA). The developed PUs mimicking the hydrogel structure could notably increase the hydrophilicity of the PU surface and solve the adhesion and wettability problems observed in plain PUs for applications to medical supplies and devices.
Conclusions
Polymeric chains with basic and acidic groups, namely poly(DAMA) and poly(AA), were grafted onto PU in the DA series to enhance the hydrophilicity of the PU surface, whereas the control CDA series contained free poly(DAMA) and poly(AA). The poly(DAMA) and poly(AA) could be partly ionized by mutual acid–base neutralization, which sharply increased the hydrophilicity of PU as demonstrated in the water contact angle, water swelling, and WVP results. The connections between the grafted poly(DAMA) and poly(AA) increased the cross-link density, and the viscosity of the DA series and notably enhanced the tensile strength and shape recovery capability. The interactions between hard segments were reduced by the grafted poly(DAMA) and poly(AA), as shown by comparing the DPS values from the IR spectra. The Tm slightly changed after the grafting of poly(DAMA) and poly(AA), but the ΔHm notably increased relative to the plain PU. The Tg for both the DA and CDA series slightly increased with increasing monomer feed content relative to that of the plain PU. Overall, the grafted or free poly(DAMA) and poly(AA) in PU significantly enhanced the surface hydrophilicity, tensile strength, and shape recovery capability.
References
Hoffman AS (2012) Hydrogels for biomedical applications. Adv Drug Deliv Rev 64:18–23
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications: a review. J Adv Res 6:105–121
Gennen S, Grignard B, Thomassin JM, Gilbert B, Vertruyen B, Jerome C, Detrembleur C (2016) Polyhydroxyurethane hydrogels: synthesis and characterizations. Eur Polym J 84:849–862
Bankoti K, Rameshbabu AP, Datta S, Maity PP, Goswami P, Datta P, Ghosh SK, Mitra A, Dhara S (2017) Accelerated healing of full thickness dermal wounds by macroporous waterborne polyurethane-chitosan hydrogel scaffolds. Mater Sci Eng C 81:133–143
Jiang G, Tuo X, Wang D, Liu J (2010) Syntheses and self-assembly of novel polyurethane–itaconic acid copolymer hydrogels. React Funct Polym 70:175–181
Li K, Zhou C, Liu S, Yao F, Fu G, Xu L (2017) Preparation of mechanically-tough and thermo-responsive polyurethane-poly(ethylene glycol) hydrogels. React Funct Polym 117:81–88
Chen Y, Wang R, Wang Y, Zhao W, Sun S, Zhao C (2017) Heparin-mimetic polyurethane hydrogels with anticoagulant, tunable mechanical property and controllable drug releasing behavior. Int J Biol Macromol 98:1–11
Lin CH, Jao WC, Yeh YH, Lin WC, Yang MC (2009) Hemocompatibility and cytocompatibility of styrenesulfonate-grafted PDMS–polyurethane–HEMA hydrogel. Colloid Surf B 70:132–141
Chung YC, Kim HY, Choi JW, Chun BC (2015) Modification of polyurethane by graft polymerization of poly(acrylic acid) for the control of molecular interaction and water compatibility. Polym Bull 72:2685–2703
Archambault JG, John L (2004) Protein resistant polyurethane surfaces by chemical grafting of PEO: amino-terminated PEO as grafting reagent. Colloid Surf B 39:9–16
Alves P, Coelho JFJ, Haack J, Rota A, Bruinink A, Gil MH (2009) Surface modification and characterization of thermoplastic polyurethane. Eur Polym J 45:1412–1419
Kim SR (2000) Surface modification of poly(tetrafluoroethylene) film by chemical etching, plasma, and ion beam treatments. J Appl Polym Sci 77:1913–1920
Huang CY, Lu WL, Feng YC (2003) Effect of plasma treatment on the AAc grafting percentage of high-density polyethylene. Surf Coat Technol 167:1–10
Tan K, Obendorf SK (2006) Surface modification of microporous polyurethane membrane with poly(ethylene glycol) to develop a novel membrane. J Membr Sci 274:150–158
Freij-Larsson C, Wesslen B (1993) Grafting of polyurethane surfaces with poly(ethylene glycol). J Appl Polym Sci 50:345–352
Huang J, Xu W (2010) Zwitterionic monomer graft copolymerization onto polyurethane surface through a PEG spacer. Appl Surf Sci 256:3921–3927
Chung YC, Park HS, Choi JW, Chun BC (2012) Characterization and low temperature test of the flexibly crosslinked polyurethane copolymer by poly(dimethylsiloxane). High Perform Polym 24:200–209
Chung YC, Jung IH, Choi JW, Chun BC (2014) Characterization and proof testing of the halochromic shape memory polyurethane. Polym Bull 71:1153–1171
Chung YC, Kim HY, Choi JW, Chun BC (2015) Preparation of water-compatible antifungal polyurethane with grafted benzimidazole as the antifungal agent. J Appl Polym Sci 132:41676–41684
Sekkar V, Gopalakrishnan S, Ambika Devi K (2003) Studies on allophanate–urethane networks based on hydroxyl terminated polybutadiene: effect of isocyanate type on the network characteristics. Eur Polym J 39:1281–1290
Sekkar V, Rama Rao M, Krishinamurthy VN, Jane SR (1996) Modeling of polyurethane networks based on hydroxy-terminated polybutadiene and poly(12-hydroxy stearic acid-co-TMP) ester polyol: correlation of network parameters with mechanical properties. J Appl Polym Sci 62:2317–2327
Cho JW, Jung YC, Chun BC, Chung YC (2004) Water vapor permeability and mechanical properties of fabrics coated with shape-memory polyurethane. J Appl Polym Sci 92:2812–2816
Pause B (1996) Measuring the water vapor permeability of coated fabrics and laminates. J Coat Fabr 25:311–320
Choi T, Weksler J, Padsalgikar A, Runt J (2010) Microstructural organization of polydimethylsiloxane soft segment polyurethanes derived from a single macrodiol. Polymer 51:4375–4382
Russo P, Lavorgna M, Piscitelli F, Acierno D, Di Maio L (2013) Thermoplastic polyurethane films reinforced with carbon nanotubes: the effect of processing on the structure and mechanical properties. Eur Polym J 49:379–388
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1D1A1B01014308).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chung, YC., Bae, J.C., Choi, J.W. et al. The preparation of hydrogel-like polyurethane using the graft polymerization of N,N-dimethylaminoethyl methacrylate and acrylic acid. Polym. Bull. 76, 6371–6386 (2019). https://doi.org/10.1007/s00289-019-02726-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02726-x