Abstract
Novel redox-responsive amphiphilic cationic multi-block copolymers PEG2000–PLA3000–PEI1200–PLA3000–PEG2000 and PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 were synthesized and self-assembled into micelles for co-delivery of siRNA and hydrophobic doxorubicin (DOX). The chemical structure and molecular weight of the copolymers were characterized by 1H nuclear magnetic resonance and gel permeation chromatography, respectively. The copolymeric micelles were examined by dynamic light scattering, and their size, zeta potential and critical micelle concentration were determined. The in vitro drug release analyses indicated that reductive environment can trigger the release of DOX and siRNA by breaking the micelles. MTT assay demonstrated that the DOX/siRNA-loaded micelles are capable of inhibiting proliferation of SGC7901 cells. The results of fluorescence microscopy and flow cytometry verify the simultaneous delivery of DOX and siRNA from the nanomicellar particles into SGC7901 cells. The reduction-responsive cationic copolymers will provide a platform for constructing drug/gene delivery system toward cancer therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past decades, polymeric micelles have attracted considerable attention due to their potential applications in nanomedicine [1]. Especially, amphiphilic copolymers have been widely used as drug delivery systems for cancer therapy. Amphiphilic copolymers can self-assemble into core–shell morphology with hydrophobic interior and hydrophilic corona in aqueous solution [2, 3]. In addition to drug delivery, cationic nanoparticles self-assembled from amphiphilic cationic copolymers have recently been employed as alternative choices and are promising for nucleic acid delivery [4]. Compared with homopolymers, copolymeric micelles used as nucleic acids delivery vectors have several unique advantages: (1) the capacity to condense and protect the nucleic acid segments [5]; (2) higher polymeric micelles stability [6]; (3) prolonged blood circulation lifetime in vivo [7]; (4) improved cell association and internalization to enhance transfection efficiency [8]. Therefore, cationic copolymers have been considered as the most prospective candidates with enormous potential in comparison with their counterparts due to their unique characteristics of forming polyelectrolyte complexes with genes and ability to protect them from various enzymes [9,10,11,12]. Besides, the structure and properties of copolymeric micelles can be sensitive to external or internal stimuli that can be utilized to control the encapsulated drug and gene release [13, 14]. The incentives include glutathione (GSH), temperature, pH, glucose and others [15].
Most cationic polymers contain amine group in their backbone [16, 17]. Polyethylenimine (PEI) has been widely applied and investigated owing to its extremely high effectivity in gene transfecting and delivering nucleic acids [18, 19]. In recent years, PEI has been used as the gold standard [20], which is low cost and easy to be derived in a wide range of molecular weight as DNA delivery vectors in vitro and in vivo [21,22,23,24]. It was found that PEI’s efficacy in transfecting genetic materials increased with increasing molecular weight [25, 26]. However, a larger molecular weight of PEI led to more severe cytotoxicity. To overcome this dilemma and break the in vivo application limitation of PEI [27, 28], studies have been carried out to enhance the biocompatibility and applicability of PEI-based gene delivery systems [29]. One effective strategy is to combine the advantageous features of PEI and poly(ethylene glycol) (PEG) to reduce the toxicity of PEI [30, 31]. PEG is a nonionic hydrophilic polymer, which could shield the surface charge of the polyplexes [32]. With the polyplexes around, PEG forms a hydration shell that can reduce the intermolecular interactions and decrease the toxicity of PEI [33,34,35]. More remarkably, a series of studies demonstrated that PEGylation (i.e., PEG modification) could hinder the interaction of polyplexes with blood components. These valuable features of the PEGylation could enhance the serum stability of polyplexes, reduce the clearance by the reticuloendothelial system and extend their blood lifetime after intravenous administration [29].
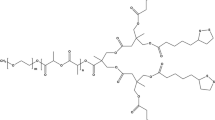
In this paper, a ABCBA-type pentablock copolymer containing PEI, PEG and poly(l-lactic acid) (PLA) was designed and synthesized based on H-bonding instructed association units with double disulfide linkage (Scheme 1). The amphiphilic and cationic pentablock copolymer PEG–PLA–PEI–PLA–PEG could self-assemble into micelles for non-viral transfection and intracellular drug delivery [36,37,38,39,40,41]. As a cationic copolymer, the pentablock PEG–PLA–PEI–PLA–PEG consisted of hydrophilic PEG segments, hydrophobic PLA segments as well as a cationic PEI segment. The copolymeric micelles were flower-like and possessed enhanced intracellular barriers penetration, with PEI as the non-viral vector for siRNA delivery, PLA as hydrophobic segments for anticancer drug encapsulation and PEG as hydrophilic segments that reduce cytotoxicity, increase serum stability and blood circulation time. Compared with the micelles from the cationic triblock copolymer PEG2000–PLA5000–PEI1800 [14], the pentablock copolymer PEG–PLA–PEI–PLA–PEG micelles have more compact spherical structure, lower critical micelle concentration (CMC) and lower nitrogen to phosphorus (N/P) ratio and may be superior therapeutic agents for cancers. The results of this study show that hydrophobic DOX and FAM-siRNA can be co-delivered by the cationic copolymer with high efficiency as well as low toxicity. Therefore, the prepared polymeric micelles are expected to be a promising platform for drug/gene delivery system.
Materials and methods
Materials
All chemicals were purchased from Aldrich or Aladdin and were used as received unless otherwise indicated. All reactions were followed by thin-layer chromatography (TLC) (precoated 0.25-mm silica gel plates from Aldrich), and silica gel column chromatography was carried out with silica gel 60 (mesh 200–400). Dichloromethane, N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO) were dried over calcium hydride and then purified by vacuum distillation. 3,5-Bis(4-(tritylthio) butanamido)benzoic acid (intermediate A), 5-amino-N1,N3-bis(2-(tritylthio)ethyl)isophthalamide (intermediate B) and PEG2000-A were synthesized according to our reported procedure [40]. Poly(ethylene glycol) (PEG) was purchased from Shanghai Yare Bio. Co. Ltd, (number average molar mass (Mn) determined by gel permeation chromatography (GPC), (Mn = 2000, Mw/Mn = 1.05)) and used as received. Polylactic acid (PLA) was purchased from Jinan Daigang Biology Co. Ltd, (for PLA viscosity average molar (Mv) determined by viscometer Mv = 3000 g/mol;). Branched polyethylenimine (PEI, Mw = 1800 g/mol, Mw = 1200 g/mol) was purchased from Sigma-Aldrich and used as received.
SGC7901 gastric cancer cells were obtained from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, and the cells were cultured in DMEM (Dulbecco’s modified Eagle’s medium) supplemented with 10% FBS (fetal bovine serum) and antibiotics (10 units mL−1 penicillin and 100 units mL−1 streptomycin) at 37 °C in a humidified atmosphere containing 5% CO2.
Measurements
Nuclear magnetic resonance (NMR) analyses were recorded on Bruker Avance III 400 MHz with deuterated chloroform (CDCl3) or dimethyl sulfoxide-d6 (DMSO-d6) as solvent. The number average molar mass (Mn), weight average molar mass (Mw) and polydispersity index (PDI) were measured by gel permeation chromatography (GPC). Spectrometer dynamic light scattering (DLS) measurements were performed in aqueous solution using a Malvern Zetasizer Nano S apparatus equipped with a 4.0-mW laser operating at λ = 633 nm. All samples of 1.0 mg mL−1 were measured at 25 °C and at a scattering angle of 173°.
Synthesis and characterization
Synthesis of hydrophobic block B-PLA3000-B
In a typical procedure, modified PLA3000(HO-PLA3000-COOH) (1.5 mmol) in 20 mL methylene chloride (DCM) was cooled in ice water and stirred for 5 min, and then, 4-dimethylaminopyridine (DMAP) (3.0 mmol) and succinic anhydride (4.0 mmol) were added. After the being stirred at room temperature for 18 h, the reaction mixture was washed with saturated sodium bicarbonate solution and dried over anhydrous Na2SO4. Subsequently, the filtrate was distilled in vacuo to give the intermediate HOOC-PLA3000-COOH. And then HOOC-PLA3000-COOH (0.2 mmol), NMM (0.8 mmol), HATU (0.8 mmol) and the intermediate B (0.6 mmol) in anhydrous DMF (20 mL) under nitrogen atmosphere were cooled in an ice water bath, stirred by a magnetic. After stirring for 1 h, the reaction mixture was warmed up to 35 °C and continued stirring for an additional 24 h. The resulting mixture was washed with water and extracted with CH2Cl2, and the combined organic extracts were washed with water and brine, dried over anhydrous Na2SO4 and then filtered. The filtrate was evaporated in vacuo. The hydrophobic block B-PLA3000-B was further purified by chromatography on silica gel by using CH2Cl2/CH3OH (60/1) as an eluent. Yield 70%; 1H NMR (400 MHz, CDCl3) δ 8.22–8.01 (m, 4H, ArH), 7.83 (s, 2H, ArH), 7.44–7.37 (m, 24H, ArH), 7.30–7.14 (m, 38H, ArH), 6.44 (s, 4H, –NH–), 5.43–5.02 (m, 21H, 1H per repeating unit, PLA–CH–), 4.43–4.33(m, 1H, PLA–CH–), 3.36 –3.28 (m, 8H, –NCH2–), 2.53 (t, J = 6.3 Hz, 8H, –SCH2–), 1.71–1.39 (m, 69H, 3H per repeating unit, PLA–CH3). IR(KBr, cm−1) 3406, 3064, 2991, 2945, 1747, 1646, 1532, 1453, 1378, 1264, 1190, 1122, 1082, 1042, 860, 746, 706, 672.
Synthesis of hydrophilic block PEG2000-A
Common procedure: PEG-OH (2.0 mmol) was dissolved in 50 mL methylene chloride solution (DCM), followed by the addition of 3,5-dinitrobenzoyl chloride (4.0 mmol) and triethylamine (8.0 mmol). The reaction mixture was stirred at ice water bath for 4 h. The reaction mixture was poured into water and extracted with CH2Cl2, the combined organic extracts were washed with water and brine, dried over anhydrous Na2SO4 and filtered, and the filtrate was evaporated to give the intermediate PEG-NO2. And then the PEG-NO2 (0.8 mmol) and 10% Pd/C (0.4 mmol) were dissolved in 50 mL methanol solution under hydrogen atmosphere. The temperature was increased to 35 °C, and the reaction mixture was stirred for 24 h. Then, the reaction mixture was cooled to room temperature, and 10% Pd/C was removed by diatomite filtration. The residue was distilled in vacuo to get the intermediate PEG-NH2. After that, 4-(tritylthio)butanoic acid (2.0 mmol), 2-(7-aza-1H-benzotriazole-1-ly)-1,1,3,3-tetra-methyluronium hexafluorophosphate (HATU) (4.0 mmol) and N-methylmorpholine (NMM) (2.0 mmol) in anhydrous DMF (20 mL) under nitrogen were cooled in ice water bath with stirring for 30 min. And then PEG-NH2 was dissolved in anhydrous DMF (5 mL) and injected into the above mixture. The reaction mixture was continued stirring at room temperature for an additional 24 h. The resulting mixture was poured into water and extracted with CH2Cl2. The combined organic extracts were washed with water and brine, dried over anhydrous Na2SO4 and filtered, and the filtrate was evaporated in vacuo. The residue was purified by silica gel column chromatography to give the hydrophilic block PEG2000-A.
PEG2000-A: isolated yield: 74%, 1H NMR (400 MHz, CDCl3) δ 8.09 (s, 1H, ArH), 7.95 (s, 2H, –NH(C = O)–), 7.85 (s, 2H, ArH), 7.44–7.34 (m, 12H, ArH), 7.28–7.23 (m, 14H, ArH), 7.18–7.12 (m, 4H, ArH), 3.74–3.54 (m, 167H, –CH2CH2–), 2.35–2.24 (m, 8H, –SCH2–, –CH2CO–), 1.82–1.70 (m, 4H, –CH2CH2CH2–); IR (KBr, cm−1) 2876, 1727, 1653, 1607, 1552, 1438, 1344, 1284, 1257, 1103, 948, 847, 746, 706, 619, 558.
Synthesis of cationic block A-PEI-A, intermediate A (2.0 mmol), NMM (2.0 mmol) and HATU (2.5 mmol) in anhydrous DMF (20 mL) under nitrogen atmosphere were cooled in an ice water bath with stirring for 1 h. PEI (1.0 mmol) was dissolved in anhydrous DMF (5 mL) and injected into the solution, while the reaction mixture was warmed up to room temperature. And then the reaction mixture was stirred for 24 h at room temperature. The resulting mixture was poured into 100 mL of ether, and the precipitate was filtered, washed thrice with ethyl ether and dried under vacuum.
A-PEI1800-A, isolated yield: 88%. 1H NMR (400 MHz, MeOD/CDCl3 = 50:50) δ 8.00 (s, 2H, ArH), 7.63 (s, 4H, –NH(C = O)–), 7.52 (s, 2H, ArH), 7.42–7.33 (m, 25H, ArH), 7.23– 7.30 (m, 25H, ArH), 7.15–7.21 (m, 12H, ArH), 2.87–2.45 (m, 157H, PEI, 2H per repeating unit, –CH2CH2–), 2.35–2.28 (t, J = 7.5 Hz, 9H, –CH2S), 2.23–2.17 (t, J = 7.2 Hz, 8H, –CH2CO–), 1.78–1.66 (m, 8H, –CH2CH2CH2–). IR (KBr, cm−1) 3433, 2963, 2862, 1653, 1546, 1431, 1385, 1270, 1122, 840, 760, 695, 606, 565.
A-PEI1200-A, isolated yield: 90%. 1H NMR (400 MHz, MeOD/CDCl3 = 50:50) δ 8.02 (s, 2H, ArH), 7.64 (s, 4H, –NH(C = O)–), 7.58 (s, 2H, ArH), 7.40 –7.32 (m, 24H, ArH), 7.29–7.17 (m, 23H, ArH), 7.17– 7.08 (m, 12H, ArH), 2.86–2.49 (m, 143H, PEI, 2H per repeating unit, –CH2CH2–), 2.34– 2.26 (t, J = 7.4 Hz, 8H, –CH2S–), 2.26–2.14 (t, J = 7.1 Hz, 8H, –CH2CO–), 1.80–1.68 (m, 8H, –CH2CH2CH2–). IR (KBr, cm−1) 3406, 3936, 2835, 1646, 1552, 1438, 1378, 1264, 1129, 847, 753, 693, 612, 559.
Synthesis of multi-block copolymer PEG2000–PLA3000–PEI–PLA3000–PEG2000, in a typical procedure, B-PLA3000-B (20 mL), A-PEI-A (20 mL) and PEG2000-A (20 mL) were taken from the CH2Cl2 stock solutions (1.0 mM) separately and mixed together in a 250-mL round bottom flask. The solvent was evaporated in vacuo, and the residue was dissolved with 60 mL of iodine solution (6 mM) in CH2Cl2. The resulting mixture was stirred at room temperature for 1 h. After that, the reaction mixture was cooled to 0 °C and a sodium thiosulfate aqueous solution (3 mM) was added until the color of iodine disappeared. The reaction mixture was then extracted with CH2Cl2, the organic extract was washed with brine and dried over anhydrous Na2SO4, filtered and evaporated, and the residue was further washed thrice with ethyl ether/acetone (35/5) and dried under vacuum to give the final product PEG2000–PLA3000–PEI–PLA3000–PEG2000.
PEG2000–PLA3000–PEI1800–PLA3000–PEG2000, isolated yield: 46%, 1H NMR (400 MHz, DMSO-d6), δ 8.91 (s, 8H, ArH), 8.12 (m, 16H, ArH), 5.28–5.07 (s, 25H, 1H per repeating unit, PLA–CH–), 3.62–3.44 (m, 191H, PEG, –CH2CH2–), 3.06–2.66 (m, 100H, PEI, 2H per repeating unit, –CH2CH2–), 1.52–1.37 (m, 75H, 3H per repeating unit, PLA–CH3). IR (KBr, cm−1) 3434, 2959, 1757, 1651, 1526, 1445, 1402, 1300, 1272, 1095, 1035, 935, 796, 706, 606, 537.
PEG2000–PLA3000–PEI1200–PLA3000–PEG2000, isolated yield: 48%, 1H NMR (400 MHz, DMSO-d6), δ 8.76 (s, 8H), 8.04 (d, J = 78.5 Hz, 16H), 5.33–5.00 (s, 25H, 1H per repeating unit, PLA–CH–), 4.13–3.22 (m, 179H, PEG, –CH2CH2–), 3.10–2.58 (m, 64H, PEI, 2H per repeating unit, –CH2CH2–), 1.57–1.33 (s, 74H, 3H per repeating unit, PLA–CH3). IR (KBr, cm−1) 3434, 2926,1757, 1649, 1546, 1452, 1400, 1272, 1095, 1035, 1011, 955, 835, 753, 701, 605.
Preparation and characterization of self-assembled micelles
Briefly, the copolymer PEG2000–PLA3000–PEI–PLA3000–PEG2000 (10 mg) was dissolved in 1.0 mL of DMSO and stirred at room temperature for 30 min. And then, the solution was slowly added to 8.0 mL of deionized water and stirred for another 1 h. After that, the solution was dialyzed against deionized water for 24 h (MWCO = 3500 g mol−1), the deionized water was changed every 4 h for 2 days, and the dialyzate was obtained through a 0.45-μm filter membrane to give a micelle solution. The critical micelle concentration (CMC) was determined using 1,6-diphenylhexa-1,3,5-triene (DPH) as a UV probe by monitoring the absorbance at 313 nm. The concentration of the block copolymer was varied from 0.5 × 10−4 to 0.5 mg mL−1, and the DPH concentration was fixed at 5 × 10−6 M. The absorbance spectra of all solution were recorded using a BioTek Synergy 2.
Preparation of DOX-loaded micelles
In brief, 10 mg of PEG2000–PLA3000–PEI–PLA3000–PEG2000 was in 1.0 mL of DMSO, followed by addition of 1 mg DOX·HCl and 2 equivalents of triethylamine (TEA, purity 99%), and the solution was stirred at room temperature for 1 h. The mixture was added slowly to 8 mL of deionized water within 10 min and stirred for another 1 h. Subsequently, the solution was dialyzed against deionized water for 24 h (MWCO = 2000 g mol−1), and the deionized water was exchanged every 4 h. To determine the total loading of drug, the DOX-loaded micelle solution was lyophilized and then dissolved in DMSO again. The UV absorbance of the solution at 485 nm was measured to determine the total loading of DOX. Drug loading content (DLC) and drug loading efficiency (DLE) were calculated according to the following formula.
In vitro drug release
In brief, DOX-loaded PEG2000–PLA3000–PEI–PLA3000–PEG2000micelles (1 mg mL−1) with treatment of DTT (0 and 1 mM) were immediately measured by fluorescence measurements (BioTek Synergy 2, EX 485 nm, EM 590 nm) at different time intervals, and 2.0 mL of the micelle solution was transferred into a membrane bag [molecular weight cutoff (MWCO = 2000 g mol−1)]. It was immersed into a glass bottle containing 100 mL of PBS (50 mM, pH 7.4) or PBS with 1.0 mM of DTT in a sharking water bath at 37 °C to ensure sink conditions. At predetermined time intervals, 10 mL of external buffer solution was withdrawn and replaced with 10 mL of fresh PBS or PBS with 1.0 mM of DTT. The amount of DOX released was measured by fluorescence measurement and calculated by the standard curve plotted in advance (BioTek Synergy 2, excitation wavelength at 485 nm, emission wavelength at 590 nm (DOX)). All DOX-release experiments were conducted in triplicate, and the results are the average date with standard deviations. The cumulative release Er is calculated according to the following formula:
Er: the total cumulative release % of DOX; Ve: the replacement of PBS volume (10 mL); V0: the total amount of phosphate buffered saline (PBS) volume (100 mL); Ci: DOX concentration of the ith replacement liquid (μg/mL) (determined by fluorescence measurement); Cn: DOX concentration of the last replacement liquid (μg/mL); mdrug: the total amount of DOX in micelle (μg).
Biophysical properties of the polyplexes
The capability of polymers to condense FAM-siRNA (GenePharma Company, Shanghai, China) was studied by gel retardation. FAM-siRNA (20 μM) was dissolved into DEPC water. And then the polymer solution was dropped into the DEPC water containing FAM-siRNA to form various mixtures at different N/P ratios. Polyplexes with various N/P ratios were mixed with 5 × loading buffer and loaded onto the agarose gel (1%). Gel electrophoresis was carried out at room temperature in 1 × tris-acetic/EDTA (TAE) buffer (tris acetate (40 mM), EDTA (1 mM)) at 60 V for 50 min in a Sub-Cell system. Free RNA was used as the control. The UV illuminator (ChemiDoc™ XRS+, Bio-Rad, CA, USA) could be used to visualize the gel and the bands of FAM-siRNA.
In vitro cytotoxicity assay
The SGC7901 cells were firstly floated in the solution of DMEM (Dulbecco’s modified Eagle’s medium) and given the supplement of 10% fetal bovine serum. And then SGC7901 cells was seeded onto 96-well plates at a density of 5000 cells per well in 100 μL of medium to preincubate in a wet circumstance with 5% CO2 at 37 °C for 48 h. The new culture medium which contained different concentrations of polyplexes was added to continue for further culture 48 h. The surviving capability of the SGC7901 cells was performed by MTT assay. Then, 20 μL of 5 mg mL−1 MTT assay stock solution was added to 100 μL 96-well plates for 4-h incubation at 37 °C, and unreacted MTT was removed. The obtained blue formazan crystals were dissolved in 100 μL per well DMSO, and the absorbance was measured at a wavelength of 490 nm using a BioTek Synergy 2.
Cell uptake
To determine the cellular uptake efficiency of the nanoparticle/siRNA (NP/FAM-siRNA) complex, the location and intensity of FAM-siRNA after cellular uptake were observed by fluorescence microscopy. SGC7901 cells (2 × 105 per well) were seeded in six-well plates and cultured for 24 h. Then, the culture medium was replaced with 500 μL DMEM without FBS containing FAM-siRNA-loaded nanomicellar particle (N/P = 15:1) with a FAM-siRNA final concentration of 200 nM, incubated for 0.5 h, 1 h, 2 h at 37 °C under 5% CO2. Cells without polyplex treatment were considered as the control.
For flow cytometric analysis, SGC7901 cells were seeded in six-well plates at 2 × 105 per well and proliferated for 36 h before the experiment. And then the cells in each well were incubated with 200 nM FAM-siRNA formulated in DOX-loaded micelleplexes (N/P = 15:1). And after transfection for 2 h, the cells were washed with PBS and trypsinized, and re-suspended in PBS. The samples were analyzed by using a flow cytometer (Becton–Dickinson, San Jose, CA, USA). Cells without polyplex treatment were considered as the control.
Results and discussion
Synthesis and characterization of pentablock copolymers PEG–PLA–PEI–PLA–PEG
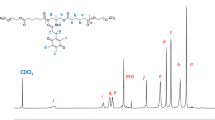
According to reported procedure [10b, 22], we synthesized pentablock copolymer PEG–PLA–PEI–PLA–PEG with double disulfide linkage complemented by double H-bonding sequence specific units efficiently. Briefly, the hydrophilic block PEG-A was synthesized via reaction of carboxyl group in the intermediate A with amino group in PEG-NH2 in the presence of HATU/NMM [22a]. The amine group in the intermediate B reacted with carboxyl group in modified PLA (COOH-PLA-COOH) in the presence of HATU/NMM, forming hydrophobic block B-PLA-B[22a]. And the amine group of PEI reacted with carboxyl group of the intermediate A in the presence of HATU/NMM in DMF solution, forming the modified polymer A-PEI-A[10b]. The desired pentablock copolymer PEG–PLA–PEI–PLA–PEG was synthesized from PEG-A, B-PLA-B as well as A-PEI-A under iodine oxidation conditions (Scheme 2). The intermediates B-PLA-B, PEG-A, A-PEI-A and the terminal pentablock copolymer PEG–PLA–PEI–PLA–PEG were characterized by 1H NMR and GPC. The 1H NMR spectra are shown in Fig. 1, and the typical 1H NMR spectrum of PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 not only reveals the signals of the PEG block (3.62–3.44 ppm from the repeating -OCH2CH2O- unit), the PLA block (repeating LA residues 5.28–5.07 ppm from the -CH- groups, and CH3- groups of the LA units 1.52–1.37 ppm), and the PEI block (3.06–2.66 ppm from the repeating -NCH2CH2NH- unit), but also those of the intermediates A and B amide linking units (-CH2- overlaps in the PEI block about 3.25–1.90 ppm). The dominant signals of the trityl groups in the spectra of PEG2000-A, B-PLA3000-B and PEI1800-A disappeared completely in the spectrum of copolymer PEG2000–PLA3000–PEI1800–PLA3000–PEG2000, suggesting the formation of disulfide cross-linking units indeed occurred. Figure 2 shows the GPC traces of B-PLA3000-B, A-PEI1800-A, PEG2000-A as well as PEG2000–PLA3000–PEI1800–PLA3000–PEG2000. Obviously, the Mn value of PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 increased significantly compared with that of B-PLA3000-B, A-PEI1800-A, PEG2000-A. The Mn and PDI of the polymers are listed in Table 1. The above results are in accordance with the structure of amphiphilic pentablock copolymer PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 and confirmed that the double disulfide linkages indeed formed. The same cross-coupling reaction also occurred with copolymer PEG2000–PLA3000–PEI1200–PLA3000–PEG2000, in which 1H NMR spectra and GPC traces are shown in Figure S1 and Figure S2 in Supporting Information.
Preparation and characterization of nanomicellar particle, DOX and siRNA binding to nanomicellar particle
Amphiphilic pentablock copolymer PEG–PLA–PEI–PLA–PEG can self-assemble into core–shell structured micelles in aqueous solution, driven by the strong hydrophobic/hydrophilic interaction between the linear chains of PLA and the shells of PEI and PEG. The hydrophobic PLA segments assembled as the inner core of the micelles, while the hydrophilic PEI and PEG segments formed the corona owning to their highly hydrophilic nature. Self-assembled blank micelles or DOX-loaded micelles were prepared by dissolving the copolymers (or DOX and the copolymers) in DMSO and dialyzed against deionized water. The CMC of the polymeric micelles was measured by UV/Vis spectroscopy, using DPH as a hydrophobic probe [42]. The absorbance of DPH as a function of the copolymer PEG–PLA–PEI–PLA–PEG concentration in aqueous solution at room temperature is shown in Fig. 3, and the CMC values are listed in Table 2. The CMC values were 0.046 mg mL−1 (Fig. 3) and 0.052 mg mL−1 (Figure S3 in SI) for PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 and PEG2000–PLA3000–PEI1200–PLA3000–PEG2000 micelles, respectively, confirming that the polymeric micelles were highly stable in dilute solution [43, 44]. The DOX loading of the micelles was evaluated by UV analysis, and the results showed that the DLC of PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 and PEG2000–PLA3000–PEI1200–PLA3000–PEG2000 micelles were 4.59% and 2.59%, respectively. (The theoretical DLC was set at 10%). Hence, the copolymer PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 was selected as the model carrier for further evaluation. To further investigate the properties of the polymeric micelles, DLS and TEM were performed. The DLS results show that PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles exhibited unimodal size distribution with a mean diameter of 25 ± 0.3 nm for blank micelles (Fig. 4a) and 37 ± 0.3 nm for DOX/siRNA-loaded micelles (Fig. 4b). Compared with that of triblock copolymer PEG2000–PLA5000–PEI1800[10b], the pentablock copolymer micelles have low CMC, which indicated the micelles are relatively stable under dilute solution (Fig. 4 and Table 2). The micelles were spherical with an average size of 22 nm for blank micelles (Fig. 4e) and 37 nm for DOX/siRNA-loaded micelles (Fig. 4f) observed by TEM. High-resolution TEM images revealed the morphology of the micelles more clearly. The size of polymeric micelles is an important parameter for intracellular drug delivery, and small size (< 200 nm) of nanomicellar particle is in favor of maintaining a lower level of the reticuloendothelial system (RES) uptake and minimal renal excretion [45, 46]. Hence, the polymeric micelles of PEG–PLA–PEI–PLA–PEG can be a promising siRNA delivery complex for cancer therapy, relying on the enhanced permeation retention effect (EPR) for passive tumor targeting.
Characterization of PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles. Size distribution of a blank micelles and b DOX and siRNA (15:1)-loaded micelles. Zeta potential of c blank micelles and d DOX and siRNA (15:1)-loaded micelles. e TEM image of blank micelles; (inset) high-resolution TEM image. f TEM image of DOX and siRNA (15:1)-loaded micelles (N/P = 15); (inset) high-resolution TEM image
Cationic nature of the nanomicellar particles was also determined by zeta potential measurements. PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles were positively charged with a zeta potential of 54.7 ± 1.4 mV (Fig. 4c) due to the presence of protonized amino groups from PEI block. When DOX and siRNA (N/P = 15:1) were bound to the nanomicellar particles, the average particle size increased to 37 ± 0.3 nm (Fig. 4b), and the zeta potential was 34.7 ± 1.7 mV (Fig. 4d).
Redox responsiveness and stimuli-triggered drug release and siRNA release
The disulfide bonds incorporated into copolymer micelles are responsive to reducing agents [47]. Here, we investigated the in vitro release of the encapsulated DOX from PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles under reductive environment. The release behavior was examined by dialyzing the micelles in 0 mM and 1.0 mM DTT solution at 37 °C, respectively. The cumulative drug release from PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles was 29% within 20 h in the absence of DTT. In contrast, the DOX release from the micelles increased to 63% in the presence of 1.0 mM DTT within the same period (Fig. 5). These results demonstrate that the disulfide linkage was readily cleaved by DTT, breaking the core–shell structure and accelerating the release of the encapsulated DOX.
Some researchers found that there exists fourfold higher level of GSH in tumor tissues compared with normal tissues in tumor-bearing mice [48, 49]. The disulfide bonds in PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles can be reduced in the cytosol due to the reductive environment provided by intracellular glutathione. Therefore, we investigated the redox-responsive siRNA release behavior of the siRNA-loaded PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles by agarose gel electrophoresis.
As shown in Fig. 6a, only a small amount of siRNA was released into the gel from PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles in the absence of GSH. When the N/P ratio ≥ 15:1, most of the siRNA was retained in the wells by complexation with PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 nanomicellar particle. In Fig. 6b, most of siRNA was released from the particles in the presence of 10 mM GSH solution. However, when the N/P ratio was 20:1 and 25:1, few siRNA molecules were released into the gel. The above results show that PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 nanomicellars particle was able to inhibit siRNA migration at an N/P ratio of 15. At the same time in redox environment, disulfide bonds in the polyplexes can be destroyed and the most of siRNA can be released at the N/P ratio of 15.
In vitro cytotoxicity
As potential drug delivery and gene carrier materials, the cytotoxicity of nanomicellars particles is a key parameter for their biomedical applications [50, 51]. Cytotoxicity of PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles and siRNA/DOX-loaded PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 in vitro was evaluated by MTT assay against SGC7901 gastric cancer cell lines. Figure 7 shows the cell viability after 24-h incubation with blank micelles or siRNA/DOX-loaded micelles at different concentrations. As shown in Fig. 7a, the cell viability remained at above 90%, indicating that the micelles have low cytotoxicity and good biocompatibility. In comparison, the viability of the cells treated with the DOX/siRNA-loaded micelles significantly decreased, indicating that the drug released from the PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles enhances proliferation inhibition of SGC7901 cells.
PEG 2000 –PLA 3000 –PEI 1800 –PLA 3000 –PEG 2000 micelles co-deliver siRNA/DOX into SGC7901 gastric cancer cells
To demonstrate that the DOX/FAM-siRNA can be delivered into SGC7901 cells simultaneously by PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles, we analyzed the cellular uptake and intracellular distribution of DOX/FAM-siRNA-loaded micelles in SGC7901 cells by fluorescence microscopy. FAM-labeled siRNA was aimed at fluorescence detection of the siRNA. Cells were incubated with DOX/FAM-siRNA-loaded micelles for 0.5 h, 1 h and 2 h, respectively. As shown in Fig. 8a, a high degree of co-localization of the red and green fluorescence signals was observed, revealing the similar distribution of DOX and siRNA in the cytoplasm.
Intracellular uptake and distribution of DOX/siRNA-loaded micelles in SGC7901cells. a SGC7901 cells were incubated with DOX/siRNA-loaded micelles for different time periods. The DOX (red) and FAM-siRNA (green) were imaged using a fluorescence microscope. b Fluorescence-activated cell storing analysis of SGC7901 cells incubated with DOX/siRNA-loaded micelles for 2 h. The cells were non-pretreated as control (color figure online)
Flow cytometry was used to investigate the cell uptake efficiency of PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 micelles. To observe the co-delivery of siRNA and DOX, we performed cell fluorescence for SGC7901 cells at 2 h after treatment with DOX/siRNA-loaded micelles as indicated in Fig. 8b. Fluorescence-activated cell storing analysis showed that most of the cells were located in the double-positive quadrant after 2-h incubation. This indicated that siRNA and DOX can be simultaneously delivered into the cells by the micelles.
Conclusion
Amphiphilic pentablock copolymer PEG2000–PLA3000–PEI–PLA3000–PEG2000 micelles with H-bonding instructed double disulfide linkage have been synthesized successively. The redox-responsive copolymer PEG2000–PLA3000–PEI–PLA3000–PEG2000 has low CMC. In the reductive environment provided by intracellular glutathione, the disulfide bonds were cleaved, which triggered the release of siRNA and drug. In vitro cell viability evaluation confirmed that the PEG2000–PLA3000–PEI1800–PLA3000–PEG2000 blank micelles have good cytocompatibility and the DOX/siRNA-loaded micelles are able to effectively inhibit the proliferation of gastric cancer cells. And in vitro assay study shows that nanomicellar particle could simultaneously deliver siRNA and drug into gastric cancer cells. These results indicate that the amphiphilic pentablock copolymer PEG2000–PLA3000–PEI–PLA3000–PEG2000 micelles provide a platform for co-delivery of hydrophobic drug and siRNA for cancer therapy.
References
Kakizawa Y, Kataoka K (2002) Block copolymer micelles for delivery of gene and related compounds. Adv Drug Deliv Rev 54:203–222
Zhang ZK, Ma RJ, Shi LQ (2014) Cooperative macromolecular self-assembly toward polymeric assemblies with multiple and bioactive functions. Acc Chem Res 47:1426–1437
Lv J, Hao XF, Yang J, Feng YK, Behl M, Lendlein A (2014) Self-assembly of polyethylenimine-modified biodegradable complex micelles as gene transfer vector for proliferation of endothelial cells. Macromol Chem Phys 215:2463–2472
Sun TM, Du JZ, Yan LF, Mao HQ, Wang J (2008) Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials 29:4348–4355
Rosler A, Vandermeulen GW, Klok HA (2012) Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv Drug Del Rev 64:270–279
Huo H, Gao YK, Wang TY, Jiang HT, Wang SL, Jiang TY (2012) The investigation on polyion complex micelles composed of diammonium glycyrrhizinate/poly(ethylene glycol)-glycidyltrimethylammonium chloride-grafted polyasparthydrazide. AAPS PharmSciTech 13:1367–1376
Petersen H, Petra MF, Alison LM, Kunath K, Stolnik S, Clive JR, Fischer D, Davies MC, Kissel T (2002) Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjugate Chem 13:845–854
Zhang Z, Yang C, Duan Y, Wang Y, Liu J, Wang L, Kong D (2010) Poly(ethylene glycol) analogs grafted with low molecular weight poly(ethylene imine) as non-viral gene vectors. Acta Biomater 6:2650–2657
Nouri N, Talebi M, Abas AP (2012) Viral and nonviral delivery systems for gene delivery. Adv Biomed Res 1:27
Schaffert D, Wagner E (2008) Gene therapy progress and prospects: synthetic polymer-based systems. Gene Ther 15:1131–1138
Burke PA, Pun SH, Reineke TM (2013) Advancing polymeric delivery systems amidst a nucleic acid therapy renaissance. ACS Macro Lett 2:928–934
Park TG, Ji HJ, Kim SW (2006) Current status of polymeric gene delivery systems. Adv Drug Deliv Rev 58:467–486
Gohy JF, Willet N, Varshney S, Zhang JXZ, Jerome R (2001) Core-shell-corona micelles with a responsive shell. Angew Chem Int Ed 40:3214–3216
He C, Zhang Z, Yang Q, Chang Q, Shao Z, Gong B, Shen YM, Liu B, Zhu Z (2016) Reductive triblock copolymer micelles with a dynamic covalent linkage deliver antimiR-21 for gastric cancer therapy. Polym Chem 7:4352–4366
Xiao RZ, Zeng ZW, Zhou GL, Wang JJ, Li FZ, Wang AM (2010) Recent advances in PEG–PLA block copolymer nanoparticles. Int J Nanomed 5:1057–1065
Prevette LE, Lynch ML, Reineke TM (2010) Amide spacing influences pDNA binding of poly(amidoamine)s. Biomacromolecules 11:326–332
Mary XT, Redemann CT, Szoka FC (1996) In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjugate Chem 7:703–714
Wong SY, Pelet JM, Putnam D (2007) Polymer systems for gene delivery-past, present, and future. Prog Polym Sci 32:799–837
Pandey AP, Sawant KK (2016) Polyethylenimine: a versatile, multifunctional non-viral vector for nucleic acid delivery. Mater Sci Eng C 68:904–918
Mintzer MA, Simanek EE (2009) Nonviral vectors for gene delivery. Chem Rev 109:259–302
Godbey WT, Wu KK, Mikos AG (1999) Size matters: molecular weight affects the efficiency of poly(ethylenimine) as a gene delivery vehicle. J Biomed Mater Res 45:268–275
Fischer D, Bieber T, Li Y, Elsässer HP, Kissel T (1999) A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res 16:1273–1279
Ogris M, Steinlein P, Kursa M, Mechtler K, Kircheis R, Wagner E (1998) The size of DNA/transferrin-PEI complexes is an important factor for gene expression in cultured cells. Gene Ther 5:1425–1433
Thomas M, Klibanov AM (2002) Proc Natl Acad Sci USA 99:14640–14645
Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T (2003) In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials 24:1121–1131
Lv P, Zhou C, Zhao Y, Liao X, Yang B (2017) Modified-epsilon-polylysine-grafted-PEI-β-cyclodextrin supramolecular carrier for gene delivery. Carbohydr Polym 168:103–111
Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A (2005) A two stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol Ther 11:990–995
Kafil V, Omidi Y (2011) Cytotoxic impacts of linear and branched polyethylenimine nanostructures in a431 cells. Bioimpacts 1:23–30
Fitzsimmons REB, Uludag H (2012) Specific effects of PEGylation on gene delivery efficacy of polyethylenimine: interplay between PEG substitution and N/P ratio. Acta Biomater 8:3941–3955
Lee M, Kim SW (2005) Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res 22:1–10
Xu L, Anchordoquy T (2011) Drug delivery trends in clinical trials and translational medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. J Pharm Sci 100:38–52
Wang W, Balk M, Deng Z, Wischke C, Gossen M, Behl M, Ma N, Lendlein A (2016) Engineering biodegradable micelles of polyethylenimine-based amphiphilic block copolymers for efficient DNA and siRNA delivery. J Control Release 242:71–79
Wang Y, Gao S, Ye WH, Yoon HS, Yang YY (2006) Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater 5:791–796
Wang Y, Wang LS, Goh SH, Yang YY (2007) Synthesis and characterization of cationic micelles self-assembled from a biodegradable copolymer for gene delivery. Biomacromolecules 8:1028–1037
Wang Y, Ke CY, Beh CW, Liu SQ, Goh SH, Yang YY (2007) The self-assembly of biodegradable cationic polymer micelles as vectors for gene transfection. Biomaterials 28:5358–5368
Yang Q, Bai L, Zhang Y, Zhu F, Xu Y, Shao Z, Shen YM, Gong B (2014) Dynamic covalent diblock copolymers: instructed coupling, micellation and redox responsiveness. Macromolecules 47:7431–7441
Yang Q, Tan L, He C, Liu B, Xu Y, Zhu Z, Shao Z, Gong B, Shen YM (2015) Redox-responsive micelles self-assembled from dynamic covalent block copolymers for intracellular drug delivery. Acta Biomater 17:193–200
Hu W, He C, Tan L, Liu B, Zhu Z, Gong B, Shen YM, Shao Z (2016) Synthesis and micellization of redox-responsive dynamic covalent multi-block copolymers. Polym Chem 7:3145–3155
Yang Q, He C, Zhang Z, Tan L, Liu B, Zhu Z, Shao Z, Gong B, Shen Y (2016) Redox-responsive flower-like micelles of poly(l-lactic acid)-b-poly(ethylene glycol)-b-poly(l-lactic acid) for intracellular drug delivery. Polymer 90:351–362
Wang X, He C, Yang Q, Tan L, Liu B, Zhu Z, Gong B, Shen Y (2017) Dynamic covalent linked triblock copolymer micelles for glutathione-mediated intracellular drug delivery. Mater Sci Eng C 77:34–44
Tian Z, Huang R, Tan L (2016) Amphiphilic drug–drug assembly via dual responsive linkages for small-molecule anticancer drug delivery. RSC Adv 6:66420–66430
Jin Y, Song L, Su Y, Zhu LJ, Pang Y, Qiu F (2011) Oxime linkage: a robust tool for the design of pH-sensitive polymeric drug carriers. Biomacromolecules 12:3460–3468
Lukyanov AN, Torchilin VP (2004) Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev 56:1273–1289
Adams ML, Lavasanifar A, Kwon GS (2003) Amphiphilic block copolymers for drug delivery. J Pharm Sci 92:1343–1355
Schmalenberg KE, Frauchiger L, Nikkhouyalbers AL, Uhrich KE (2001) Cytotoxicity of a unimolecular polymeric micelle and its degradation products. Biomacromolecules 2:851–855
Chow EK, Ho D (2013) Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med 5:214–216
Saito G, Swanson JA, Lee K-D (2003) Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev 55:199–215
Ilangovan G, Li H, Zweier JL, Kuppusamy P (2002) In vivo measurement of tumor redox environment using EPR spectroscopy. Mol Cell Biochem 234–235:393–398
Kuppusamy P, Li H, Ilangovan G, Cardounel AJ, Zweier JL, Yamada K, Krishna MC, Mitchell JB (2002) Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res 62:307–312
Gary DJ, Puri N, Won YY (2007) Polymer-based siRNA delivery: perspectives on the fundamental and phenomenological distinctions from polymer-based DNA delivery. J Control Release 121:64–73
Koo AN, Lee HJ, Kim SE, Chang JH, Park C, Kim C (2008) Disulfide-cross-linked PEG-poly (amino acids) copolymer micelles for glutathione-mediated intracellular drug delivery. Chem Commun 48:6570–6572
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81671802) and the SJTU Biomedical Engineering Joint Project (YG2017QN55).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, B., Tan, L., He, C. et al. Redox-responsive micelles self-assembled from multi-block copolymer for co-delivery of siRNA and hydrophobic anticancer drug. Polym. Bull. 76, 4237–4257 (2019). https://doi.org/10.1007/s00289-018-2600-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2600-y