Abstract
In the present work, amoxicillin drug-loaded films of poly (vinyl alcohol)-g-poly (acrylamide) of varying compositions were prepared by graft copolymerization method. The as-prepared drug-loaded grafted hydrogels were characterized by various analytical techniques such as Fourier transform infrared spectroscopy, differential scanning calorimetry, scanning electron microscopy, and X-ray diffraction analysis, respectively. The grafted hydrogels were investigated for their water intake behavior under varying experimental conditions of their chemical composition, pH and temperature of the swelling bath, and simulated biological fluids. The water sorption data of hydrogels were used to calculate network parameters of the grafted hydrogel such as average molecular weight between crosslinks (Mc), crosslink density (Ve), number of elastically effective chains (qe), swelling exponent (n), and diffusion constant (D), respectively. The swelling controlled drug release behavior of amoxicillin loaded hydrogel was investigated under in vitro conditions, and the influence of various factors such as chemical composition of grafted hydrogel, percent loading of drug (amoxicillin), pH and temperature of the release media was studied on the release profiles of the drug. The amoxicillin loaded grafted hydrogels were also examined for their antibacterial activity against gram-negative bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ongoing advancements in materials science have greatly benefited biomedical fields and medicine by providing unique kind of implantable materials to treat complex diseases and other physiological disorders through surgery and drug delivery [1]. In spite of the emergence of new and novel materials, the microbial infections due to bacteria and fungi have posed a serious threat to patients undergoing surgical or other health-care treatments like implantation and wound healing. [2]. The infection can either prolong the healing response of wound or lead to implant failure. Thus, the control of infection at material-tissue interfaces necessitates exploring new strategies either by augmenting biocompatibility of the implant or delivering antibiotic drugs at the infected site [3]. One of the effective methods of suppressing infections includes delivery of antibiotic drugs at the implanted organ, diseased tissue, or device insertion site. An appropriate drug can be impregnated into the material and may be delivered desirably over the times [4].
Hydrogels are versatile and most suitable materials for controlled delivery of antibiotic drugs and other biomedical applications [5]. These are 3D materials having strong tendency to accommodate water and other bioactive molecules into their internal molecular structure. The water sorption tendency of hydrogels greatly depends on internal structural parameters such as molecular weight between crosslinks, crosslink density, elasticity of polymer network, and presence of hydrophilic functional groups. [6].
PVA is a biocompatible and nontoxic water-soluble synthetic polymer [7]. It absorbs water, swells easily and has been extensively used in controlled drug delivery applications [8]. This polymer has a number of interesting properties like, apparent adiabatic compressibility in solution, critical surface tension, solubility in water, and ethylene glycol. The mechanical strength and biocompatibility of PVA account for its inclusion in a number of other polymer matrices to yield variety of materials finding numerous biomedical applications [9,10,11].
There are a variety of polymer hydrogels which have been frequently used in different biomedical applications. For instance, Singh and co-workers synthesized carbopol-crosslinked poly (2-hydroxyethyl methacrylate) hydrogel and studied the release behavior of amoxifloxacin [12]. In another study by Alvarase et al. [13], electrospun nanofibers based on PVA and chitosan were prepared and loaded with tetracycline drug. The as-prepared device was investigated for controlled release of drug and suggested for wound-healing applications. Manju et al. [14] reported fabrication of hydrogel based on PVA and poly (3-aminophenylboronic acid) for ciprofloxacin loading and suggested that the drug-loaded hydrogel could be a good candidate to treat wound healing in diabetic patients. Posadowska et al. fabricated an injectable drug delivery system, which consists of GENloaded poly(lactide-co-glycolide) (PLGA) NPs embedded in the gellan gum hydrogel. The system was suitable for injection and was antibacterially active against Staphylococcus saprophyticus without affecting the bone-forming cells [15]. The photo-crosslinked methacrylated dextran and poly (l-glutamic acid)-graft-hydroxyethylmethacrylate (PGA-g-HEMA) hydrogels were studied [16] and both exhibited excellent antibacterial properties and desirable release capabilities.
Polyacrylamide (PAM) is a known hydrophilic polymer and has been extensively investigated due to ease of polymerizability, ability to get copolymerized with other monomers, and grafted onto a variety of naturally occurring and synthetic polymers. The major applications of polyacrylamide and its copolymers include gel electrophoresis, artificial implants, drug delivery systems, etc. [17,18,19]. Thus, looking to the vast biomedical and technological utility of PVA and PAM, both of these polymers have been chosen to fabricate PAV-g-PAM matrices intended for delivery of antibiotic drug amoxicillin [20, 21].
Amoxicillin, also called as amino penicillin, is a semisynthetic drug belonging to Penicillin family (Fig. 1) [22, 23]. This drug is well proven and recognized in terms of its potential in fighting against a variety of gram-positive and gram-negative bacteria in both human and animals. Amoxicillin does not have a strong affinity for binding with proteins, and its elimination half life in renal patients has been reported to lay from 0.7 to 1.4 h showing rapid clearance through the urine [24]. Thus, the major objectives of the present work include preparing PVA-g-PAM hydrogels and encapsulating amoxicillin drug into the hydrogel matrix for designing a swelling controlled drug delivery system.
Experimental
Materials
Polyvinyl alcohol (PVA) (Mol. wt. 30,000 Da, degree of hydrolysis 98%) was obtained from Merck India, Mumbai and used without purification. Acrylamide (Merck India, Mumbai) was recrystallized twice in methanol to make it free from the inhibitor and stored in a cool place. N,N′-methylene-bis-acrylamide (MBA) was used as a crosslinking agent of acrylamide and obtained from Sigma-Aldrich. Potassium metabisulfite (activator) and potassium persulfate (initiator) were obtained from Merck India, Mumbai. The amoxicillin drug in capsule form was used for this study and purchased from retail pharmacy shop (Cipla, Mumbai, India). Distilled water was used for the preparing of solutions. The simulated gastric fluid (SGF, pH 1.2) and PBS solution (pH 7.4) were prepared for conducting drug delivery experiments. The simulated gastric fluid was used in the present study. This simulated fluid was obtained by dissolving 3.2 g of purified pepsin (derived from porcine stomach mucosa, with an activity of 800–2500 units per mg of protein), and 2.0 g of sodium chloride in 7.0 mL of hydrochloric acid and making up with water to 1 L. The pH of this solution was found to be 1.2.
Methods
Preparation of grafted hydrogel
The PVA-g-PAM hydrogels of varying compositions were prepared following a redox polymerization procedure. In a typical experiment, into a 25 mL of 8% w/v solution of PVA, 1.0 g of acrylamide, 10 mg of MBA and 1 mL each of potassium metabisulfite (0.01 M) and potassium persulfate (0.001 M) was added and the reaction mixture was thoroughly homogenized. The solution was then kept in a Petri dish (dimension 2 inch, corning) and placed at room temperature (35 °C) for four days. The whole reaction mixture converted into thick white slab indicating the completion of polymerization reaction and formation of hydrogel. The grafted hydrogel film was peeled off and freed from the impurities and unreacted chemicals by allowing the hydrogel to swell in the water bath for 1 week to attain equilibrium swelling. During equilibrium swelling process, along with impurities and unreacted chemicals, ungrafted PVA also leaches out since native PVA is fully soluble in water. Now the grafted hydrogel was dried at room temperature for a week, and its weight was recorded which accounts for the weights of both the grafted polymer and homopolymer (PAM). Now in order to remove the homopolymer, the grafted copolymer was thoroughly washed with acetone, dried for a week and its weight was recorded which represents the weight of the PVA-g-PAM (excluding homopolymer). Similar procedures were followed to prepare grafted hydrogels of different compositions as summarized in Table 1. The purified grafted hydrogel film was dried at room temperature for a week, cut into square pieces and stored in air-tight polyethylene bags for subsequent studies. The thickness of the hydrogel was found to be 0.048 cm.
The grafting percentage (GP) and grafting efficiency (GE) were calculated using the following equations,
Here, the weight of substrate in Eq. (2) implies for that of PVA. The product obtained was a graft copolymer with 62.8% grafting efficiency and 76.4% grafting percentage. The digital images of dry and swollen PVA-g-PAM films are shown in Fig. 2 which provides clear evidences for the formation and swelling of PVA-g-PAM hydrogel.
Characterization of grafted hydrogel
The grafted hydrogels prepared as above were characterized by techniques such as Fourier transform infrared (FTIR) spectroscopy, differential scanning calorimetry (DSC), scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis as described below:
FTIR analysis
The FTIR analysis enables to confirm the presence of functional groups present in the PVA-g-PAM grafted hydrogel. The FTIR spectral analysis was carried out by scanning a thin film of the hydrogel in the range 4000–450 cm−1 on a FTIR Spectrophotometer (Shimadzu, Japan). In all, total 25 cumulative scans were captured with a resolution of 2 cm−1. For this purpose, a thin film of grafted hydrogel was cast and mounted on the window of the spectrophotometer.
Differential scanning calorimetry
A 10-mg piece of thin grafted film was put into aluminum pans, and DSC measurements were recorded on a DSC instrument (21000, DuPont) in the temperature range 250–400 °C under nitrogen atmosphere and at a heating rate of 10 °C min−1.
Scanning electron microscopy
Morphological features of amoxicillin loaded and unloaded grafted hydrogel were examined using a scanning electron microscope equipped with a field-emission gun, after the samples were vacuum-coated with thin layers of gold to minimize the charging effect. The external morphology and chemical composition of the samples were examined using a JEOL JSM-5600 field emission scanning electron microscope (SEM) equipped with a Phoenix EDX energy dispersive X-ray spectrometer (EDX) to determine the elemental contents of the synthesized material.
X-ray diffraction spectroscopy
The X-ray diffraction studies of native and grafted hydrogel were carried out on D8 Advance X-ray diffractometer. The native and grafted hydrogel films were fixed on an aluminum holder which was then mounted on the rotating stage of the diffractometer. The diffraction data were collected from 10° to 60°, 2-theta values with a step size of 0.020 and counting times of 2 s/steps.
In order to determine the nature of amorphous and crystalline properties of the pure and PVA-g-PAM hydrogel, the degree of crystallinity was also calculated by Eq. (3), where Ac, area of crystalline; and Aa, amorphous phases.
Swelling measurements
The dried and pre-weighed samples of grafted copolymer films were soaked in different pH solutions (1.8, 7.4 and 8.6 pH), simulated biological fluids (glucose, artificial urine, KI-solution and urea) and at various temperatures ranging from 10 to 60 °C for different time periods. After swelling, the hydrogel samples were taken out and pressed gently in between two filter papers to remove excess of water adhered on the hydrogel surfaces and weighed again. The swelling ratio was calculated by the following equation,
where Wd and Ws are the masses of dry and wet hydrogels, respectively.
Network parameters of hydrogels
The swelling property of a hydrogel is intimately dependent on its internal structure which is quantitatively designated by the network parameters such as average molecular weight, Mc, defined as the average molar mass present between the two crosslinks in the crosslinked network of the hydrogel, crosslinked density q and number of elastically effective chains Ve, respectively. The value of Mc has great practical relevance, and it is one of the factors that decide the mechanical and rheological behavior of the polymer materials. The application of Flory and Rehner theory of swelling of network helps to determine evaluation of Mc using the following relationship [25],
here Mc represents the average molar mass present between the crosslinks, V1 is the molar volume of water (mL/mole), dp is the polymer density (g/mL), Vs is the volume fraction of the polymer in the swollen gel, χ is the Flory–Huggins’s interaction parameter between the solvent and the polymer. The value of V1 was 18, and the Flory–Huggins’s interaction constant χ was taken from the literature [26].
As an approximation, the volume fraction Vs is taken as reciprocal of the swelling ratio. The crosslink density q is calculated by the following equation:
Here, Mo represents the molar mass of the repeat unit. Some workers, however, have defined another characteristic parameter, Ve, defined as the number of elastically effective chains which may be calculated by the following relationship,
The value of V1 and χ are adopted from the literature and NA is the Avogadro number.
Drug activity
In order to demonstrate that the biological and chemical activity of the entrapped amoxicillin was not lost during the device preparation, following two tests were performed.
UV spectral study
For determining the structural stability of amoxicillin drug, solutions of known concentration of amoxicillin were prepared and their UV spectra (Shimadzu, 1800) were recorded at different pH and time periods. The obtained UV spectra were compared with those of the drug released from the hydrogels.
Drug loading on to the hydrogel
The amoxicillin drug was loaded onto the PVA-g-PAM hydrogel following an equilibrium swelling method. In a typical experiment, a pre-weighed piece of hydrogel was allowed to swell in a drug solution of known concentration for 72 h so that the hydrogel was fully loaded with the amoxicillin solution. The swollen piece of hydrogel was taken out, washed with distilled water and dried at room temperature for a week. The following equation was used to calculate percent (%) loading.
where Wd and Wo are the dry weights of drug-loaded and unloaded grafted hydrogel pieces, respectively.
In vitro release experiments
In order to determine the amount of released drug, the amoxicillin loaded hydrogels were gently shaken in the release medium (PBS, 7.4), and gastric fluid (pH 2.4) for predetermined timer period. The hydrogels were taken out after definite time period and the amount of drug remaining in the solution was quantified spectrophotometrically (UV–VIS Shimadzu, Japan) by measuring the absorbance of the solution at definite wavelength of 234 nm (λmax).
Antibacterial study
The antibacterial activities of drug-loaded grafted hydrogel was determined against Escherichia coli by agar well diffusion method. Briefly, pure isolate of each bacterium was first sub-cultured in nutrient broth at 37 °C for 24 h. One hundred microliters of the standardized inoculum (106 CFU/mL; 0.5 Mac-Farland) of each test bacterium was spread with the help of sterile spreader on to a sterile Mueller–Hinton Agar plate Media, to achieve a confluent growth. The plates were allowed to dry, and a sterile cork borer of diameter 6.0 mm was used to bore wells in the agar plates. Furthermore, a 50 μL volume of the oil was introduced in triplicate wells into Mueller–Hinton Agar plate. Sterile DMSO served as negative control. A positive control in the form of amoxicillin (10 mg/mL) was also included in the study. The plates were allowed to stand for at least 1 h for diffusion to take place and then incubated at 37 °C for 24 h. The zone of inhibition was recorded to the nearest size in mm. Test microorganisms used were E. coli, and different concentrations (2.5% (V/V), 5% (V/V), 15% (V/V) and 25% (V/V) of each drug solutions were made in distilled water and the zone of inhibition was calculated for each concentration.
Results and discussion
Mechanism of grafted hydrogel formation
The mechanism of formation of grafted hydrogel of PVA and N,N′-methylene bisacrylamide—crosslinked poly (acrylamide) is shown in Fig. 3. According to this mechanism, metabisulfite and persulfate act as activator and initiator, respectively, thus constituting a redox system for graft copolymerization of acrylamide onto the PVA backbone. The mechanism reveals that the bisulfite and persulfate ions react together to form bisulfite and sulfate ion radicals which further abstract a hydrogen atom from the methylene groups of PVA creating a PVA macroradical. The primary free radicals also react with acrylamide molecules and crosslinker (MBA) to produce crosslinked macroradicals of poly (acrylamide) which further reacts with PVA macroradicals to form PVA-g-poly (acrylamide) molecules.
FTIR spectra
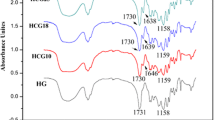
The FTIR spectra of native PVA, native grafted hydrogel, and drug-loaded grafted hydrogel are depicted in Fig. 4a–c, respectively. The presence of specific functional groups and existing intermolecular interactions in the grafted hydrogel was confirmed by recording transmission IR spectra of thin films of native PVA and PVA-g-PAM. A broad band that appears around 3348 cm−1 in all the spectra may be attributed to the O–H stretching vibrations of the hydroxyl group of PVA. A sharp band at 1094 cm−1 corresponds to the symmetrical stretching of C–O–C group of un-hydrolyzed poly (vinyl acetate) present along the PVA backbone. The asymmetric N–H stretching vibration of the primary amide groups of poly (acrylamide) overlaps with the stretching vibrations of O–H groups of PVA.
In the spectra (b), a broad band appears at 3485 cm−1 which suggests that the hydrogel is hydrophilic in nature and contains hydrogen bonded water molecules. The spectra also confirm that OH and CH2 groups are also present as evident from the peaks observed 3439 cm−1 in the spectra (a) and at 3424 cm−1 in spectra (c), respectively.
Furthermore, the peaks appeared in the range 3000–2900 cm−1 reveal the presence of CH2 groups in both the native and drug-loaded hydrogels. The presence of C=O group in the native grafted hydrogel is also evident from the band observed at 1698 cm−1. It is worth to mention here the intensity of the C=O band in the spectra (b) of the hydrogel is quite intense in comparison to that of the spectra (a) of native PVA film which may be attributed to the fact that many of the hydroxyl groups of PVA are oxidized to carbonyl groups by the free radicals thus confirming the graft copolymer nature of the hydrogel.
Moreover, the broadness of the spectra (c) suggests for hydrogen bonding between the two hydrophilic polymers (PVA AND PAM), and the appearance of prominent bands at 3439 cm−1 (O–H stretching), 3424 cm−1 (N–H stretching) and 1698 cm−1 (C–O stretching) indicates the presence of hydroxyl and amide groups in the grafted hydrogel.
The aliphatic C–H stretching vibrations appear at 2923 cm−1. A strong band at 1659 cm−1 may be assigned to the stretching of carbonyl (C=O) groups in the carboxamide functional groups of the PVA-g-PAM hydrogel. The presence of strong band at 1616 cm−1 may be attributed to the asymmetric bending of N–H groups. From the FTIR spectral results, it can be concluded that during formation of PVA-g-PAM hydrogel, the hydrogen bonding interactions exist.
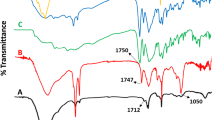
In the (FTIR) spectrum of the amoxicillin loaded grafted hydrogel, the bands at 3456 cm−1 due to υ(N–H) primary amine stretching vibration, and 3525 cm−1 for –OH stretching confirm the presence of PVA and PAM, while the peaks observed at 3176 cm−1 (amide N–H) and phenol (OH stretch) confirm that of the amoxicillin in the grafted hydrogel. Furthermore, the bands at 3039 cm−1 due to υ(C–H) stretching vibration of benzene ring, 1774 cm−1 to υ (C=O) β-Lactam group, 1452 cm−1υ(N–H) to combination of C–N stretch and (NH3+) symmetric deformation also confirm the entrapment of drug within the grafted polymer matrix. The bands at 1585 cm−1, and 1396 cm−1 may also be assigned to stretching vibrations, υ(COO–) asymmetric and symmetric stretching vibrations, respectively. The bands at 1519 cm−1, 3039 cm−1, 1178 cm−1, and 2970 cm−1 may be attributed to υ(C=C) aromatic, υ(C–H) aromatic, υ(C–C) aliphatic, and υ(C–C) stretching vibrations, respectively. The band at 1282 cm−1 is assigned to υ(C–N) cm−1 stretching vibration whereas that at 1249 cm−1 may be attributed to υ(C–O) stretching vibration amoxicillin molecule. Moreover, the band at 557 cm−1 may also be due to υ(C–S) stretching vibration of the amoxicillin drug.
DSC study
The thermal parameters of PVA-g-PAM and native PVA films were also investigated using the DSC technique, and thermal properties of polymers like the Tg and Tm were evaluated. The DSC curves are shown in Fig. 5 which represent the thermal behavior of native PVA and PVA-g-PAM, respectively. The curve (a) shows a broad endotherm that represents a transition from the glassy to rubbery state beginning at 60 °C and ending at 160 °C with maxima at 110 °C that may be regarded as the glass transition temperature (Tg) of the native PVA. The curve (a) also shows a sharp endotherm at 210 °C which represents crystalline melting temperature of the native PVA as reported in the literature also [27]. The presence of both the Tg and Tm confirms the semicrystalline nature of the native PVA film. The DSC curve of grafted hydrogel (PVA–PAM) is shown in Fig. 5 which reveals a broad endotherm representing transition of the grafted hydrogel from glassy state to rubbery state showing a significant shift to higher temperature. The Tg of the grafted hydrogel was observed at 140 °C which is much greater than that of the native PVA obtained at 110 °C. The observed increase in glass transition temperature (Tg) may be attributed to the fact that due to grafting of PAM chains on to the PVA backbone, the mobility of the macromolecular chains of PVA is restrained which consequently requires higher temperature for producing relaxation of the macromolecular chains thus resulting in a higher value of Tg. In a study by Lu et al. [28], solid-state graft copolymerization of PAM was carried out onto PVA and the thermal stability of the grafted polymer was evaluated using thermogravimetric analysis (TGA) technique. The authors also noticed an enhanced thermal stability of grafted polymer which was attributed to greater extent of hydrogen bonding in the grafted copolymer as compared to native PVA. Similar types of results were also reported by other workers [29].
The crystalline melting temperature (Tm) of the grafted hydrogel was found to be slightly greater than that of the native PVA and was found to be at 220 °C. The higher melting temperature of the grafted hydrogel may be explained by the fact that due to grafting of PAM chains onto the PVA, the intermolecular forces between the macromolecular chains of the PVA and PAM become stronger and demand greater thermal energy for transforming into liquid state. Another remarkable feature of the crystalline melting peak of the grafted hydrogel is that it is quite small and thus suggests for reduced crystallinity of the hydrogel in comparison with the native PVA. The reduced crystalline nature of the grafted hydrogel in comparison with the native PVA may be attributed to the fact that due to grafting of PAM chains, the tendency of PVA chains to acquire an ordered array decreases which results in a decrease in the crystalline nature. In addition, it is also to notice that the area under the melting endotherm also decreases as the PAM content increases. This suggests for a smaller fraction of crystalline phase and a larger fraction of amorphous phase.
Scanning electron microscopy (SEM)
The morphology of the native PVA and PVA-g-PAM hydrogels has been investigated by recording SEM images of the two polymer films. The SEM images are shown in Fig. 6a, b, which clearly show that whereas the morphology of the native PVA film is quite smooth and does not contain any cracks or voids on its surfaces, the grafted PVA-g-PAM hydrogel, however, shows a network type of morphology which could be attributed to the grafting of PAM chains onto the backbone of the PVA macromolecules. The grafting of PAM chains onto PVA results in a three-dimensional macromolecular structure in which the polymer chains create pores or voids in the grafted hydrogel which is also confirmed by the SEM image. The image also reveals that the grafted PAM chains build up pores varying in the size from nearly 50–100 µm.
X-ray diffraction study (XRD)
The X-ray diffraction analysis provides valuable information on the structure and configuration of molecules and orientation and size of ordered regions in the material. In the present study, the XRD spectra were recorded in the range from 10° to 60°, 2θ and the results are shown in Fig. 7. It is clear from Fig. 7a that the native PVA hydrogel shows a sharp peak at 19.2° and 43°, 2θ, while Fig. 7b shows the typical X-ray diffraction pattern of PVA-g-PAM representing a peak at 15°, 17°, and 19.2°, 2θ, respectively. It was observed that the intensities of diffraction peaks are weakened after the graft copolymerization of PAM chains onto the PVA backbone, especially the peak at 43° almost disappeared and the peak at 19.2° also weakened. It reveals that the grafted chains of PAM tend to reduce the crystal nature of PVA. The reduction in crystallinity was further confirmed by determining degree of crystallinity of native PVA and PVA-g-PAM grafted polymers, respectively, using Eq. (3). The percent crystallinity of native PVA was calculated to be 48.6%, while that of the grafted polymer was found to be 22.4%. Similar types of results have also been reported by other workers [28] who observed that the percent crystallinity of native PVA was reduced from 50.5 to 10.4% after grafting of PAM onto the native PVA. The observed reduction in intensity was attributed to a reduction in ordered packing.
Swelling measurements
Effect of acrylamide variation
Poly (acrylamide) is known for its water solubility due to the presence of large number of amide groups [30]. Because of hydrophilic nature of this polymer, its varying concentration causes large influence on the water sorption capacity of the grafted hydrogel. The impact of polyacrylamide content in the hydrogel on its swelling performance has been studied by varying the concentration of acrylamide in the range of 1.5–3.0 g for definite concentrations of PVA and MBA. The results are shown in Fig. 8 which reveals that the equilibrium swelling ratio constantly decreases in the studied range of acrylamide concentration.
The fall in the swelling ratio is quite apparent as due to the presence of a large number of amide groups in the polyacrylamide chains, the extent of inter- and intramolecular hydrogen bonding between the amide groups lying along the PAM chains increases which produces a compact polymer network. In this way due to an enhanced number of crosslinks and intermolecular forces between grafted PAM chains, the mobility of polymer chains is restrained which permits less permeation of water molecules into the polymer matrix resulting in a fall in the water sorption capacity of the grafted hydrogel. Another reason for the observed decrease in water sorption capacity is that with increasing concentration of acrylamide in the reaction mixture, the length of the grafted PAM chains increases thus resulting in an increase in the molecular weight of the polymer and consequently its solubility decreases. Thus, a reduction in solubility of polyacrylamide chains brings about a lower water sorption capacity of the grafted hydrogel. Lower water sorption with increasing polymer content has also been reported by other workers.
Effect of polyvinyl alcohol
PVA is well-known hydrophilic polymer with adequate solubility in water in both the cold and hot conditions depending on its degree of hydrolysis [31]. It is, therefore, necessary that the highly hydrophilic PVA must be crosslinked either chemically or physically to make it insoluble [32]. The increase in the amount or molecular weight of PVA is also known to result in a viscous solution that hinders the movements of the polymer chains in the solution and, consequently, alters the ultimate properties of the end material [33].
The effect of the PVA content in the grafted hydrogel on its water sorption capacity has been investigated by varying the amount of PVA in the feed mixture in the range of 1.5–3.0 g. The results are shown in Fig. 9 which shows that the water sorption capacity of the hydrogel increases with increase in the PVA content in the grafted hydrogel and finally acquires an optimum swelling. The observed enhanced swelling of the grafted hydrogel may also be attributed to the large mesh sizes of the hydrogel network which accommodate greater amount of water into their macroporous pockets and results in large water sorption values. An alternate explanation may be that increasing content of PVA results in an enhanced hydrophilicity of the grafted hydrogel which results in an increase in water sorption capacity.
Effect of pH on swelling of grafted hydrogel
Swelling characteristics of a hydrogel depends greatly on the pH of the swelling reservoir, and this property of pH dependence is of great biomedical significance especially in designing drug delivery systems. What actually happens is that as the physiological pH changes inside the human body, the swelling behavior of the hydrogel correspondingly changes thus delivering desired amount of the entrapped drug. In the present investigation, the effect of pH has been investigated on water sorption characteristics of the hydrogel at the pH values of 1.6, 7.4, and 8.6. The results are depicted in Fig. 10, which clearly indicates that as the pH increases the swelling ratio also increases. The results may be explained by the fact that as the swelling medium acquires an alkaline nature, the amide groups of the PAM chains may undergo slight hydrolysis that produces carboxylate ions along the PAM chains. The anionically charged carboxylate ions repeal each other and produce frequent relaxation of the macromolecular chains thus allowing greater number of water molecules to enter the polymer network and, consequently, the swelling ratio of the grafted hydrogel increases.
Effect of temperature on swelling of grafted hydrogel
The impact of temperature on water sorption capacity of the grafted hydrogel has been studied by varying the temperature of the swelling medium from 10 to 60 °C. The obtained results are depicted in Fig. 11 which reveals that the water sorption capacity of the hydrogel increases with increasing temperature. The observed enhanced water sorption by the hydrogel may be attributed to the fact that at low temperature the segmental mobility of hydrogel chains is quite low, and therefore, the swelling ratio is also low. However, upon increasing the temperature, both the relaxation of polymer chains as well as the diffusion of water molecules into the hydrogel also increase which brings about an increase in the overall water sorption capacity of the grafted hydrogel. Similar increase in water sorption capacity of cryogel comprising of poly (2-hydroxyethyl methacrylate-co-acrylonitrile) was also obtained in a previous publication of the authors [34].
Effect of biological fluids on swelling of grafted hydrogel
It is a known fact that the there are certain factors such as charge, valency, and concentration of salt ions present in the swelling bath which significantly affect the overall water sorption by a polymer hydrogel. The theory of swelling of polymer network reveals that the phenomenon of swelling of polymer networks in a solution is the result of competing effects of osmotic pressure and restoring tendency of elastic chains of the polymer network [25]. When salt ions are present in the swelling bath, they might be able to tilt this balance and may either increase or decrease in the swelling of hydrogel. Thus, to study the effect of nature of the swelling media on water uptake capacity of the grafted hydrogel, the swelling experiments were performed in various simulated biological fluids and the results are shown in Fig. 12. The observed results reveal that each biofluid shows certain equilibrium swelling ratio at room temperature. It is also observed that the grafted hydrogel shows less water sorption capacity in comparison to that in the PBS.
The obtained lower swelling ratio in simulated biological fluids may be attributed to the presence of salt ions and other compounds in the swelling bath which affect the difference in osmotic pressure between the bulk of the swelling grafted hydrogel and outer environment and consequently alters the water sorption capacity as predicted by theoretical considerations. It is, however, noticed that an optimum swelling is obtained in artificial urine which may be attributed to the reason that some of the salt ions present in the artificial urine may diffuse into the hydrogel and result in an increased osmotic pressure that may lead to enhanced swelling of the grafted hydrogel. Similar types of results has also been reported elsewhere [35].
Network parameters
The values of Mc, q and Ve of the prepared hydrogel networks of varying compositions have been calculated and summarized in Table 2. It is clear from the data that the network parameters greatly vary with the changing chemical composition of the hydrogel.
When the amount of PVA varies from 1.5 to 3.0 g, the value of Mc constantly increases which reveals that larger chains are formed between the crosslink points when increasing amount of PVA is added into the polymerizing reaction mixture. The increasing average lengths of macromolecular chains may be attributed to the fact that with increasing amount of PVA, greater numbers of polymerization initiating free radicals (R) are available per PVA chains to abstract H atom to produce PVA radical and, as a consequence, average number of crosslinks per PVA macromolecule decreases resulting in higher molecular weight of polymer chains between crosslinks. However, an opposite trend is observed in the case of variation of acrylamide concentration. It is clear from Table 2 that the average molecular weight Mc between the crosslinks decreases with increasing concentration of acrylamide in the range 1.5–3.0 g. The reason for the observed decrease in Mc values may be due to the fact that with increasing concentration of acrylamide a larger number of PAM macroradicals are produced which tends to enhance the chances of mutual termination of macroradicals chains of acrylamide to produce a polymer and, thus, resulting in shortening of macromolecular chains between two crosslinks.
In the case of variation of crosslinking agent MBA, the average molecular weight Mc constantly decreases with increasing concentration of MBA in the studied range of 60–120 mg. The observed results are quite expected since increasing concentration of MBA results in greater degree of crosslinking of macromolecular chains of PAM, and consequently, the length of PAM chains present between the two crosslinks decreases. The other network parameters, i.e., qe and Ve, are obviously self-explained in the light of the above explanation.
Amoxicillin release study
The PVA-g-PAM hydrogel loaded with the amoxicillin drug forms a swelling controlled drug delivery system that delivers drug in a swelling controlled manner. The following section discusses the influence of various factors on the release of amoxicillin from the grafted hydrogel.
Effect of percent loading on released amoxicillin
One of the important aspects in the use of hydrogel as drug vehicles is the effect of the drug loading levels on the release profile of the drug. The amount of drug encapsulated inside the hydrogel does affect drug-polymer interactions and has significant impact on the amount and rate of the released drug. In the present study, the grafted hydrogels of definite composition were loaded with different amounts of amoxicillin drug by allowing the hydrogels to swell in the drug solutions of concentrations varying from 2.5 to 25 mg/mL. The amount of drug imbibed by the hydrogel results in fabrication of drug-loaded devices having percent loading of amoxicillin varying in the range 22–87%. The drug-loaded hydrogels were allowed to release the entrapped drug into a definite volume of the release medium (PBS, pH 7.4 and SGF, pH 2.4) which was monitored spectrophotometrically. The drug release results are shown in Fig. 13 which clearly indicates that the amount of released amoxicillin increases from 2 to 8 mg/mL when the drug loading increases from 22 to 87%. The higher drug release with increasing percent loading may be explained by the fact that higher entrapment of amoxicillin results in rapid diffusion of water molecules into the grated hydrogel network which leads to greater amount of released drug. Another reason for the observed greater release of amoxicillin with increasing drug loading may be that when the water molecules penetrate the hydrogel device, they dissolve the drug molecules and develop a concentration gradient at the device-release medium interface which derives drug molecules to diffuse from the bulk of the hydrogel into the outer release medium thus enhancing the drug release rate. Similar type of observation has also been reported previously [36].
However, a reduced drug release was observed by Sanli et al. [37] who investigated the effect of increasing drug-to-polymer ratio on the release of diclofenac sodium. The authors reported that a smaller amount of drug was released when the drug/polymer ratio was increased from 1:5 to 1:1. In the present study, however, no such observations were found.
Effect of composition of hydrogel
The chemical composition of hydrogel is an important parameter that may drastically affects the release profiles of the drug since the amount of polymers present, number of crosslinks, the lengths of the polymer chains in the network, hydrophilicity of the hydrogel are the key factors that determine how much drug will go into the release medium when the drug-loaded device is put into the release medium. The amounts of drug released from various hydrogels of definite compositions are summarized in Table 3, which clearly reveal that the chemical composition of the hydrogel has large impact on the quantity of the released drug.
It is clear from the results that when the amount of PVA is varied from 1.5 to 3.0 g, the amount of released amoxicillin increases constantly in the studied range of PVA. The observed enhanced release of amoxicillin may be explained by the fact that the greater hydrophilicity of the hydrogel with increasing PVA causes larger imbibitions of water molecules into the hydrogel network which, in turn, releases greater amount of drug entrapped within the grafted hydrogel matrix. It is also likely that due to increased amount of PVA, the average length of molecular chains of PVA also increases which due to larger mesh sizes of the network may accommodate greater amount of water that expels drug molecules.
The release data summarized in Table 3 also reveal that the amount of released amoxicillin decreases with increasing amount of polyacrylamide in the grafted hydrogel network. The observed findings may be explained by the fact that due to polar nature of PAM, the amoxicillin molecules will be firmly held by the PAM chains and may not lose them when water molecules enter the hydrogel network. This clearly results in reduced amount of released drug.
It is also clear from the data that when the concentration of crosslinker varies from 60 to 120 mg, the amount of released amoxicillin is significantly suppressed which may be attributed to the reason that the hydrogel network with higher degree of crosslinking will have low water sorption tendency and smaller mesh sizes due to higher crosslink density of the hydrogel network.
Drug activity
In vitro antibacterial activity
The amoxicillin loaded grafted hydrogel which showed optimum drug release was taken for further evaluation using antimicrobial activity. The antimicrobial assay of amoxicillin loaded grafted hydrogel was performed using agar plate well diffusion method. The different concentrations of drug solutions ranging from 2.5 to 25 mg/mL were taken, and the hydrogels were allowed to swell in the drug solutions so that the drug was loaded onto the grafted hydrogel disks. The drug-loaded disks were carefully transferred into the wells prepared with sterile borer on solidified nutrient agar media plate in Petri dishes inoculated with test gram-negative E. coli (ATCC25922). After inoculation, Petri dishes were kept in an incubator at 37 °C for 24 h. After incubation, the zone of inhibition (ZOI) for time-dependent release bilayer tablet, marketed preparation, and standard dilution of antibiotic was measured with the help of slide calliper scale in mm. The concentration of AMX in aliquot samples was calculated using the following equation:
where MIC is the minimum inhibitory concentration, x is the zone of inhibition (mm), c is the concentration of antibiotic (mg/mL), D is the diffusion coefficient, and t is the time required for antibiotic diffusion. A plot was made between x2 versus In (c) (Fig. 14) for standard dilutions of the antibiotic using each test organism. Figure 15 shows that zone of inhibition increases with the amount of drug loaded in the grafted hydrogel.
UV spectral analysis
In order to ascertain chemical and structural integrity of the released amoxicillin drug, its UV spectra were recorded at 334 nm. The obtained results are shown in Fig. 16a–c, which represent the spectral pattern of the released drug in SGF (pH 2.4), PBS (pH 7.4), and pH 8.6, respectively. It is clear from Fig. 16 that the spectral patterns are almost identical thus showing nearly same λmax values which suggest that the chemical, structural, and biological activities of the native and released drugs are intact and identical, and there is no change in these properties of drug when they are released. Other significant information that may be obtained from these spectra is that the drug molecules do not undergo any chemical or structural changes with time and, thus, warrant their antibacterial activity even after 6 h.
Conclusions
The graft copolymerization of acrylamide onto polyvinyl alcohol using a redox couple results in the formation of grafted hydrogel in which PAM chains are grafted onto PVA backbone. The FTIR spectral study offers convincing evidences of grafting of PAM chains onto the PVA backbone and confirms the presence of various functional groups in the drug-loaded grafted hydrogel. The DSC studies show that the crystalline nature of the native PVA is reduced due to grafting of PAM chains and the glass transition temperature of native PVA is significantly increased due to grafting of PAM chains onto PVA. The reduced crystallinity is also confirmed by the XRD studies which reveal that the percent crystallinity of native PVA is reduced from 50.5 to 10.4. The morphological study of the native PVA and grafted hydrogel using SEM studies reveals that whereas the native PVA is quite smooth in surfaces, the grafted hydrogel shows network type of morphology having pore sizes in the range of 50–100 µm.
The hydrogel shows enhanced swelling when PVA content of the hydrogel increases from 1.5 to 3.0 g, while a fall in swelling ratio is noticed when the amount of PAM increases from 1.5 to 3.0 g. The grafted hydrogel also shows a slight increase in swelling when pH of the swelling medium increases from 1.8 to 8.6. An increase in temperature from 10 to 60 °C results in a significant increase in swelling ratio of the grafted hydrogel. The hydrogel also shows a suppressed level of swelling in various simulated biological fluids. Based on the swelling data, the network parameters of the hydrogels were also calculated and it was found that the increase in the amount of PVA tends to form longer grafted PAM chains resulting in an increase in the average molecular weights between the crosslinks. The grafted hydrogel shows greater crosslink density when the amounts of acrylamide and crosslinker are increased in the grafted hydrogel.
The grafted hydrogel also offers potential to encapsulate amoxicillin drug which is released when the drug-loaded hydrogel undergoes swelling in the release medium. The amount of released drug increases with increasing percent loading from 22 to 87% of the drug and varies significantly with varying composition of the grafted hydrogel. The amount of released amoxicillin increases with increasing PVA content, while it decreases when PAM content increase in the grafted hydrogel. The release of amoxicillin from the grafted hydrogel also depends on pH and temperature of the release media. The amoxicillin also offers fair stability in acidic, neutral, and alkaline release media. The amoxicillin loaded grafted hydrogel also shows antibacterial nature against gram-positive bacteria.
References
Bajpai AK, Shukla SK, Bhanu S, Kankane S (2008) Responsive polymers in controlled drug delivery. Prog Polym Sci 33(11):1088–1118
Viega AS, Schneider JP (2013) Antimicrobial hydrogels for treatment of infection. Biopolymers 100(6):637–644
Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine. Adv Mater 21:3307–3329
Siafaka PI, Zisi AP, Exindari MK, Karantas ID, Bikiaris DN (2016) Porous dressings of modified chitosan with poly (2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr Polym 143:90–99
Eid M, Hegazy SA (2009) Radiation synthesis of stimuli-responsive hydrogels for biological applications. J Radiat Res Appl Sci 2:717–736
Nguyen MK, Alsberg E (2014) Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog Polym Sci 39:1234–1265
Yang D, Li Y, Nie J (2007) Preparation of gelatine-PVA nanofibers and their potential application in controlled release of drugs. Carbohydr Polymers 69:538
Varshosaz J, Koopaie N (2002) Cross-linked poly (vinyl alcohol) hydrogel: study of swelling and drug release behaviour. Iran Polym J 11(2):123
Paradossi G, Cavalieri F, Chiessi E, Ponassi V, Martorana V (2002) Tailoring of physical and chemical properties of macro- and microhydrogels based on telechelic PVA. Biomacromolecules 3:1255–1262
Mishra S, Bajpai R, Katare R, Bajpai AK (2007) Radiation induced crosslinking effect on semi interpenetrating polymer networks of poly(vinyl alcohol). Express Polym Lett 1(7):407–415
Stammen JA, Williams S, Ku DN, Guldberg RE (2001) Mechanical properties of a novel PVA hydrogel in shear and unconfined compression. J Biomater Sci 22:799–806
Singh B, Sharma A, Sharma A, Dhiman A (2017) Design of antibiotic drug loaded carbopol crosslinked-poly(2-hydroxyethylmethacrylate) hydrogel for wound dressing applications. Am J Drug Deliv Ther 4:1–9
Alavarse AC, de Oliveira Silva FW, Colque JT et al (2017) Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater Sci Eng C77:271–281
Manju S, Antony M, Sreenivasan K (2010) Synthesis and evaluation of a hydrogel that binds glucose and releases ciprofloxacin. J Mater Sci 45:4006–4012
Posadowska U, Wloch MB, Drozdz A, Krok-Borkowicz M, Wlodarczyk-Biegun M, Dobrzynski P, Chrzanowski W, Pamula E (2016) Injectable hybrid delivery system composed of gellan gum, nanoparticles and gentamicin for the localized treatment of bone infections. Drug Deliv 13:613–620
Zhang J, Xiao C, Wang J, Zhuang X, Chen X (2013) Photo cross-linked biodegradable hydrogels for enhanced vancomycin loading and sustained release. Chin J Polym Sci 31:1697–1705
Arvanitoyannis IS, Dionisopoulou N (2014) Acrylamide: formation, occurrence in food products, detection methods, and legislation. Crit Rev Food Sci Nutr 54:708–733
Nagpal M, Singh SK, Mishra D (2013) Synthesis characterization and in vitro drug release from acrylamide and sodium alginate based superporous hydrogel device. Int J Pharm Investig 3(3):131
Becerra-Bracamontes F, Sánchez-Díaz JC, González-Álvarez A, Ortega-Gudiño P, Michel-Valdivia E, Martínez-Ruvalcaba A (2007) Design of a drug delivery system based on poly(acrylamide-co-acrylic acid)/chitosan nanostructured hydrogels. J Appl Polym Sci 106(6):3939
Bajpai AK, Bajpai J, Soni SN (2008) Preparation and characterization of electrically conductive composites of poly (vinyl alcohol)-g-poly(acrylic acid) hydrogels impregnated with polyaniline (PANI) express. Polym Lett 2(1):26–39
Rihawy MS, Alzier A, Allaf AW (2011) PIXE investigation of in vitro release of chloramphenicol across polyvinyl alcohol/acrylamide hydrogel. Nucl Instrum Methods Phys Res Sect B 269:1892
Aziz Taghreed H, Al-Noor Manhel Reemon, Ahmed T, Jeboori AL (2014) Synthesis, characterization and antimicrobial activities of [Fe(II), Co(II), Ni(II), Cu(II) and Zn(II)] mixed ligand complexes schiff base derived from amoxicillin drug and 4-(dimethylamino)benzaldehyde with nicotinamide. J Chem Pharmaceut Res 6(4):1225–1231
Rahman S, Ahuja A, Ali J, Khar K (2004) Simultaneous spectrophotometric determination of amoxycillin trihydrate and metronidazole in dental films. Indian J Pharm Sci 66:135–136
Tripathi GK, Singh S, Gupta M (2014) UV Spectroscopy technique for analysis of amoxicillin trihydrate in pH stimuli sensitive formulation. Der Pharma Sin 5(1):29–33
Flory PJ, Rehner J (1943) Statistical mechanics of cross-linked polymer networks, II Swelling. J Chem Phys 11:521–526
Brandrup J, Immergut EH, Grulke EA (eds) (1999) Polymer handbook. Wiley, New York
Çavus S, Durgun E (2016) Poly(vinyl alcohol) based polymer gel electrolytes: investigation on their conductivity and characterization. Acta Phys Pol A 4:129
Lu Y, Jing R, Q-m Kong, P-x Zhu (2014) Solid state grafting copolymerization of acrylamide onto poly(vinyl alcohol) initiated by redox system. J Appl Polym Sci 131:39938
Abdel-Razik HH, Abbo M, Almahy HA (2012) Polymer-based metal adsorbents via graft copolymerization of polyvinyl alcohol with diaminomaleonitrile: a green chemistry approach. J Appl Polym Sci 125:2102–2109
Demadis KD, Stathoulopoulou A (2006) Solubility enhancement of silicate with polyamine/polyammonium cationic macromolecules: relevance to silica-laden process waters. Ind Eng Chem Res 45:4436
Byfield MP, Abuknesha RA (1994) Biochemical aspects of biosensors. Biosens Bioelectron 9(4–5):373–400
Dinh PV, Bach LT (2014) Immobilized bacteria by using PVA (polyvinyl alcohol) crosslinked with sodium sulphate. Int J Sci Eng 7(1):41–47
Hossein H (2013) Synthesis and swelling properties of a poly (vinyl alcohol)-based superabsorbing hydrogel. Curr Chem Lett 2:153–158
Jain A, Bajpai J, Bajpai AK (2017) Structural, morphological and thermal characterization of poly (2-hydroxyethyl methacrylate-co-acrylonitrile) (P (HEMA-co-AN)) cryogels: evaluation of water sorption potential and cytotoxicity. J Polym Res 24:111–125
Chaturvedi A, Bajpai AK, Bajpai J, Singh SK (2016) Evaluation of poly (vinyl alcohol) based cryogel–zinc oxide nanocomposites for possible applications as wound dressing materials. Mater Sci Eng C 65:408–418
Mahobia S, Bajpai J, Bajpai AK (2018) Glutaraldehyde crosslinked and alkaline denaturation induced self association of haemoglobin to design nanocarriers for In vitro release of insulin in simulated gastrointestinal fluids (SGFs). J Drug Deliv Sci Technol 44:71–81
Sanli O, Ay N, Isiklan N (2007) Release characteristics of diclofenac sodium from (vinyl alcohol)/sodium alginate and poly(vinyl alcohol)-grafted-poly(acrylamide)/sodium alginate blend beads. Eur J Pharm Biopharm 65:204–214
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bajpai, A.K., Vishwakarma, A. & Bajpai, J. Synthesis and characterization of amoxicillin loaded poly (vinyl alcohol)-g-poly (acrylamide) (PVA-g-PAM) hydrogels and study of swelling triggered release of antibiotic drug. Polym. Bull. 76, 3269–3295 (2019). https://doi.org/10.1007/s00289-018-2536-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2536-2