Abstract
A series of poly(l-lactide)-block-poly(tetramethylene oxide) glycol (PLLA-b-PTMG) cross-linked by 3-isocyanatopropyltriethoxysilane (IPTS) as the coupling agent were synthesized. Triethoxysilane-terminated oligolactide (OLLA) and PTMG precursors were synthesized by reacting IPTS with hydroxyl-terminated PLLA and PTMG oligomers, respectively. Cross-linked PLLA-b-PTMG (C-PLLA-b-PTMG) was then prepared via one-pot hydrolysis–condensation of the triethoxysilane-functionalized precursors and casted into films in situ. The cross-linked reactions were verified by 29Si-NMR and FTIR. The effects of PLLA/PTMG ratio on the thermal and mechanical properties of the resulting C-PLLA-b-PTMG films were investigated. It was found that the block copolymer containing 40 wt% PTMG exhibited the highest elongation at break of 240% and shape recovery ratio of 95%, showing decent toughness and resilience. The procedure has great potential in preparing high molecular weight PLLA-based copolymers in that the stringent water-free or oxygen-free conditions could be obviated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(l-lactide) (PLLA) as a bio-based material has received numerous attention due to its excellent biodegradability, biocompatibility, renewability, and compatibility. It is primarily produced from corns and other natural resources [1,2,3,4]. PLLA has been successfully employed in medical and packaging fields [5,6,7,8]. However, there is still a great need to improve PLLA’s properties for its inherent brittleness greatly limited its potential applications [9,10,11]. Therefore, toughening of PLLA has been extensively explored over the recent years [12, 13].
Blending is a facile way to improve polymer toughness. Blends of PLLA with various polymers such as poly (ε-caprolactone) [14], poly (butylene succinate) [15], and polyhydroxy butyrate [16] have been reported to show enhanced toughness, but the improvement was generally limited due to poor interfacial adhesion and phase separation of the two immiscible components. Toughening of PLLA by copolymerization is considered to be one of the most effective methods, because the miscibility problem is solved and specific properties could be achieved through design of the segment structure [17,18,19,20,21]. Maleic anhydride-grafted PLLA polymers have been used as a reactive intermediary to graft flexible compounds such as citrate [22] or poly(ethylene glycol) [23] onto PLLA to increase its toughness.
PLLA can also be toughened by introducing an appropriate level of thermoplastic polymers by cross-linking. Specifically, vinyl alkoxysilane grafting of PLLA was considered to offer an excellent combination of copolymerization and backbone grafting approaches to the cross-linking of PLLA [24, 25]. In addition to being more efficient and energy saving, this two-step method also had the advantage of producing the more stable Si–O–Si cross-links.
The objective of our study was to prepare PLLA copolymerized with soft segments. PTMG, an outstanding flexibility, biological stability, and biocompatibility material [26,27,28], was chosen as the soft segment. Oligomeric PLLA and PTMG segments sized 5000 and 2000 Da, respectively, were terminal-modified by 3-(triethoxysilyl)propyl isocyanate (IPTS), which could provide multiple triethoxysilane functionalities for the subsequent coupling step and was readily available and highly reactive [29]. The C-PLLA-b-PTMG films were prepared by in situ casting the hydrolysis–condensation reaction mixtures. To our knowledge, blocked copolymers of PLLA prepared by triethoxysilane hydrolysis–condensation had not been previously reported in the researches. This approach (as illustrated in Scheme 1) took the advantage of the triethoxysilane hydrolysis–condensation reaction to avoid the stringent water-free or oxygen-free conditions in preparing high molecular weight PLLA copolymers traditionally.
Experimental
Materials
PTMG (2000 g mol−1) and stannous octoate (Sn(Oct)2, 95%) were purchased from Aldrich Co. l-Lactide was purchased from Shenzhen Esun Industrial Co., Ltd. IPTS and 1,4-butanediol were supplied by TCI (Shanghai) Development Co., Ltd. Methanol, dichloromethane (DCM), and tetrahydrofuran (THF) were purchased from Sinopharm Chemical Reagent Co., Ltd. All solvents were analytical grade. THF was distilled over a sodium benzophenone complex before use. 1,4-Butanediol was distilled over calcium hydride before use [16].
Synthesis of hydroxyl-terminated oligolactide (HO-OLLA)
HO-OLLA was synthesized by the ring-opening polymerization (ROP) of l-lactide following a previously reported method [30]. Typically, l-lactide (10 g, 69.4 mmol) was placed in a flame-dried round-bottom flask equipped with a magnetic stirring bar, and then, an exhausting–refilling process was repeated three times. After that, BD (0.19 g, 2.08 mmol) was injected into the flask, and the mixture was stirred and heated to 135 °C. Finally, Sn(Oct)2 (0.83 mL, 0.42 mmol) was injected into the mixture to initiate the ROP as soon as the reactants were melt completely and the reaction was kept at 135 °C in an argon atmosphere for 12 h to yield the crude product. The crude product was purified by precipitation from DCM/methanol and dried in a vacuum oven at 40 °C for 12 h. 1H NMR (400 MHz, CDCl3, δ, ppm): 5.05 (H, –CH–), 1.26 (3H, −CH3), 4.18 (2H, –CH2–).
General procedure for preparing C-PLLA-b-PTMG films
HO-OLLA and PTMG were dried under vacuum for 12 h at 80 °C before use. Predetermined HO-OLLA and PTMG (1.5 g) were dissolved in 15 mL THF solution. A stoichiometric amount of IPTS (the ratio of –NCO to –OH was 1.1:1) and (1% mmol) Sn(Oct)2 were added to the reaction vessel with a syringe. The mixture was refluxed, stirred for 3 h under nitrogen and then cooled to room temperature. Nitric acid (1 wt%) was added to the mixture, which was further stirred at room temperature for 12 h. The solution was then poured into a Teflon casting plate (10*10 cm) to allow slow evaporation of THF (12 h) and formation of the films. Finally, films were removed from the Teflon plate and dried in a vacuum oven at 40 °C for 12 h to ensure complete removal of any residual THF. The samples containing 0–50 wt% PTMG were marked as C-PLLA-b-PTMG0, C-PLLA-b-PTMG10, C-PLLA-b-PTMG20, C-PLLA-b-PTMG30, C-PLLA-b-PTMG40, and C-PLLA-b-PTMG50, respectively.
In order to characterize the triethoxysilane-terminated oligolactide (Si-OLLA), HO-OLLA was reacted with IPTS in the presence of Sn(Oct)2 in THF. After the reaction completed, THF was removed under reduced pressure and the obtained product was characterized by NMR. 1H NMR (400 MHz, CDCl3, δ, ppm) 3.11 (1H, –NH–CH2–), 0.56 (2H, –CH2–Si–), 3.75 (2H, –Si–OCH2), 1.15 (2H, –Si–O–CH2–CH3). Triethoxysilane-terminated PTMG (Si-PTMG) was prepared and characterized similarly. 1H NMR (400 MHz, CDCl3, δ, ppm): 3.15 (1H, –NH–CH2–), 0.61 (2H, –CH2–Si–), 3.81 (2H, –Si–OCH2), 1.22 (3H, –Si–O–CH2–CH3).
Measurements
Fourier-transform infrared spectra (FTIR) were recorded on a PerkinElmer Spectrum-2 using the compression membrane samples in the range of 400–4000 cm−1.
1H NMR spectra were obtained on a Bruker DRX-400 at room temperature using CDCl3 as the solvent. The molecular weights of HO-OLLA were estimated from the integrals of methenyl C–H peak (δ 5.1 ppm) on the PLA chain and the methylene C–H peak (δ 4.09 ppm) of the BD moieties.
Thermogravimetric analysis (TGA) was performed on a Netzsch 209 F1 under nitrogen atmosphere from room temperature to 600 °C with the heating rate of 10 °C/min.
Differential scanning calorimeter (DSC) was carried out using a Netzsch DSC204F1. The samples were subjected to two consecutive DSC runs. The samples were heated up to and kept at 200 °C for 3 min, cooled down to 0 °C, and finally heated up again to 200 °C. All runs were conducted at a heating rate of 20 °C min−1 under nitrogen atmosphere.
Mechanical properties including tensile strength and elongation at break were determined using a Hounsfield H5K-S instrument at a strain rate of 500 mm/min. The measurements were taken at 25 °C. The tensile toughness as depicted by the area under the stress–strain curves was evaluated from five repeats for each sample. The film samples had thickness of 0.2 mm and length of 30 mm, conformed to the ASTM D5034 standard. The data presented were an average of at least five different measurements.
Scanning electron microscopy (SEM) was performed on a VE-7800 electron microscope (KEYENCE Corp., Osaka, Japan) operated at 10 kV. The stretched fractured samples were surface-coated with gold on an aluminum stage prior to observation.
Elastic recovery analysis of the C-PLLA-b-PTMG films was carried out on a DMAT Q800 (TA Corp. USA). All measurements were taken at 25 °C. The film strips (width = 5 mm, thickness = 0.2 mm) were examined by step cycle tensile deformation, which was conducted stepwise to progressively higher tensile strains. The samples were stretched from the initial strain (εi) to a pre-set strain (εm) (corresponding to stress = 5 MPa) by increasing the stress from 0 to 5 Mpa at 0.2 MPa/min. The recover strain (εr) was measured immediately after the stress was removed, and the deformed samples were allowed to relax for 10 min before the next cycle was applied. Three cycles were conducted for each sample, and the elastic recovery ratios were calculated using the following equation:
Results and discussion
Synthesis of cross-linked copolymers
As shown in Scheme 1, hydroxyl-terminated polylactide (HO-OLLA) was synthesized by general ring-opening polymerization of l-lactide initiated by butanediol and catalyzed by Sn(Oct)2. OLLA and PTMG were subsequently end-capped with triethoxysilyl groups by reacting with two equivalents of IPTS to yield Si-OLLA and Si-PTMG. Generally, when the average functionality of the pre-polymers was no less than 3, the resulting polymer became cross-linked. In this study, the Si-OLLA and Si-PTMG both had functionality of 6.0. Therefore, the C-PLLA-b-PTMG films casted in situ from mixing these pre-polymers were cross-linked in nature and were confirmed experimentally. The dried C-PLLA-b-PTMG films were insoluble in common organic solvents such as tetrahydrofuran, dichloromethane, toluene, dimethyl sulfoxide, and methanol, in agreement with that common notion that cross-linked polymers are insoluble in any solvents.
To confirm the formation of copolymer, the ATR-FTIR spectra of the C-PLLA-b-PTMG were recorded and compared with those of Si-OLLA, Si-PTMG, and the triethoxysilane cross-linked autopolymer of PLLA (C-PLLA-b-PTMG0). The results are summarized in Fig. 1. The IR spectrum of C-PLLA-b-PTMG0 containing no PTMG segments was very similar to that of its precursor Si-OLLA, and both showed peaks at 1080, 1180, 1518, 1753, 2994, and 2940 cm−1 characteristic for C–O stretching, C=O bending, N–H bending, C=O stretching, methenyl C–H asymmetric stretching, and methyl C–H symmetric stretching, respectively. In the spectra of C-PLLA-b-PTMG (10-50), on the other hand, all featured absorption peaks at 2944, 2853, and 1720 cm−1 corresponded to methylene C–H asymmetric, symmetric stretching, and carbamate C=O bending from the PTMG moiety. The intensity of these peaks increased with increasing PTMG contents. Since all the samples tested were pre-washed with THF to remove any unreacted pre-polymers, the coexistence of peaks characteristic to both PLLA and PTMG segments in the IR spectra confirmed the coupling of the pre-polymers via Si–O–Si cross-links.
The comparison of the 29Si NMR spectra of Si-OLLA and C-PLLA-b-PTMG50 is shown in Fig. 2. The different tri-substituted silicon species were denoted as Tn, where T designates the tri-functional status of the centered silicon and n is the number of neighboring silicon atoms interconnected via a single Si–O–Si bridge. The 29Si NMR spectrum of Si-OLLA exhibits a single peak at δ = − 45 ppm, which is assigned to the triethoxysilyl silicon species having none interconnected neighboring silicon ( T0). The T0 peak was completely absent in the spectrum of CL50 and replaced by two peaks at δ = − 49 and − 57 ppm corresponding to T1 and T2 silicon species. These results also confirmed the successful synthesis of the C-PLLA-b-PTMG.
Thermal properties of the cross-linked films
Figure 3 shows the TGA (3a) and DTG (3b) curves of HO-OLLA and the Si–O–Si cross-linked polymers of PLLA and the PLLA-b-PTMG copolymers. The oligomeric HO-OLLA started to lose weight at around 180 °C and its maximum decomposition temperature is 239 °C, whereas the onsets of decomposition temperature and the maximum decomposition temperature for all the cross-linked polymers were around 235 and 284 °C, respectively, and an improvement by about 45 °C attributed mainly to their stable three-dimensional structures from cross-linking [31]. Two-step decomposition profiles were observed for all C-PLLA-b-PTMG copolymers (10–50). In the first step, the onset degradation temperature was invariant at 235 °C to the mass ratio of OLLA and PTMG. The temperature likely corresponded to the thermal degradation due to breaking of the OLLA ester linkages. The percent weight loss during this step decreased with increasing PTMG contents. The decomposition of the ether bonds of PTMG took place at 370 °C in a later stage.
The DSC thermograms of the C-PLLA-b-PTMG films are presented in Fig. 4. It was noticed that the melting point of the C-PLLA-b-PTMG0 was at 131 °C, while the copolymers showed increasingly lower melting points of 128, 126, 126, 122, and 120 °C as more PTMG segments were incorporated. The observation could be understood in that polymers with more flexible and softer backbones tend to melt at lower temperatures. It is worth noting that the presence of endothermic melting peaks in all of the samples in the first DSC scan (Fig. 4a) indicated that they had rather low degree of cross-linking due to incomplete hydrolysis–condensation. The exothermic peaks ranging from 160 to 171 °C could be attributed to further condensation reaction between the residual triethoxysilanes. The observations that the endothermic melting peak weakened and broadened, while the exothermal reaction peak sharpened and intensified with increasing Si-PTMG content also agreed with this argument. As the samples were cured during the first scan, they became truly cross-linked leading to the disappearance of the exothermic melting peaks in the second scan (Fig. 4b).
Mechanical properties of cross-linked films
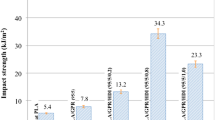
The stress–strain curves of the C-PLLA-b-PTMG films are shown in Fig. 5, and the calculated tensile strength (MPa) and elongation at break (%) are presented in Table 1. The autopolymer C-PLLA-b-PTMG0 had the highest tensile strength but lowest elongation at break of 12% with an obvious yield point indicative of being a brittle polymer with poor ductility. However, with increasing contents of the soft PTMG segment, the elongation at break rapidly increased and peaked at 244% for sample C-PLLA-b-PTMG40, which contained 40 wt% of PTMG. Further increase in PTMG content to 50 wt% led to a slight decrease to 218%. The trend observed for tensile strength of the cross-linked polymers was the opposite, but to a much smaller extend. When considering the film tensile toughness, the C-PLLA-b-PTMG40 film showed a sixfold increase in toughness. Tensile strength measured for all samples fell in the range of 9–15 MPa making them viable for applications as packaging materials. Therefore, toughening of PLA could be achieved by preparing C-PLLA-b-PTMG copolymers.
Resilience properties
Resilience is a measure of the ability of a material to deform reversibly without loss of energy, which is often used to characterize elastic materials. The cross-linked copolymers featured the flexible PTMG chains as the elastic recoil component, the hard OLLA chains as the stiff component, and the Si–O–Si as the cross-linking points. The combination of the soft and stiff joined up with a cross-linked network structure may endow the material with superior resilience.
As illustrated in Fig. 6, the resilience properties of C-PLLA-b-PTMG30, C-PLLA-b-PTMG40, and C-PLLA-b-PTMG50 were investigated using dynamic mechanical analysis under a controlled force mode at 25 °C and the elastic recoveries were recorded. The cross-linked films with PTMG content lower than 30% did not appear to be elastic and, therefore, were not included.
The results showed that deformed films could not completely recover the original shape, indicating entanglement of PTMG chains may exist to some extent in the network. Interestingly, over 90% of the maximum strain was recovered right after the samples were released from the strain, likely because the cross-linked structures provided a strong resilience [32].
As shown in Fig. 7, after three cycles of tensile deformation, the elastic recovery increased greatly from 54 to 91% for C-PLLA-b-PTMG30, from 80 to 95% for C-PLLA-b-PTMG40, and from 75 to 96% for C-PLLA-b-PTMG50. The small residual strain of less than 10% after three cycles could be caused by the deformation-induced chain disentanglements [33,34,35]. In other words, during stretching, the cross-linked entanglement network could be disentanglement and cross-linked entanglement network could be entanglement after the stress was removed, resulting in the small residual strain of less than 10%. The C-PLLA-b-PTMG 40 has the highest elastic recovery. This probably owes to the slight excessive cross-linking of films.
Stretched fractured morphologies of the cross-linked films
The morphologies for the stretched fractured surfaces of the cross-linked films are shown in Fig. 8. The stretched neat PLLA film (Fig. 8a) presented a flat fractured surface indicating typical brittle fractured during the mechanical test, which was consistent with previous report [36]. The stretched fractured surfaces of both the triethoxysilane cross-linked C-PLLA-b-PTMG0 and C-PLLA-b-PTMG40 were dramatically different to that of the neat PLLA. It can be seen that a large amount of voids were present on the fractured surfaces of C-PLLA-b-PTMG0 and C-PLLA-b-PTMG40 films, indicative of porous structures. The voids could play an important role in recovery of the materials from deformation by shrinking in response to the applied stress.
Conclusions
A series of cross-linked PLLA/PTMG copolymers were successfully prepared by triethoxysilane hydrolysis–condensation at room temperature. The tensile toughness properties could be modulated by varying the ratio of the hard and soft segments. The break at elongation of films casted from the cross-linked polymers ranged from 12% for C-PLLA-b-PTMG0, which contained no soft PTMG segment to up to 250% for C-PLLA-b-PTMG40 with 40 wt% PTMG added. The tensile strength of all cross-linked films ranged from 9 to 15 MPa, which was lower than of neat PLLA but sufficiently high for the materials to be used in packaging. For the more elastic C-PLLA-b-PTMG30, C-PLLA-b-PTMG40, and C-PLLA-b-PTMG50 films, DMA analysis was performed. The results showed that elastic recovery of 91–95% could be achieved after three deformation cycles. In summary, a simple one-pot procedure was developed to toughen PLLA via triethoxysilane cross-linking. The method was applied successfully to obtain tough cross-linked PLLA/PTMG copolymer films that showed greatly improved resilience and reasonable tensile strengths. The procedure has great potential in preparing high molecular weight PLLA-based copolymers in that the stringent water-free or oxygen-free conditions could be obviated.
References
Drumright RE, Gruber PR, Henton DE (2000) Polylactic acid technology. Adv Mater 12(23):1841–1846
Garlotta D (2001) A literature review of poly(lactic acid). J Polym Environ 9(2):63–84
Gwon J-G, Cho H-J, Chun S-J, Lee S, Wu Q, Li M-C, Lee S-Y (2016) Mechanical and thermal properties of toluene diisocyanate-modified cellulose nanocrystal nanocomposites using semi-crystalline poly(lactic acid) as a base matrix. RSC Adv 6(77):73879–73886
Dong J, Li M, Zhou L, Lee S, Mei C, Xu X, Wu Q (2017) The influence of grafted cellulose nanofibers and postextrusion annealing treatment on selected properties of poly(lactic acid) filaments for 3D printing. J Polym Sci Polym Phys 55(11):847–855
Shalgunov V, Zaytseva-Zotova D, Zintchenko A, Levada T, Shilov Y, Andreyev D, Dzhumashev D, Metelkin E, Urusova A, Demin O, McDonnell K, Troiano G, Zale S, Safarova E (2017) Comprehensive study of the drug delivery properties of poly(l-lactide)-poly(ethylene glycol) nanoparticles in rats and tumor-bearing mice. J Control Release 261:31–42
Oh JK (2011) Polylactide (PLA)-based amphiphilic block copolymers: synthesis, self-assembly, and biomedical applications. Soft Matter 7(11):5096–5108
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromol Biosci 4(9):835–864
Armentano I, Bitinis N, Fortunati E, Mattioli S, Rescignano N, Verdejo R, Lopez-Manchado MA, Kenny JM (2013) Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog Polym Sci 38(10–11):1720–1747
Farah S, Anderson DG, Langer R (2016) Physical and mechanical properties of PLA, and their functions in widespread applications: a comprehensive review. Adv Drug Deliv Rev 107:367–392
Rasal RM, Janorkar AV, Hirt DE (2010) Poly(lactic acid) modifications. Prog Polym Sci 35(3):338–356
Lim LT, Auras R, Rubino M (2008) Processing technologies for poly(lactic acid). Prog Polym Sci 33(8):820–852
Anderson KS, Schreck KM, Hillmyer MA (2008) Toughening polylactide. Polym Rev 48(1):85–108
Liu H, Zhang J (2011) Research Progress in Toughening Modification of Poly(lactic acid). J Polym Sci Pol Phys 49(15):1051–1083
Wang X, Gao Y, Li X, Xu Y, Jiang J, Hou J, Li Q, Turng L-S (2017) Selective localization of graphene oxide in electrospun polylactic acid/poly(ε-caprolactone) blended nanofibers. Polym Test 59:396–403
Shibata M, Inoue Y, Miyoshi M (2006) Mechanical properties, morphology, and crystallization behavior of blends of poly(l-lactide) with poly(butylene succinate-co-l-lactate) and poly(butylene succinate). Polymer 47(10):3557–3564
Abdelwahab MA, Flynn A, Chiou B-S, Imam S, Orts W, Chiellini E (2012) Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym Degrad Stabil 97(9):1822–1828
Li X, Kang H, Shen J, Zhang L, Nishi T, Ito K, Zhao C, Coates P (2014) Highly toughened polylactide with novel sliding graft copolymer by in situ reactive compatibilization, crosslinking and chain extension. Polymer 55(16):4313–4323
Jing F, Hillmyer MA (2008) A bifunctional monomer derived from lactide for toughening polylactide. J Am Chem Soc 130(42):13826–13827
Gramlich WM, Robertson ML, Hillmyer MA (2010) Reactive compatibilization of poly(l-lactide) and conjugated soybean oil. Macromolecules 43(5):2313–2321
Theryo G, Jing F, Pitet LM, Hillmyer MA (2010) Tough polylactide graft copolymers. Macromolecules 43(18):7394–7397
Jiang Z, Chang Y, Chen Z (2017) Catalyst free synthesis of poly(l-lactic acid)-poly(propylene glycol) multiblock copolymers and their properties. J Appl Polym Sci 134(37):45299
Hassouna F, Raquez J-M, Addiego F, Toniazzo V, Dubois P, Ruch D (2012) New development on plasticized poly(lactide): chemical grafting of citrate on PLA by reactive extrusion. Eur Polym J 48(2):404–415
Hassouna F, Raquez J-M, Addiego F, Dubois P, Toniazzo V, Ruch D (2011) New approach on the development of plasticized polylactide (PLA): grafting of poly(ethylene glycol) (PEG) via reactive extrusion. Eur Polym J 47(11):2134–2144
Han C, Bian J, Liu H, Han L, Wang S, Dong L, Chen S (2010) An investigation of the effect of silane water-crosslinking on the properties of poly(l-lactide). Polym Int 59(5):695–703
Schneider J, Bourque K, Narayan R (2016) Moisture curable toughened poly(lactide) utilizing vinyltrimethoxysilane based crosslinks. Express Polym Lett 10(10):799–809
Zhang J, Niu Y, Huang C, Xiao L, Chen Z, Yang K, Wang Y (2012) Self-healable and recyclable triple-shape PPDO–PTMEG co-network constructed through thermoreversible Diels–Alder reaction. Polym Chem 3(6):1390
Cai S, Zeng C, Zhang N, Li J, Meyer M, Fink RH, Shi D, Ren J (2016) Enhanced mechanical properties of PLA/PLAE blends via well-dispersed and compatilized nanostructures in the matrix. RSC Adv 6(30):25531–25540
Yeganeh H, Ghaffari M, Jangi A (2009) Diaminobisbenzothiazole chain extended polyurethanes as a novel class of thermoplastic polyurethane elastomers with improved thermal stability and electrical insulation properties. Polym Adv Technol 20(5):466–472
Helminen A, Korhonen H, Seppälä JV (2001) Biodegradable crosslinked polymers based on triethoxysilane terminated polylactide oligomers. Polymer 42(8):3345–3353
Xie H, Cheng C-Y, Du L, Fan C-J, Deng X-Y, Yang K-K, Wang Y-Z (2016) A facile strategy to construct PDLLA-PTMEG network with triple-shape effect via photo-cross-linking of anthracene groups. Macromolecules 49(10):3845–3855
Liu M, Yin Y, Fan Z, Zheng X, Shen S, Deng P, Zheng C, Teng H, Zhang W (2012) The effects of gamma-irradiation on the structure, thermal resistance and mechanical properties of the PLA/EVOH blends. Nucl Instrum Methods B 274:139–144
Klueppel M, Heinrich G (1994) Network structure and mechanical properties of sulfur-cured rubbers. Macromolecules 27(13):3596–3603
Mahajan DK, Singh B, Basu S (2010) Void nucleation and disentanglement in glassy amorphous polymers. Phys Rev E 82(1):011803
Boukany PE, Wang S-Q (2008) Use of particle-tracking velocimetry and flow birefringence to study nonlinear flow behavior of entangled wormlike micellar solution: from wall slip, bulk disentanglement to chain scission. Macromolecules 41(4):1455–1464
Zuo F, Keum JK, Chen X, Hsiao BS, Chen H, Lai S-Y, Wevers R, Li J (2007) The role of interlamellar chain entanglement in deformation-induced structure changes during uniaxial stretching of isotactic polypropylene. Polymer 48(23):6867–6880
Shi X, Chen Z, Yang Y (2014) Toughening of poly(l-lactide) with methyl MQ silicone resin. Eur Polym J 50:243–248
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Number: 51503029).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B., Ren, R. & Chen, Z. Synthesis of cross-linked polylactide–poly(tetramethylene oxide) copolymers with enhanced toughness. Polym. Bull. 76, 1531–1545 (2019). https://doi.org/10.1007/s00289-018-2452-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2452-5