Abstract
The present research work is an in vivo study that aimed to evaluate the potential role of bioglass–chitosan (BG–CS) and bioglass–chitosan–20%ciprofloxacin (BG–CS–20Cip) in antioxidant profile and osteointegration. These scaffolds were implanted in the defect bone of femoral condyles in ovariectomized rats. The treatment with BG–CS–20Cip has shown a significantly higher stress proteins concentration in comparison with that implanted with BG–CS group. The thiol and vitamin C in BG–CS–20Cip group were significantly enhanced when compared with those in BG–CS group. The histological and physicochemical analyses highlight the BG–CS implications in the bone construction. This property was found to decrease with the presence of ciprofloxacin that caused the delay of this phenomenon. ICP-OES has revealed that the introduction of this antibiotic to the composite led to decrease bone mineralization by evaluating Ca/P ratio. The SEM results have confirmed a progressive degradation of BG–CS and BG–CS–20Cip. However, such bioresorbability and bioactivity of BG–CS was proven to be faster than those of BG–CS–20Cip. Therefore, the incorporation of ciprofloxacin in BG–CS was characterized by a delaying effect of composite dissolution and the formation of apatitic phase. The development of BG–CS as a therapeutic biomaterial protector against oxidative stress is likely to make an effective choice for the application in tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tissue engineering (TE) is a quite new interdisciplinary field of science, involving cell biology, biomaterial science and medicine. It aims to regenerate damaged tissue and/or promote the growth of new tissues using biomaterials alone or in combination with biopolymers and antibiotics [1].
In the last couple of decades, significant advances in the development of biomaterial-based scaffolds for hard and soft tissue regeneration have been achieved, including the manufacture of biocomposites that combine natural or synthetic polymers with bioactive glasses or glass–ceramics. These novel biomaterials offer the possibility of tailoring a variety of parameters and properties such as degradation kinetics, mechanical properties, and chemical composition according to the intended application [2]. This approach involves the use of a biodegradable substrate, called scaffold for cell attachment, proliferation, migration, differentiation to ultimately support the formation of new bone tissues [3]. Bioglass (BG) is well-known for its ability to regenerate or repair bone defects due to its excellent osteogenic bioactivity in stimulating the proliferation and osteogenic differentiation of osteoblastic cells or stem cells, and in promoting the mineralization of the extracellular matrix [4]. To improve the reactivity of bioactive glass, it is necessary to combine them with those chitosan. This natural polymer has free amine groups and, therefore, possesses positive ionic charges, thus facilitating its chemical binding with negatively charged proteins and macromolecules such as glycosaminoglycans and proteoglycans [5]. This ability of chitosan to combine with other materials has allowed its use for the development of a wide range of scaffolds. This polymer that belongs to the group of semi-synthetic cationic polymers with cyclodextrin, dextran, cellulose often retain their biodegradability. Aside from the introduced cationic functionalities, polymers such as cationic dextran or chitosan possess additional hydroxyl as well as aldehyde groups, which are suitable for functionalization. The hydroxyl functionalities are easily accessible, although such polymers possess a definite 3D structure that is mainly based on the character of the glycosidic bond. Utilizing these functionalities, the cationic polymers can be acylated or alkylated to introduce additional properties or functional groups. Cellulose and chitosan have been proven to have antibacterial activity [3]. In fact, chitosan is the only positively charged, naturally occurring polysaccharide [4]. It is important to mention that chitosan is poorly soluble at physiological pH and it readily swells in aqueous solutions such as cellulose, resulting in rapid drug release in its application as a continuous matrix for controlled drug release [5, 6]. This biopolymer presents higher biocompatibility than polyethylenimine, poly(l-lysine), polyamidoamine. Actually, these materials can regenerate many organ tissues such as bone, liver, cartilage skin as well as neural and vascular tissues [7, 8].
Ciprofloxacin is a fluoroquinolone derivative, widely used in osteomyelitis thanks to its bactericidal effect on all the probable osteomyelitis pathogens [9]. Antibiotics cause various side effects when used systemically. To avoid such effects, we must synthesize composites containing this drug, and aiming local treatment in vivo, while evaluating their oxidative stress profile and their mineralization in the femoral condyle of ovariectomized rats. The implant/drug combination or implant materials with antibacterial properties would represent an excellent approach for the prevention of potential postoperative infections.
For this reasons, this work aimed to study the influence of incorporated ciprofloxacin in BG(M)–PVA activity, to investigate both its biocompatibility behavior, by the evaluation an oxidative stress phenomena which is an important parameter to evaluate the tissue regeneration process by the crucial role of osteoblasts (which caused the antioxidant enzymes) and osteoclasts (which caused the reactive oxygen species), and its degradation after its implantation in ovariectomized rats [10]. In fact, the remains of biomaterial and newly formed bone can well be differentiated from the histological slices [11]. Physicochemical techniques (ICP-OES and SEM) were engaged to highlight the influence of antibiotic on the structure, porosity and bioactivity of a porous glass–PVA before and after implantation [12].
Materials and methods
BG–CS synthesis of bioactive glass by melting technique
The bioactive glass 46S6 (BG) powder was synthesized by melting process. The used materials were calcium silicate (Alfa Aesar, molecular weight = 233–250 g/mol, Germany), trisodium trimetaphosphate (molecular weight = 305.9 g/mol, Sigma, Germany) and sodium metasilicate pentahydrate (molecular weight = 212.1 g/mol, Sigma, Germany). It was mixed by a mechanical mixer for 1 h. The batch was melted in a platinum crucible Rh–Pt through the following firing regime [11] heating to 900 °C/1 h at a rate of 10 K/min, firing at 1350 °C/3 h at a rate of 20 K/min. The melted glass was poured into a preheated die at 500 °C near the glass transition temperature. The resulting bioactive glass was crushed and ground in a mechanical agate mortar and sieved to a grain size of less than 63 μm.
BG–CS and BG–CS–20Cip composites preparation
BG–CS composites scaffolds were prepared by freeze–drying [12]. First, chitosan (CS) (ALDRICH, medium molecular weight, Germany) was dissolved in acidified water using acetic acid (Analar Normapur, molecular weight = 60.05 g/mol, Germany) at room temperature (25 °C) for 3 h using a polymer concentration of 3%. The dissolved CS was normalized by the addition of a few droplets of sodium hydroxide solution until white precipitate was achieved. After the removal of this precipitate, the 50% of BG was added to the CS solution and continuously stirred overnight using a magnetic stirrer to ensure a better (homogenous) distribution of BG particles to form BG–CS composite. A concentration of 20% ciprofloxacin was added to the above mixture and stirred continuously for 1 h to form BG–CS–20Cip. This second scaffold was casted in 24 well plates and kept at − 18 °C overnight and freeze–dried for 24 h. Then, it was removed from the well plates and kept in the desiccator for future analysis.
Animal model
Female Wistar rats (aged 18–20 weeks) were delivered from the Central Pharmacy of Tunisia. The experiment was carried out in the animal house of the Faculty of Sciences of Sfax, University of Sfax, meeting the international conditions of “ethics of animal experimentation”: surface, dimensions of cages, ventilation, air conditioning, temperature, humidity, light cycle darkness, treatment isolation and sacrifice-sampling. The rats were acclimatized to their new environment for 10 days before the beginning of the study and fed on a pellet diet (Sicco, Sfax, Tunisia) and tap water ad libitum. All the animals were kept under climate-controlled conditions (25 °C; 55% humidity; 12 h of light alternating with 12 h of darkness). All rats were randomly divided into four groups (24 animals per group):
-
Group I: used as negative control (T); which were neither ovariectomized nor implanted. Sixty days after bilateral ovariectomy, the rats developed the bone disorder, and thus could be used as the animal model for osteoporosis:
-
Group II: used as positive control (T+); with ovariectomy and without a surgical creation of bone defects.
-
Group III: the bone defects were implanted with BG–CS.
-
Group IV: the bone defects were implanted with BG–CS–20Cip.
Surgical and postoperative protocol
All surgical interventions were performed under general anesthesia in aseptic conditions Anesthesia was induced with chloral hydrate (3.5 g/100 ml) depending on the body weight. Indeed, the injected volume was 0.4 ml for 100 g. The preoperative preparation of the surgical sites was routinely carried out by cleaning with 96% alcohol and antiseptic solutions (PROLABO; AnalaR Normapur®, France). The resulting bone defects were irrigated profusely with physiological saline solution (0.9 wt% NaCl; Ref. 091214; Siphal, Tunisia) to eliminate bone debris. A drilled hole of 4 mm in diameter and 5 mm in depth was created on the lateral aspect of the femoral condyle using a refrigerated drill to avoid necrosis. Scaffolds prepared in molds were cut with the same dimension of the burr holes and then implanted. The drill-hole was filled with 10 mg of implants. The filling was done carefully and retrogradely to ensure both minimal inclusion of air bubbles and direct implant—bone contact. The closure of the wounds was performed in layers (i.e. fasciae and the subcutaneous tissue), using resorbable material (Vicryl 3/0; Ethicon, Germany) in a continuous manner. After the surgical intervention, all rats received subcutaneous analgesia (carprofen 10 mg/kg I CRimadyl®) for three postoperative days, and they were allowed unrestricted mobility. During this period, they were checked daily for clinical lameness or other complications. On days 15, 30, 60 and 90 after implants insertion, all rats were sacrificed and specimens were harvested for biological and physicochemical evaluation. At the end of treatment, 24 animals from each group were rapidly sacrificed by decapitation to avoid stress. All samples of bone marrow were extracted from different groups (T, T+, BG–CS and BG–CS–20Cip). To verify the correction of oxidative stress, we should compare the groups implanted versus the negative and positive controls. These bones marrow were removed and homogenized (10% w/v) with an Ultra Turrax homogenizer in ice-cold, 1.15% KCl 0.01 M sodium, potassium phosphate buffer. Homogenates were centrifuged at 10,000×g for 20 min at 4 °C. The resulting supernatants were used for immediate lipid peroxidation and protein oxidation determination. Homogenate aliquots were stored at − 80 °C for further biochemical assays. Other parts of these livers were fixed and processed for paraffin sectioning and histological studies.
Oxidative stress measurements
Preparation of cytosolic extracts
Bone marrow samples were ground in 2 ml TBS with pH 7.4 using an Ultra-Turrax homogenizer. After homogenization, the tissue homogenate was centrifuged at 9000 rpm at 4 °C and for 25 min to recover the supernatant: the cytosolic extract was used for the various assays.
Oxidant stress markers in bone marrow
-
Determination of protein carbonyl (PC) levels: protein carbonyls (PCO) were measured using the method of Reznick and Packer [13]. Briefly, 100 μl of cytosolic extract was placed in glass tubes. Then, 500 μl of 10 mM 2,4-dinitrophenylhydrazine (DNPH) in 2N HCl was added. Tubes were incubated for 1 h at room temperature. Samples were vortexed every 15 min. Then, 500 μl TCA (20%) were added and the tubes were left on ice for 5 min followed by centrifugation for 10 min. The protein precipitates were collected. The pellet was then washed twice with ethanol–ethyl acetate (v/v). The final precipitate was dissolved in 600 μl 6 M guanidine hydrochloride solution and incubated for 15 min at 37 °C. The absorbance of the sample was measured at 370 nm. The carbonyl content was calculated based on the molar extinction coefficient of DNPH (ε = 2.2 × 104 cm−1 M−1) and expressed as nmol/mg protein [13].
-
Determination of advanced oxidation of protein products (AOPP) levels: AOPP levels were determined according to the method of Kayali et al. [14]. Briefly, 0.4 ml of extract was treated with 0.8 ml phosphate buffer (0.1 M; pH 7.4). After 2 min, 0.1 ml 1.16 M potassium iodide was added to the tube followed by 0.2 ml of acetic acid. The absorbance of the reaction mixture was immediately recorded at 340 nm. The concentration of AOPP for each sample was calculated using the extinction coefficient of 261 cm−1 mM−1 and the results were expressed as nmol/mg protein.
Determination of antioxidant activities
-
Determination of thiol levels: the thiol levels were determined using the method reported by Ellman [15]. An amount of 500 μl aliquot of the supernatant was mixed with 10% trichloroacetic acid (1 v/1 v). After centrifugation, the protein pellet was discarded and free-SH groups were determined in a clear supernatant. A quantity of 100 μl aliquot of the supernatant was added to 850 μl of 1 M potassium phosphate buffer and to 50 μl of DTNB (10 mM). The absorbance of the colorimetric reaction was measured at 412 nm. The total Thiol content was expressed as µmol/mg of protein [16].
-
Determination of vitamin C (Vit C) levels: The Vit C determination was performed as described by Jacques-Silva et al. [17]. The protein in the plasma was precipitated in 10 volumes of a cold 4% trichloroacetic acid solution. An aliquot of sample (300 μl) was adjusted with H2O to a final volume of 1 ml and incubated at 38 °C for 3 h, then 1 ml of H2SO4 65% (v/v) was added to the medium. The reaction product was determined using color reagent containing 4.5 mg/ml dinitrophenylhydrazine and CuSO4 (0.075 mg/ml). The data were expressed as nmol/mg protein [13].
Histological assay
The pieces of controls and implanted bone were fixed in a Bouin solution and then embedded in paraffin. Sections of 3 mm in thickness were then stained with Trichrome of Masson (TM).
Inductively coupled plasma optical emission spectrometry (ICP-OES)
After different periods of implantation, the rats were sacrificed and the implants were excised by a specific equipment of mark DREMEL. Implanted and non-implanted composites were dried for 24 h at 80 °C, weighed accurately and placed in 25 ml tubes (0.5 ml of added nitric acid and 24.5 of distilled water). The standard solutions of Ca, P, Si, and Na were used to prepare the working standard solution and the blank solution. The element concentrations were detected using inductively coupled plasma optical emission spectrometry ICP-OES (Ciros; Spectro Analytical Instrument, Germany).
Scanning electron microscopy (SEM) characterization
Implanted and non-implanted composites were excised by a specific equipment of mark DREMEL and then analyzed by SEM, using a JEOL JSM 6301F (Tokyo, Japan).
Statistical analysis
The data were analyzed using the statistical package program Stat view 20 Soft Ware for Windows (SAS Institute, Berkley, CA). The statistical analysis was conducted using one-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test as a post hoc test for comparison between groups. All values were expressed as mean ± SE. Differences were considered significant if p < 0.05.
Results
Oxidative stress results
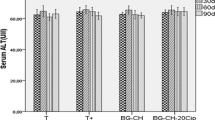
The aim of this study was to evaluate the oxidative stress after a bone filling in ovariectomized rats and to determine the role of release of ciprofloxacin from bioactive composite synthesized by melting and associated with chitosan. The evaluation of oxidative stress profile was performed on the bone marrow and shown in Figs. 1, 2, 3 and 4.
After 60 days of bilateral removal of the ovaries, the analysis of markers of oxidative stress in ovariectomized rats (T+) has an acute stress state following the evaluation of the advanced oxidation protein products (AOPP) and protein carbonyl (PC). These markers have been used by several authors as indices for the measurement of osteoclast activity. In parallel, there was a significant decline of the activities of thiols and Vit C in this group compared with those of control rats. After 15, 30, 60 and 90 days of implantation, the oxidative stress was significantly decreased by measuring the levels of AOPP and PC in rats implanted by BG–CS compared to ovariectomized rats. However, after these different periods, the rats implanted by BG–CS–20Cip showed slight decreases of these markers. The control of antioxidant concentrations seems to be particularly important for the preservation of cellular integrity. During the treatment, a significant evolution of these antioxidants was reported for rats implanted by composite without ciprofloxacin. Nevertheless, this evolution is less important for the rats implanted by composite containing 20% of the antibiotic (BG–CS–20Cip), possibly due to free radicals generated. The introduction of ciprofloxacin in the composite delays oxidative stress correction and, therefore, retards the mineralization and bone formation (later confirmed by the histological study).
Histological results
The histological examination on bone parts without previous decalcification, cut and stained with Masson’s trichrome can track different aspects of bone tissue before and after implantation with BG–CS and BG–CS–20Cip (Fig. 5). The bone negative control T shows a highly mineralized aspect, against the positive control T+ which is characterized by the presence of thin spans. In our study, the implants were all retained in bone defects and no signs of toxicity could be observed after 30 days. The loss of bone defects treated with BG–CS implants has demonstrated a more advanced healing than those treated with BG–CS–20Cip. Mineralized tissue was more abundant in the case of BG–CS than in that of BG–20Cip. This mineralization was initiated by a fibrocartilage tissue. Nonetheless, a structural alteration in the general organization of the femoral condyle treated with BG–CS–20Cip was reported. Ciprofloxacin allows the appearance of invasive implant fibrosis spans causing a reduced osteointegration. After 90 days of implantation, the BG–CS has proven to generate mature bone tissue, which is better integrated than the other implant. The bone repair by this implant (BG–CS) is crucial with the appearance of a normal trabecular bone.
Such composite is a good antiosteoporotic implant, and 90 days of implantation is a sufficient period to achieve a perfect osteointegration. At the same period, the BG–CS–20Cip material has different bone growth zones with a less advanced healing process than BG–CS. The incorporation of ciprofloxacin, thus, causes the delay of mineralization and consequently osteogenesis.
ICP-OES analysis
The study of the kinetics of degradation of the BG–CS and BG–CS–20Cip composites was undertaken by ICP-OES method. During the first 15 days, the content of Ca in the BG–CS increases from 124 to 180 µg/g (Fig. 6). However, a smaller increase of this element was detected in the BG–CS–20Cip (from 110 to 135 µg/g). In addition, the implementation of BG–CS and BG–CS–20Cip composites caused a significant increase in bone phosphorus content (Fig. 7). In fact, the obtained calcium phosphate ratio was 1.72 and 1.87 for the group implanted with BG–CS and BG–CS–20Cip, respectively, 60 days after implantation, compared to the control bones with a ratio of 1.69 (Fig. 8). This ratio was stabilized at normal levels for BG–CS and BG–CS–20Cip, respectively, after 60 and 90 days of implantation. This is the result of a redistribution of Ca and P, which varied after contact between the biomaterials and the biological environment around them to form an apatite layer showing the progress and bone healing. The latter is less advanced in the presence of ciprofloxacin. For the evolution of Si in the BG–CS implant, we observed a highly significant decrease of this element compared to the BG–CS–20Cip, which has very slow kinetics on its biodegradation (Fig. 9). Na levels are increasingly important with time but the changes between the two implants are not significant (Fig. 10).
SEM analysis
The morphology of the prepared composite (BG–CS and BG–CS–20Cip) is shown in Fig. 11A0, B0. BG–CS material shows the association of two forms of bioactive glass and chitosan particles. The incorporation of ciprofloxacin reveals the appearance of a neighboring stem BG–CS of the particles to form a new biocomposite which is the BG–CS–20Cip. After 15 days of implantation, an apatite deposit was detected more abundantly on the surface of these implants in the case of BG–CS than in that of the BG–CS–20Cip (Fig. 11A1, B1). This phenomenon may be explained by the presence of close contact between the composite and bone tissue favoring the beginning of their degradation to the detriment of an apatite deposit. The latter is delayed with the presence of the antibiotic. In this case, the process of degradation is very slow. After 60 days of implantation, we noted that the BG–CS surface was covered with the biological apatite to form a new bone tissue (Fig. 11A2). Nevertheless, the BG–CS–20Cip shows hydroxyapatite crystals with the persistence of a few fragments of our composite which is gradually resorbed (Fig. 11B2). Hence, the retarding effect of ciprofloxacin on the formation of the apatite biological layer is clearly noted.
Discussion
Recently, biotechnology has focused on composite loaded with therapeutic drugs and generators a dual function for bone matrices. First, these composites are characterized by their effect on the delivery of cells and the growth of new tissues [18, 19] and second, they are considered as carriers for the controlled delivery of antibiotics [19, 20]. These therapeutic drugs used in the treatment of bone diseases and administered locally have more advantages than systemic administration which causes liver [21, 22] and renal toxicity [23] and the risk of overdose, while increasing their bioavailability with an appropriate therapeutic concentration to effectively reach the target site [19, 24].
Recent studies have shown that the implantation of orthopedic devices in vivo induces a series of toxicological activities such as oxidative stress [25]. We evaluate the oxidative stress profile in vivo after the implantation of these composites. As an effect of ovariectomy, a substantial increase in the level of lipid peroxidation and a significant decrease in the activities of antioxidant enzymes bone were reported [26,27,28,29], which is in agreement with our study. Indeed, the estrogen deficiency was found to cause osteoclastic hyperactivity by lowering antioxidant defenses osteoblastic cells, thereby increasing ROS [29, 30]. A previous research work has shown that the increase of the antioxidant defenses in bone marrow is used to stop or slow down the bone degeneration process [33]. Indeed, the implantation of bioactive glass associated with chitosan in ovariectomized rats was proven to reduce the phenomenon of oxidative stress in the bone marrow. The experimental analysis revealed a significant decrease in the markers level of lipid peroxidation and a considerable increase in the activities of antioxidant enzymes in the bone marrow of rats implanted by the BG–CS compared to ovariectomized rats, which exhibited oxidative stress acute status. The dissolved ionic bioglass products (e.g., Si, Ca and P) stimulated the expression of several genes in osteoblastic cells [34] containing these enzymes. These are used to suppress osteoclast differentiation [30, 31]. A large variety of molecules, such as chitosan, provide protection for biological sites, as a result of the elimination of pro-oxidant molecules and acceleration of fracture healing [32]. Studies have shown that the chitosan oligosaccharides significantly inhibits the production of systemic levels of pro-inflammatory cytokines TNF-alpha and IL-1β at the tissue level. The BG–CS implant is well-known to prevent the deleterious effects of oxidative stress. To effectively fight against oxidative damage, the body has multiple defense systems such as repair or elimination systems of molecules damaged by ROS. Chitosan also stimulates various antioxidant enzymes that play an important role in maintaining the cellular redox potential. This administration, therefore, restores a normal antioxidant status in ovariectomized and implanted rats. The implanted composite BG–CS act as free radical scavengers and react with ROS. In this model, and in the light of these results, it is clearly seen that this reactivity reports powerful antioxidants and potential therapeutic agents for the treatment of osteoporosis (confirmed by histology analysis).
However, the incorporation of ciprofloxacin in the composite locally induces oxidative stress at the beginning of treatment. The drug causes significantly higher levels of lipid peroxidation and a substantial depletion of antioxidant enzymes, due to their rapid penetration into the bone [33], which is in accordance with the work of Talla and Veera reddy [34]. Most antibiotics generate ROS to kill bacteria in the bone [35], thus leading to cellular damage [34]. These free radicals have been induced by these exogenous chemicals in the direct redox cycle of the agent or its metabolism by cytochrome P450 [36]. Indeed, with the progress of the implantation time of BG–CS–20Cip, oxidative stress is reduced with the kinetics of release of the antibiotic. Our results have been confirmed by other studies that have stated that the in vivo degradation of the implanted biocomposite increases the release of ciprofloxacin for having a full salting in 6 weeks [37] causing a stabilization of oxidation state to a level similar to that of normal animals [28]. Ciprofloxacin exerts a negative effect on the metabolism of collagen type 1 and the signaling proteins of the cytoskeleton [38]. This causes disturbances in the integrity of the cells, leading to cell damage/death. Research studies have shown that the fluoroquinolones induce early stimulation of oxidative metabolism in chondrocytes immature rabbits [34].
In fact, the histological and physicochemical analyses confirm these results. These data, showing the progression and cell organization in each of the implanted biomaterials, allow the understanding of the results of bone repair. Combining bioglass and chitosan promotes the differentiation of osteoprogenitor cells and facilitates bone formation. Chitosan is a highly charged organic molecule that allows the creation of ionic bonds between the free carboxyl groups of the collagen, negatively charged, and the amine groups of the glucosamine units of the chitosan, positively charged [39]. This property seems to be favored by the attraction of growth factors through the polycationic properties of chitosan and their capture by gel formation [40].
At first, immature fibrocartilaginous microcalcifications appeared inside the implants. With the progress of implantation period, the appearance of trabecular bone and the increase of osteoblasts were reported. At the end of treatment (90 days), a formation of large zones of calcification was observed. Consequently, the inserted implant was invaded by a new bone. Our physiological and physicochemical results are proven to be complementary. A high intensity of ions of Ca and P was detected in the implant without ciprofloxacin because of the increase in its dissolution rate. A series of physicochemical reactions in the periphery of the material leads to a gradual degradation of our implants and its transformation into an apatite layer [27, 41] following the ion exchange with the surrounding tissue. At the end of treatment, the low rate of Si, and Na may cause a deposit of amorphous calcium phosphate layer, which can then be crystallized to create a layer apatite. In fact, the composition of bioactive glass makes the surface of the implant very reactive when exposed to an aqueous environment, leading to in vitro and in vivo biological activities [42]. The bioactive glass dissolution of the products has a positive effect on the expression of genes regulating osteogenesis [42,43,44]. Besides, chitosan penetrates through endocytosis in osteoclast where they act on different metabolic pathways to result in the inhibition or even apoptosis of the cell [45]. The bone bonding is attributed to the formation of an HA layer, which cooperates with the collagen fibrils of the damaged bone to form a bond causing the proliferation and attachment of progenitor cells of the bone cell differentiation and excretion of the bone extracellular matrix, followed by its mineralization [46].
The incorporation of ciprofloxacin in the implant retards osteogenesis, which in agreement with our previous work changing the chitosan by polyvinyl alcohol [10]. Although the resorption of the implant is made, it is slower than implants without ciprofloxacin. It has been suggested that the accumulation of ROS stimulates the apoptosis of human mesenchymal stem cells, and thereafter limits the formation of adipose tissue as well as bone and cartilage formation. Previous work have shown that chondrotoxicity caused by fluoroquinolone is a multifactorial event related to oxidative stress (lipid peroxidation and oxidative DNA damage chondrocytes and collagen, inhibition of proteoglycan synthesis…) [47, 48]. Other studies have reported that ciprofloxacin also leads to the reduction of the length of the limbs of rats [49] because of the inhibition of mitosis in the zone of proliferation. This antibiotic has declined with the thickness of the articular cartilage of the femoral condyle through the inhibition of proliferation of cultured chondrocytes and secretion of the soluble proteoglycans dependently of concentration and time [38]. In addition, it induces cartilage lesions during certain stages of development by changes in the morphology of the cytoskeleton. These articular damages were induced following the formation of the chelate–ciprofloxacin complex, causing a magnesium deficiency to the cytoskeleton. In fact, the magnesium deficiency could induce arthropathogenic effects with cartilage lesions [47]. Therefore, the introduction of ciprofloxacin causes a loss of bioactivity of the composite to the formation of the apatite layer, and consequently on bone formation. The advantage of drug incorporation is essential to treat various bone infections and prevent their propagation throughout the bone [9] by a retarding effect of the ossification. The introduction of antimicrobial agents in osteoconductive biomaterials (calcium sulphate, calcium phosphate, hydroxyapatite or tricalcium phosphate) was used for the local treatment of osteomyelitis [50]. This shows that the presence of ciprofloxacin with bioactive glass and chitosan leads to the delay of the formation of the hydroxyapatite layer, and subsequently the ossification of the bone. It is worthy to mention that this antibiotic belongs to the fluoroquinolone family that affects the function of osteoblasts in vitro [51, 52]. This family, except for gentamicin or vancomycin, has also been shown to impair fracture healing in vivo [52, 53], which is consistent with our results on the delayed ossification. After the antibiotics’s release, the composite behaves as devoid of drug to be effective as a material to protect bones against local osteoporosis, and is likely to help accelerating the healing of damaged bone through the creation of a chemical link. Then, these composites are proven to support bone growth.
Conclusion
The present paper has shown that, in vivo, BG–CS implant presents an excellent oxidative balance correction. The association of chitosan with BG is an efficient strategy for bone repair/antioxidation therapies. Furthermore, a progressive degradation and an accelerated mineralisation were confirmed by histological, ICP-OES and SEM analyses. However, the introduction of ciprofloxacin in this composite has been proven to cause a retardatory effect of hydroxyapatite layer formation, and consequently osteogenesis at the beginning of treatment. Nevertheless, the use of antibiotics in the implant is crucial to prevent osteomyelitis during the surgical intervention of implantation.
References
Goonoo N, Jeetah R, Bhaw-Luximon A, Jhurry D (2015) Polydioxanone-based bio-materials for tissue engineering and drug/gene delivery applications. Eur J Pharm Biopharm 97(Part B):371–391. https://doi.org/10.1016/j.ejpb.2015.05.024
Ding Y, Souza MT, Li W et al (2015) Bioactive glass-biopolymer composites. In: Antoniac IV (ed) Handbook of bioceramics and biocomposites. Springer International Publishing, Cham, pp 1–26
Tabujew I, Peneva K (2014) Chapter 1 Functionalization of cationic polymers for drug delivery applications. 1–29. https://doi.org/10.1039/9781782620105-00001
Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J 49:780–792
Park JH, Saravanakumar G, Kim K, Kwon IC (2010) Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv Drug Deliv Rev 62:28–41. https://doi.org/10.1016/j.addr.2009.10.003
Bhattarai N, Gunn J, Zhang M (2010) Chitosan-based hydrogels for controlled, localized drug delivery. Adv Drug Deliv Rev 62:83–99. https://doi.org/10.1016/j.addr.2009.07.019
Gentile P, Nandagiri VK, Daly J et al (2016) Localised controlled release of simvastatin from porous chitosan–gelatin scaffolds engrafted with simvastatin loaded PLGA-microparticles for bone tissue engineering application. Mater Sci Eng C 59:249–257. https://doi.org/10.1016/j.msec.2015.10.014
Shi C, Zhu Y, Ran X et al (2006) Therapeutic potential of chitosan and its derivatives in regenerative medicine 1. J Surg Res 133:185–192. https://doi.org/10.1016/j.jss.2005.12.013
Nayak AK, Sen S (2009) Hydroxyapatite–ciprofloxacin minipellets for bone-implant delivery: preparation, characterization, In-vitro drug adsorption and dissolution studies. Int J Drug Dev Res 1:47–59
Boulila S, Oudadesse H, Badraoui R et al (2016) Antioxidative/oxidative effects and retarding osteoconductivity of ciprofloxacin-loaded porous polyvinyl alcohol/bioactive glass hybrid. Med Biol Eng Comput. https://doi.org/10.1007/s11517-016-1473-1
Automatic registration of 2D histological sections to 3D microCT volumes: trabecular bone—ScienceDirect. https://www.sciencedirect.com/science/article/pii/S8756328217303162. Accessed 24 Mar 2018
Boulila S, Oudadesse H, Lefeuvre B et al (2015) Retarding bioactivity effect of ciprofloxacin incorporated in polyvinyl alcohol/bioglass scaffold as bone graft. Adv Mater Res 1104:163–167. https://doi.org/10.4028/www.scientific.net/AMR.1104.163
Driss D, Soudani N, Boudawara T et al (2014) Toxicological study and oxidative stress evaluation for safety assessment of xylanase preparations in Wistar rats. J Biochem Mol Toxicol 28:490–500. https://doi.org/10.1002/jbt.21589
Kayali R, Cakatay U, Akçay T, Altuğ T (2006) Effect of alpha-lipoic acid supplementation on markers of protein oxidation in post-mitotic tissues of ageing rat. Cell Biochem Funct 24:79–85. https://doi.org/10.1002/cbf.1190
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Ghorbel I, Khemakhem M, Boudawara O et al (2015) Effects of dietary extra virgin olive oil and its fractions on antioxidant status and DNA damage in the heart of rats co-exposed to aluminum and acrylamide. Food Funct 6:3098–3108. https://doi.org/10.1039/c5fo00342c
Jacques-Silva MC, Nogueira CW, Broch LC et al (2001) Diphenyl diselenide and ascorbic acid changes deposition of selenium and ascorbic acid in liver and brain of mice. Pharmacol Toxicol 88:119–125
Guarino V, Gloria A, Raucci MG et al (2012) Bio-inspired composite and cell instructive platforms for bone regeneration. Int Mater Rev 57:256–275. https://doi.org/10.1179/0950660812Z.00000000021
Mouriño V, Cattalini JP, Li W et al (2014) Multifunctional scaffolds for bone tissue engineering and in situ drug delivery. In: Boccaccini AR, Ma PX (eds) Tissue engineering using ceramics and polymers, 2nd edn. Woodhead Publishing, Sawston, pp 648–675
Hum J, Boccaccini AR (2012) Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: a review. J Mater Sci Mater Med 23:2317–2333. https://doi.org/10.1007/s10856-012-4580-z
Zimpfer A, Propst A, Mikuz G et al (2004) Ciprofloxacin-induced acute liver injury: case report and review of literature. Virchows Arch Int J Pathol 444:87–89
Alshammari TM, Larrat EP, Morrill HJ et al (2014) Risk of hepatotoxicity associated with fluoroquinolones: a national case–control safety study. Am J Health Syst Pharm AJHP Off J Am Soc Health Syst Pharm 71:37–43. https://doi.org/10.2146/ajhp130165
Lomaestro BM (2000) Fluoroquinolone-induced renal failure. Drug Saf 22:479–485
Mouriño V, Boccaccini AR (2010) Bone tissue engineering therapeutics: controlled drug delivery in three-dimensional scaffolds. J R Soc Interface R Soc 7:209–227. https://doi.org/10.1098/rsif.2009.0379
Jebahi S, Oudadesse H, Jardak N et al (2013) Biological therapy of strontium-substituted bioglass for soft tissue wound-healing: responses to oxidative stress in ovariectomised rats. Ann Pharm Fr 71:234–242. https://doi.org/10.1016/j.pharma.2013.05.003
Muthusami S, Ramachandran I, Muthusamy B et al (2005) Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta Int J Clin Chem 360:81–86. https://doi.org/10.1016/j.cccn.2005.04.014
Jebahi S, Oudadesse H, El Feki H et al (2012) Antioxidative/oxidative effects of strontium-doped bioactive glass as bone graft. In vivo assays in ovariectomised rats. J Appl Biomed 10:195–209. https://doi.org/10.2478/v10136-012-0009-8
Jebahi S, Oudadesse H, Saleh GB et al (2014) Chitosan-based bioglass composite for bone tissue healing: oxidative stress status and antiosteoporotic performance in a ovariectomized rat model. Korean J Chem Eng 31:1616–1623. https://doi.org/10.1007/s11814-014-0072-9
Lean JM, Davies JT, Fuller K et al (2003) A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 112:915–923. https://doi.org/10.1172/JCI18859
Lean JM, Jagger CJ, Kirstein B et al (2005) Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 146:728–735. https://doi.org/10.1210/en.2004-1021
Jebahi S, Oudadesse H, Trabolsi O et al (2014) Effect of hydroxyapatite and strontium-doped bioactive glass on the antioxidant defense mechanism after bone graft in Wistar rat model. Open J Biomed Mater Res 1:21. https://doi.org/10.12966/ojbmr.04.02.2014
Prasad AS, Bao B, Beck FWJ et al (2004) Antioxidant effect of zinc in humans. Free Radic Biol Med 37:1182–1190. https://doi.org/10.1016/j.freeradbiomed.2004.07.007
Meissner A, Borner K (1993) Concentration of ciprofloxacin in bone tissue. Aktuelle Traumatol 23:80–84
Talla V, Veerareddy P (2011) Oxidative stress induced by fluoroquinolones on treatment for complicated urinary tract infections in indian patients. J Young Pharm JYP 3:304–309. https://doi.org/10.4103/0975-1483.90242
Becerra MC, Eraso AJ, Albesa I (2003) Comparison of oxidative stress induced by ciprofloxacin and pyoverdine in bacteria and in leukocytes to evaluate toxicity. Lumin J Biol Chem Lumin 18:334–340. https://doi.org/10.1002/bio.742
Afolabo OK, Oyewo EB (2014) Effects of ciprofloxacin and levofloxacin administration on some oxidative stress markers in the rat. World Acad Sci Eng Technol Int J Biol Food Vet Agric Eng 8:72–76
Castro C, Sánchez E, Delgado A et al (2003) Ciprofloxacin implants for bone infection. In vitro–in vivo characterization. J Control Release 93:341–354. https://doi.org/10.1016/j.jconrel.2003.09.004
Adikwu E, Brambaifa N (2012) Ciprofloxacin induced chondrotoxicity and tendinopathy. Am J Pharmacol Toxicol 7:94–100. https://doi.org/10.3844/ajptsp.2012.94.100
Damour O, Gueugniaud PY, Berthin-Maghit M et al (1994) A dermal substrate made of collagen–GAG–chitosan for deep burn coverage: first clinical uses. Clin Mater 15:273–276
Jin R, Moreira Teixeira LS, Dijkstra PJ et al (2009) Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 30:2544–2551. https://doi.org/10.1016/j.biomaterials.2009.01.020
Greenspan DC, Hench LL (1976) Chemical and mechanical behavior of bioglass-coated alumina. J Biomed Mater Res 10:503–509. https://doi.org/10.1002/jbm.820100405
Huang J, Best SM (2007) Ceramic biomaterials. In: Boccaccini AR, Gough JE (eds) Tissue engineering using ceramics and polymers. Woodhead Publishing, Swaston, pp 3–31
Xynos ID, Edgar AJ, Buttery LD et al (2000) Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Biophys Res Commun 276:461–465. https://doi.org/10.1006/bbrc.2000.3503
Xynos ID, Edgar AJ, Buttery LD et al (2001) Gene-expression profiling of human osteoblasts following treatment with the ionic products of bioglass 45S5 dissolution. J Biomed Mater Res 55:151–157
Nancollas GH, Tang R, Phipps RJ et al (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627. https://doi.org/10.1016/j.bone.2005.05.003
Jones JR (2013) Review of bioactive glass: from Hench to hybrids. Acta Biomater 9:4457–4486. https://doi.org/10.1016/j.actbio.2012.08.023
Halawa AM (2010) Effect of ciprofloxacin on the articular cartilage and epiphyseal growth plate cartilage in the growing Albino rats and the possible protective role of vitamin E (α-tocopherol): a histological and morphometric study. Egypt J Histol 3:569–582
Li Q, Peng S, Sheng Z, Wang Y (2010) Ofloxacin induces oxidative damage to joint chondrocytes of juvenile rabbits: excessive production of reactive oxygen species, lipid peroxidation and DNA damage. Eur J Pharmacol 626:146–153. https://doi.org/10.1016/j.ejphar.2009.09.044
Gao C, Gao Q, Li Y et al (2012) Preparation and in vitro characterization of electrospun PVA scaffolds coated with bioactive glass for bone regeneration. J Biomed Mater Res A 100:1324–1334. https://doi.org/10.1002/jbm.a.34072
Nelson CL, McLaren SG, Skinner RA et al (2002) The treatment of experimental osteomyelitis by surgical debridement and the implantation of calcium sulfate tobramycin pellets. J Orthop Res Off Publ Orthop Res Soc 20:643–647. https://doi.org/10.1016/S0736-0266(01)00133-4
Isefuku S, Joyner CJ, Simpson AHRW (2003) Gentamicin may have an adverse effect on osteogenesis. J Orthop Trauma 17:212–216
Perry AC, Prpa B, Rouse MS et al (2003) Levofloxacin and trovafloxacin inhibition of experimental fracture-healing. Clin Orthop 414:95–100. https://doi.org/10.1097/01.blo.0000087322.60612.14
Mäkinen TJ, Veiranto M, Lankinen P et al (2005) In vitro and in vivo release of ciprofloxacin from osteoconductive bone defect filler. J Antimicrob Chemother 56:1063–1068. https://doi.org/10.1093/jac/dki366
Acknowledgements
This research was supported by the Tunisian Ministry of Higher Education and Scientific Research and the Tunisian Ministry of Public Health, University of Rennes 1 and CNRS France. The authors would like to thank Mr. M. Mabrouk, a PhD student, and Pr. A. Mostapha, from the National Research Centre, Egypt. They also extend their thanks to Mrs. Leila Mahfoudhi, Emeritus teacher of English in the Sfax Faculty of Sciences, for proofreading and polishing the language of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boulila, S., Oudadesse, H., Kallel, R. et al. The performance of a scaffold bioglass–chitosan in the treatment of bone defect. Polym. Bull. 75, 5567–5586 (2018). https://doi.org/10.1007/s00289-018-2342-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-018-2342-x