Abstract
Thermal degradation of three biodegradable polyesters: poly(caprolactone), poly(lactic acid), and poly(hydroxybutyrate), was studied by thermogravimetry coupled to Fourier Transform Infrared Spectroscopy TGA/FTIR before and after they were partially degraded. TGA curves and Gram–Schmidt plots showed only one decomposition stage for both poly(caprolactone), PCL, and poly(lactic acid), PLA. In contrast, poly(hydroxybutyrate), PHB, exhibited two degradation stages by TGA, but only one region of evolved gases was appreciated in the Gram–Schmidt plot. It was established that hexenoic acid, ε-caprolactone, and small fragments of polymeric chains are the main degradation products of PCL, which were simultaneously released during thermal decomposition of this polymer. Meanwhile, carboxylic acid, aldehydes, and lactide monomer and/or oligomers were evolved from degradation of PLA. Finally, carboxylic acids and ester moiety were detected in the course of degradation of PHB; thus, random chain scission reaction took place during thermal decomposition of this polymer. Results from the spectroscopic characterization (FTIR and 1H NMR) of partially degraded samples supported the degradation mechanisms suggested by TGA/FTIR studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, biodegradable polyesters such as poly(caprolactone), PCL, poly(lactic acid), PLA, and poly(hydroxybutyrate), PHB, have received a great deal of attention as they have emerged as potential substitutes of the non-biodegradable synthetic polymers. This fact has triggered the publication of many papers on the thermal degradation of these polymers; however, there is still controversy about the mechanisms involved during their thermal decompositions. For instance, Persenaire et al. [1] proposed a two-stage degradation mechanism for PCL, where the first degradation stage implies a statistical rupture of the polyester chain via ester pyrolysis reaction yielding water, carbon dioxide, and 5-hexenoic acid. Su et al. [2] reported hexanoic acid and that the second stage is an unzipping depolymerization process at chain ends with hydroxyl end-groups to form ε-caprolactone. In contrast, Aoyagi et al. [3] suggested a single degradation mechanism for this polymer involving an unzipping depolymerization mechanism beginning at the polymer-chain ends.
Reports on the thermal degradation of poly(lactic acid), PLA, also show ambiguous and debatable results. Aoyagi et al. [3] reported that PLA decomposes to yield acetaldehyde, lactide monomer, and/or oligomers, whereas Vogel et al. [4] pointed out that carbon dioxide, carbon monoxide, water, and methane are also produced during thermal decomposition of PLA, in addition to acetaldehyde. They also noticed that lactide becomes the major degradation product as temperature is increased. Kopinke et al. [5] suggested that degradation of this polymer at temperatures above 200 °C, not only includes intramolecular transesterification leading to lactide and cyclic oligomers, but also cis-elimination leading to acrylic acid and acyclic oligomers.
On the other hand, Aoyagi et al. [3] and Vogel et al. [4] have reported that the thermal degradation mechanism of the polyhydroxybutyrate, PHB, almost exclusively involves a random chain scission reaction of the ester groups to form crotonic acid and its oligomers. However, Li et al. [6] observed that thermal decomposition of PHB produces not only carboxylic acids such as 2-butenoic acid (crotonic acid), but also esters as propenyl-2-butenoate and butyric-2-butenoate and even propene.
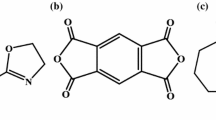
Keeping in mind these discrepancies founded in the literature about thermal degradation mechanism of the aforementioned biodegradable polymers, the aim of this work was to study the thermal decomposition pathways of PLC, PLA, and PHB using Thermogravimetry coupled with Fourier Transform Infrared Spectroscopy (TGA–FTIR) to analyze the evolved gases during their thermal decomposition. In addition, the partially degraded samples were studied by FTIR and 1H NMR. Chemical structures of polymers are displayed in Fig. 1.
Experimental
Materials
Poly(ε-caprolactone) was purchased from Sigma-Aldrich; poly(lactic acid) Ingeo™ 2002D, was obtained from NatureWorks, and poly(3-hydroxybutyrate), and was kindly supplied by Goldfellow. Polymers were used as received without further purification for the experiments. PCL has Tg = − 58 °C, Tm = 63 °C, and Mw = 8 × 104 g/mol; PLA has Tg = 59 °C, Tm = 164 °C, and Mw = 6 × 104 g/mol; and finally, PHB has Tg = 41 °C, Tm = 161 °C, and Mw = 1.6 × 105 g/mol.
Thermal degradation study
Thermal degradation of the polymers was carried out using a Perkin Elmer TGA/FTIR system consisting of a TGA Pyris 1 coupled to a Spectrum GX FTIR System. Samples (25–30 mg) were heated from 50 to 650 °C at a rate of 10 °C/min. Dry nitrogen gas with a flow of 60 mL/min carried the decomposition products through capillary tubing into the gas cell for IR detection. Both the transfer line and the gas cell were kept at 250 °C to prevent gas condensation.
IR spectra were recorded in the spectral range from 4000 to 650 cm−1 with a resolution of 4 cm−1 averaging 8 scans. Forty-eight spectra were obtained for each polymer sample.
Characterization of partially degraded samples
As TGA/FTIR studies only provide information regarding the released gases during thermal degradation of studied biopolymers, partially degraded samples were also examined by FTIR and 1H NMR to gain insight in the degradation mechanism of the mentioned polymers. For these experiments, residues of the polymer were taken from the TGA pan at the maximum rate decomposition temperature and characterized. For FTIR measurements, samples were analyzed using a Ge ATR accessory, whereas 1H NMR spectra were recorded with a Varian 600 MHz NMR spectrometer using CDCl3 as a solvent and tetramethylsilane as the internal standard in all cases.
Results and discussion
TGA results
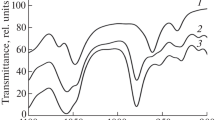
Figure 2 shows the TGA mass loss curve and the corresponding derivate curve (DTGA) obtained for the studied biopolymers (PCL, PLA, and PHB). Both poly(caprolactone) and poly(lactic acid) exhibit only one mass loss step with onset ca. 300 °C (see Fig. 2a), although the maximum rate decomposition temperature depends on the type of polymer (see Fig. 2b). This value was located at 430 °C for PCL and 395 °C for PLA. In contrast, PHB presents two well-defined degradation stages at 303 and 410 °C, being the first one the main transition as it comprises ca. 90 wt%. Gram–Schmidt plots show only one region of evolved gases (see Fig. 3) clearly related to the peaks of the DTG curves. This was observed even for PHB, despite the fact that this polymer shows two degradation stages, and could be attributed to the small amount of mass evolved during second mass loss and/or the emission of compound possessing poor absorbance in the last event.
It has been reported in the literature that the thermal decomposition of PCL occurs in one stage [2, 7] just as it was observed in this work. However, Persenaire et al. [1] proposed a mechanism involving two degradation stages for this polymer with basis on thermal degradation results with a high-resolution TGA. Regarding to PHB, our results revealed two degradation stages, which is contrary to a single-step degradation reported for this polymer [8]. Our results also show that PLA follows a similar mechanism reported by other authors [3, 4, 6, 9] with only one stage of mass loss.
FTIR spectra evolved during thermal degradation
Poly(caprolactone)
Figure 4a displays the stack plot of FTIR spectra of evolved gases from thermal degradation of PCL as a function of temperature. Figure 4b shows the IR spectrum of the evolved gases at the maximum evolution rate (430 °C), and those obtained at 444 and 456 °C as the latter exhibited interesting features. As can be seen, the spectrum of the evolved gases at the maximum evolution rate exhibits bands at 3576 and 3086 cm−1, due to O–H-stretching vibration and C=C–H stretching, respectively; bands in the range of 2944–2872 cm−1 related to C–H stretching of methylene groups and a band at 1164 cm−1 associated with C–O stretching. Besides, an intense band in the carbonyl region was also detected. A closer inspection of this band (see inset) demonstrated that the carbonyl peak is composed of three overlapped absorptions located at 1772, 1756, and 1736 cm−1, being the first of them more intense than the other ones in the evolved gases at 432 °C. Interestingly, the first two bands tend to disappear at 456 °C, remaining that located at 1736 cm−1. The peak at 1772 cm−1 can be associated with carboxylic acids in gaseous phase, and in fact, some authors have related this absorption to compounds such as hexenoic acid [1, 4] and/or hexanoic acid (caproic acid) [2]. Indeed, according to our findings, the presence of hexenoic acid seems to be more likely if one considers that evolved gases exhibited a band a 3086 cm−1 related to C=C–H-stretching vibration. Although the band at 1736 cm−1 has been related to the emission of ε-caprolactone by some authors [1, 4], we suggest that this signal is associated with ester groups of small chain fragments of PCL. In contrast, we believe that the band owing to ε-caprolactone should be situated at 1756 cm−1 as the internal tension in the cyclic structures increase the carbonyl-stretching absorption frequency in comparison with the acyclic ones.
Vogel et al. [4] and Persenaire et al. [1] pointed out that thermal degradation of PCL proceeds in two stages; the first process implies a statistical rupture of the polyester chains yielding H2O, CO2, and hexenoic acid (although the authors did not suggest that fragments of PCL were released during this process), while in the second, ε-caprolactone is evolved as results of an unzipping depolymerization process. These results were similar to those observed in this work, although our results suggest that both processes occur simultaneously together with the emission of small chain fragments of PCL, being the later event, the most important mechanism at 456 °C. In addition, Persainare et al. [1] reported that significant amounts of carbon dioxide were released during degradation of PCL; however, our results indicate that CO2 is produced (see bands at 2358 and 672 cm−1) during this process, but its amount is lower.
Poly(lactic acid)
Stack plot of FTIR spectra of evolved gases as a function of temperature for thermal degradation of PLA and FTIR spectrum of gases released at the maximum evolution rate are shown in Fig. 5. An intense band in the carbonyl region, which was formed by overlapping of several absorptions, was clearly observed; from this, absorptions at 1790, 1764, 1746, and 1728 cm−1 were identified. Spectrum also showed a band at 3576 cm−1 (related to O–H stretching), bands in the 3005–2600 cm−1 region (associated with C–H stretching), and peaks at 1244 and 1106 cm−1 (due to C–O-stretching vibrations). In addition, bands at 2360 and 2310 cm−1 owing to carbon dioxide, as well as signals in the range of 2200–2100 cm−1 due to carbon monoxide were also detected.
Several authors have studied the thermal decomposition of poly(lactic acid), and although some of them have found similar results, the process is still far from being fully understood. For example, Vogel et al. [4] and Aoyagi et al. [3] stated that acetaldehyde and lactide monomer are the main products of thermal degradation of PLA, though carbon dioxide and carbon monoxide were also detected but in minor proportions. The presence of acetaldehyde could be confirmed by bands at 2740 and 2696 cm−1 which are typical for O=C–H-stretching vibration and a band at 1728 cm−1 owing to C=O-stretching vibrations of the aldehyde group. Similarly, bands at 3004, 2980–2860, 1764, and 1244 cm−1 could be due to lactide. In this sense, it should be mentioned that some reports [4] have claimed that the carbonyl group of the lactide structure is located at 1790 cm−1 (not at 1764 cm−1), but Nikolic et al. [10] set this band at lower wavenumbers. Then, based on the previous results, it is possible to claim that the band at 1790 cm−1 is related to the release of carboxylic acids in gaseous state, in accordance with the findings of Kopinke et al. [5], who reported the presence of acrylic acid monomer and oligomers during thermal decomposition of this polyester.
Poly(hydroxybutyrate)
Figure 6 shows the stack plot of FTIR spectra obtained during thermal degradation of PHB as well as the IR spectrum of the evolved gases at the maximum evolution rate. As noted, the spectrum exhibited signals at 3578 and 970 cm−1 owing to O–H-stretching vibration of carboxylic acids. In addition, it can be observed that the carbonyl band, which is the most intense signal displayed in the spectrum, is composed of two absorption bands: 1770 and 1756 cm−1 (see inset). The former seems to confirm the presence of carboxylic acids in the evolved gases generated during thermal degradation of PHB. In fact, several authors [3, 6, 7, 9] have reported that the crotonic acid (2-butenoic acid) is one of the main decomposition products of this polymer. The presence of this compound is supported by the presence of bands at 3064, 1662, and 1148 cm−1, which are attributed to =C–H-, C=C-, and C–O-stretching vibrations, respectively. A band at 1098 cm−1 could also be related to C–O-stretching vibration and that at 1756 cm−1 could indicate the presence of ester groups in the released products during degradation of this polymer as suggested by Li et al. [6].
Spectroscopic characterization of partially degraded samples
Since the TGA–FTIR studies only provide partial information regarding the released gases, the polymers were also partially degraded and examined by FTIR and 1H NMR to acquire additional information on the degradation behavior of these biopolymers.
Poly(caprolactone)
Figure 7a shows the FTIR spectra of the non-degraded PCL as well as that after degraded at 430 °C. It can be seen that the spectrum of partially degraded PCL is very similar to that of the non-degraded polymer, and only a slight increase in intensity of the band at 3470 cm−1, related to O–H-stretching vibration, can be noted. Spectra in Fig. 7a show absorption bands at 2944 and 2865 cm−1 corresponding to the asymmetric and symmetric C–H-stretching vibration of methylene groups; an intense band at 1723 cm−1 due to carbonyl of ester group, and bands at 1240 and 1183 cm−1 related to asymmetric and symmetric C–O–C stretching, respectively, are clearly appreciated.
The proton nuclear magnetic resonance (1H NMR) spectra of the PCL samples (undegraded and degraded at 430 °C) exhibited a similar behavior than displayed by FTIR spectra (see Fig. 7b); i.e., no relevant changes were observed when these were compared each other. Indeed, spectra present signals at 4.0 and 2.25 ppm assigned to CH2–O and O=C–CH2, respectively, whereas peaks related to methylene proton of PCL backbone at 1.6 ppm (CO–CH2CH2CH2CH2CH2O) and 1.33 ppm (CO–CH2CH2CH2CH2CH2O) were also detected [11, 12]. In addition to the typical PCL signals, the 1H NMR spectra of the polymers present a small signal at 3.6 ppm, which was attributed to protons of CH2OH from end-chains [12]; these signals are more evident in degraded sample. These results confirm that thermal degradation of PCL involves statistical rupture of the polyester chains and/or an unzipping depolymerization process, since both processes yield similar FTIR and NMR spectra.
Poly(lactic acid)
FTIR spectrum of non-degraded PLA (see Fig. 8a) exhibits an intense peak at 1746 cm−1 corresponding to carbonyl group of ester group. In addition, signals at 2995 and 2945 cm−1 related to C–H-stretching vibration from methyl groups and bands at 1452, 1382, and 1358 cm−1 associated with CH3- and C–H-bending vibrations can also be observed. In the range of 1200–1000 cm−1, there are peaks mainly related to asymmetric and symmetric C–O–C-stretching vibrations. At 868 and 755 cm−1, bands corresponding to C–COO stretching and C=O deformation modes, respectively, were also noted [13].
In contrast with the findings for PCL, FTIR spectrum of PLA heated at 395 °C was very different to that recorded for the non-degraded sample (Fig. 8a top). A broad band centered at 3420 cm−1 and bands at 2630 and 2550 cm−1 were present in the partially degraded sample, but absent in non-degraded polymer. These bands support the hypothesis that carboxylic groups (it has been reported acrylic acid, but lactic acid cannot be discarded) are produced during thermal decomposition of PLA. The carbonyl band located at 1746 cm−1 in the non-heated PLA was shifted to 1724 cm−1 in the degraded one; in addition, a shoulder situated at 1642 cm−1 was also detected in the last sample. These results could be due to the appearance of carboxylic acids and aldehyde groups replacing the ester groups in the main polymeric chain of the PLA. Besides, some bands related to methyl groups and/or C–O linkages displayed by non-degraded sample were reduced in intensity (1452, 1083, and 1040 cm−1) or disappeared (868 and 755 cm−1) in the degraded sample.
In the 1H NMR spectrum of pristine PLA, peaks at 5.15 ppm and 1.56 ppm can be attributed to CH and CH3 protons (Fig. 8b). These signals remain in the spectrum of the degraded sample, but reduced in intensity. Moreover, new peaks at 4.36 and 1.50 ppm were also observed in the degraded sample that can be attributed to CH and CH3 protons from lactic acid oligomers [14, 15]. This fact supports the findings by Aoyagi et al. [3] and Kopilke et al. [5] who pointed out that lactide oligomers are produced from thermal decomposition of PLA. Finally, a small broad signal at 3.08 ppm was detected and related to OH protons from end-groups of heated PLA [14].
Poly(hydroxybutyrate)
Figure 9a shows the FTIR spectra of non-degraded PHB and that obtained from residue resulting after the sample was heated at 303 °C. As noted, the spectrum of heated PHB was very similar to the non-degraded sample, although subtle differences are showing mainly in the region of 1400–1000 cm−1, which includes the stretching vibration of the C–O–C linkage. The spectra show bands in the range of 3000–2900 cm−1, which are associated with C–H-stretching vibrations from methyl, methylene, and methyne groups. Bands at 1455 and 1379 cm−1, related to the asymmetric and symmetric deformation of the methyl groups, respectively, are also presented. As expected, an intense band at 1723 cm−1 due to carbonyl-stretching vibration of an ester group [16] is also evident.
1H NMR spectra of the PHB samples are shown in Fig. 9b. For non-degraded sample, the methyne (–CH2–CH–) and methylene (–CH2–CH–) protons of the main chain are observed at 5.25 and 2.56–2.47 ppm, respectively, while the side chain methyl protons are appreciated at 1.26 ppm [17]. Minor peaks are also noted in the spectra related to end-groups of the PHB polymer, which are typical for polymers produced by microorganisms [18]. The spectrum of degraded polymer exhibits the same main peaks, but the minor signals were increased in intensity due to the presence of non-volatile oligomers in the degraded sample [17, 19].
Conclusions
Thermal degradation of three biodegradable polyesters was investigated by means of the analysis of the evolved gases using the TGA/FTIR technique and through the study of partially degraded samples. Poly(caprolactone) degrades in only one stage where hexenoic acid, ε-caprolactone monomer, and small fragments of polymeric chains are simultaneously released, with the last ones predominating at higher temperatures. Meanwhile, the poly(lactic acid) also decomposes in one step releasing carboxylic acid, aldehydes, and lactide monomer and/or oligomers. Finally, although the degradation of polyhydroxybutyrate occurs in two stages, only in the former, carboxylic acids and ester moiety were detected being possible suggest that a random chain scission reaction takes place during thermal decomposition of this polymer. Results from spectroscopic characterization (FTIR and 1H NMR) of partially degraded samples support the degradation mechanisms suggested by TGA/FTIR studies.
References
Persenaire O, Alexandre M, Degee P, Dubois P (2001) Mechanisms and kinetics of thermal degradation of poly(ε-caprolactone). Biomacromolecules 2:288–294
Su T-T, Jiang H, Gong H (2008) Thermal stability and thermal degradation kinetics of poly(ε-caprolactone). Polym Plast Technol Eng 47:398–403
Aoyagi Y, Yamashita K, Doi Y (2002) Thermal degradation of poly[(R)-3-hydroxybutyrate], poly[ε-caprolactone] and poly[(S)-lactide]. Polym Degrad Stab 76:53–59
Vogel C, Siesler HW (2008) Thermal degradation of poly(ε-caprolactone), poly(L-lactic acid) and their blends with poly(3-hydroxybutyrate) studied by TGA/FTIR spectroscopy. Macromol Symp 265:183–194
Kopinke F-D, Mackenzie K (1997) Mechanistic aspects of the thermal degradation of poly(lactic acid) and poly(β-hydroxybutyric acid). J Anal Appl Pyrolysis 40–41:43–53
Li S-D, He J-D, Yu PH, Cheung MK (2003) Thermal degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) as studied by TG, TG-FTIR, and Py-GC/MS. J Appl Polym Sci 89:1530–1536
Vogel C, Morita S, Sato H, Noda I, Ozaki Y, Siesler HW (2007) Thermal degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) in nitrogen and oxygen studied by thermogravimetric-fourier transform infrared spectroscopy. Appl Spectrosc 61:755–763
Abe H (2006) Thermal degradation of environmentally degradable poly(hydroxyalkanoic acid)s. Macromol Biosci 6:469–486
Grassie N, Murray EJ (1984) The thermal degradation of poly(-(d)-β-hydroxybutyric acid): part 1—identification and quantitative analysis of products. Polym Degrad Stab 6:47–61
Nikolic L, Ristic I, Adnadjevic B, Nikolic V, Jovanovic J, Stankovic M (2010) Novel microwave-assisted Synthesis of Poly(D, L-lactide): the influence of monomer/initiator molar ratio on the product properties. Sensors 10:5063–5073
Messersmith PB, Giannelis EP (1993) Polymer-layered silicate nanocomposites: in situ intercalative Polymerization of ε-caprolactone in layered silicates. Chem Mater 5:1064–1066
Zoltowska K, Sobczak M, Oledzka E (1025) Novel zinc-catalytic systems for ring-opening polymerization of ε-caprolactone. Molecules 20:2816–2827
Kister G, Cassanas G, Vert M (1998) Effect of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 39:267–273
Marques DS, Santos JMC, Ferreira P, Correia TR, Correia IJ, Gil MH, Baptista CMSG (2016) Photocurable bioadhesive based on lactic acid. Mater Sci Eng C 58:601–609
Jarmelo S, Marques DAS, Simoes PN, Carvalho RA, Batista RA, Araujo-Andrade C, Gil MH, Fausto R (2012) Experimental (IR/Raman and 1H/13C NMRE) and theoretical (DFT) studies of the preferential conformations adopted by L-lactic acid oligomers and poly(L-lactic acid) homopolymer. J Phys Chem B 116:9–21
Parra DF, Forster PL, Lyszczek R, Ostasz A, Lugao AB, Rzaczynska Z (2013) Thermal behavior of the highly luminescent poly(3-hydroxybutyrate: eu(tta)3(H2O)2 red-emissive complex. J Therm Anal Calorim 114:1049–1056
Michalak M, Kwiecien M, Kawalec M, Kurcok P (2016) Oxidative degradation of poly(3-hydroxybutyrate). A new method of synthesis for the malic acid copolymers. RSC Advances 6:12809–12818
Naheed N, Jamil N, Hasnain S, Abbas G (2012) Biosynthesis of polyhydroxybutyrate in Enterobacter sp. SEL2 and Enterobacteriaceae bacterium sp PFW1 using sugar cane molasses as media. African J Biotech 11:3321–3332
Morikawa H, Marchessault RH (1981) Pyrolysis of bacterial polyalkanoates. Can J Chem 59:2306–2313
Acknowledgements
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT), Grant 169398.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrera-Kao, W.A., Loría-Bastarrachea, M.I., Pérez-Padilla, Y. et al. Thermal degradation of poly(caprolactone), poly(lactic acid), and poly(hydroxybutyrate) studied by TGA/FTIR and other analytical techniques. Polym. Bull. 75, 4191–4205 (2018). https://doi.org/10.1007/s00289-017-2260-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-017-2260-3