Abstract
A series of flame-retardant waterborne polyurethanes were synthesized with 4,4′-diphenylmethane diisocyanate and polyethylene glycol600 (PEG600) and modified with different level of diethylbis(2-hydroxyethyl)aminomethylphosphonate (fyrol-6); besides, 1,1,1-Tris(hydroxymethyl)propane was introduced as chain extender and crosslinking agent. The structures of these waterborne polyurethanes were confirmed by Fourier transform-infrared spectroscopy. Thermal degradation properties and flame retardancy were investigated by thermal gravimetric analysis (TGA), limiting oxygen index (LOI), and vertical burning test (UL-94). Wettability and mechanical property of film were measured with contact angle analyzer and universal mechanical machine, respectively. The results showed that when the mass of fyrol-6 was 12.0 % and the TMP was 1.7 %, an LOI value of 28.1 % and a UL-94V-2 rating could be achieved. Besides, the mechanical properties, including tensile strength, 100 % modulus and impact strength, are also relatively good. Simultaneously, the TGA analysis indicated that the incorporation of fyrol-6 enhanced the carbon residue from 8.9 to 19.9 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A waterborne polyurethane (WPU) is constituted by a linear thermoplastic polyurethane which is dispersible in water [1, 2]. Waterborne polyurethane (WPU) is tasteless, non-toxic, does not pollute the environment and cause no danger to the health of operators [3–5]. Furthermore, polymers derived from WPU dispersions exhibit an excellent combination of physical properties such as high tensile and tear strength, high elasticity, a range of hardness, excellent abrasion resistance, good resistance to chemicals and good low-temperature stability [6]. These properties allow WPU to be used in extensive commercial applications including coatings and adhesives for automobiles and wood as well as for numerous flexible substrates such as leathers, synthetic leathers, textiles, membranes and rubbers [7]. In the past decades, the preparation of WPUA has been mainly focused on expensive diisocyanates, such as isophorone diisocyanate (IPDI), tetramethylxylylene diisocyanate (m-TMXDI), and 1,6-hexamethylene diisocyanate (HDI) [1, 8–10]. Compared with these, MDI-based PU shows better mechanical properties and thermal stability [11, 12].
In the past decades, studies of WPU have been mainly focused on the enhancement of the nano materials. E. Orgilés-Calpena et al. mixed the carbon nanotubes (CNTs) with waterborne polyurethane, which greatly enhanced the mechanical properties of the waterborne polyurethane film [13]. Sang Hyop-Choi et al. fed graphene sheets (FGSs) into WPU with agitation at 100 rpm to get a mixture of FGS and WPU and conductivity and thermal resistance of WPU were obviously improved [14]. However, noticeable attention should be paid in controlling the inherent flammability of WPU. Because the major application fields of WPUs all have a significant demand for flame retardancy, the flammability restricts its further applications in stringent fire situation [15–17]. Various flame retardants have been developed to improve the flame retardancy of WPU over the years. Traditionally, the halogenated compounds which have advantages of higher efficiency and lower price can endow the polyurethane excellent flame retardancy. Simultaneously, they guarantee the polyurethane exhibiting mechanical performance similar to the analogs without flame retardancy [18]. However, despite these benefits, flame-retardant WPU with bromine and chlorine can emanate obscuring, toxic and corrosive gases like dioxins and dense black smoke along with super-toxic halogenated dioxins and furans [19–21], which do lots of harm to the environment and health of humans. The phosphorus-containing flame retardants are considered to be the most promising candidate owing to their multifold advantages of notable high-efficiency flame resistant and less toxic gases when burning [22]. Most studies paid more attention to investigate additive type of phosphorus-based materials to improve the flammability of WPU, but heterogeneous dispersion and poor compatibility with WPU were shown [23]. Different from additive-type phosphorus-containing flame retardants, the reactive-type phosphorus-containing flame retardants can be introduced into the molecule, which enhances compatibility between the flame retardant and WPU. Therefore, the development of the reactive-type flame-retardant WPUs becomes the direction of flame-retardant WPU studies [24]. The research of Doris Pospiech et al. indicated that the incorporation of DOPO monomers can greatly improve the fire behavior of these bio-based aliphatic polyesters [25].
In this part of work, we presented a reactive-type phosphorus-containing halogen-free flame-retardant diethylbis (2-hydroxyethyl) aminomethylphosphonate (fyrol-6, shown as Fig. 1) as the flame-retardant extenders and introduced it into WPU. Besides, as a crosslinker, the 1,1,1-Tris (hydroxymethyl) propane (TMP) which replaced part of 1,4-butanediol (BDO) was used to enhance the mechanical property and water resistance. Subsequently, several measurements had been conducted to evaluate the flame retardancy and other related performance.
Experimental
Material
Polyethylene glycol (PEG, AR; Tianjin Kermel, Tianjin China), which relative molecular mass is 600, 1,1,1-Tris (hydroxymethyl) propane (TMP, AR; Ainopharm chemical reagent, China) and 2,2-bis (hydroxymethyl) propionic acid (DMPA, AR; Sigma-Aldrich, USA) were vacuum dried at 100 °C for 3 h before use. Triethylamine (TEA, AR; Aladdin reagent, China), acetone (AR; Sigma-Aldrich, USA), dibutyltin dilaurate (DBTDL, AR; Aladdin reagent, China) and 1,4-butanediol (BDO, AR; Aladdin reagent, China) were used after dehydrating with 4-Å molecular sieves. Diethyl bis(2-hydroxyethyl)aminomethylphosphonate (fyrol-6, GR; Changzhou chemical research institute co., Ltd, China) and 4,4′-diphenylmethane diisocyanate (MDI, 97 %; TCI, Japan) were used as received.
Synthesis and modification of waterborne polyurethane
First, prepolymer of waterborne polyurethane was synthesized. 12 g MDI and 12 g PEG 600 were dissolved in acetone which was dried by 4-Å molecular sieves for a week. The solution of PEG600 was put into a 250-mL four-necked flask equipped with spherical condenser, thermometer, pressure-equalizing dropping funnel and a tube linked to nitrogen. The four-necked flask was placed in the oil bath. Then MDI was slowly dropped into four-necked flask when the temperature of reaction system is 60 °C. 10 min after the MDI was added, 1.2 g DMPA was added to the reaction system and the temperature was maintained at 60 °C for another 10 min. Then 0.072 g of catalyst DBTDL was added to the flask dropwise. Next, the temperature of the reaction system was raised to 80 °C and maintained for 3 h until the -NCO content was constant. The prepolymer would be obtained. Then the temperature of prepolymer was cooled to 65 °C and the mixture of fyrol-6 and BDO was added. The chain extension reaction lasted for 1.5 h. When the reaction was over, the temperature was cooled to 30 °C and 0.9 g of TEA was added to the mixture with a higher stirring (300–500 rpm). At last, polymer was emulsified under a strong stirring of 2500–3000 rpm for more than 30 min. After evaporation of acetone, a series of FWPU were prepared and named PU1, PU2, PU3, PU4, PU5 and PU6. The synthesis process and detailed chemical composition of WPU are shown in Scheme 1 and Table 1, respectively.
On the other hand, compared to the above steps, the steps of crosslinking modification of FWPU were somewhat different. After reducing the temperature of prepolymer to 65 °C, the TMP was added to the reaction system together with fyrol-6 and BDO. The next steps were the same as the steps of synthesizing the ordinary FWPU. Then, a series of FWPU which were modified by TMP (C-FWPU) were prepared and named PUa, PUb, PUc, PUd, PUe, and PUf. The synthesis process and detailed chemical composition are shown in Table 2 and Scheme 2, respectively.
Preparation of WPU films
The obtained waterborne polyurethane emulsions were first allowed to be placed at room temperature for 7 days in a mold made of Teflon. After the water evaporated, the films were placed in a vacuum oven and dried for 24 h at 60 °C until the weight of films did not change any more. The dried films should be stored in a desiccator to ensure the purity of the films.
Analysis of the WPU films
The chemical structures of the different simples were analyzed using Fourier transform-infrared spectroscopy (FT-IR; Nicolet 510, Nicolet, America) in the range of 400–4000 cm−1. A particle size analyzer (Mastersizer 2000, Malvern) was used to determine the particle size of emulsions. The wettability and contact angles could be obtained using a contact angle analyzer (JC2000, Shanghai Zhongchen Digital Technic Apparatus co., ltd, Shanghai China). X-ray diffractometer (Rigaku Corporation, Japan) was used to determine the effect of modification on the crystallization of polyurethane. The viscosity of waterborne polyurethane emulsion was tested using NDJ-I (Tianjin Yonglida laboratory equipment Co., Ltd.) viscometer at 25 °C according to GB/T2794.
The thermal stability was analyzed using a thermogravimetric analyzer (Pyris-1, PE, America) in a surrounding of 50 mL/min of flowing nitrogen with a heating rate of 10 °C/min between 30 and 600 °C. The LOI test was carried out using oxygen index instrument (JF-3, Jiangning Analysis Instrument Factory, Jiangning, China) according to the standard (150 × 20 × 0.3 mm; length, width, thickness) of ASTM D2863. UL-94 of WPU film was conducted by a CZF-3 horizontal and vertical burning tester (Jiangning Analysis Instrument Factory, Jiangning China) according to the standard of GB/T 2408-2008. The cone calorimetry test was carried out using a cone calorimeter (Stanton Redcroft, England) according to ISO5660-1. Each specimen, with the dimensions of 100 mm × 100 mm × 4 mm, was wrapped in an aluminum foil and exposed horizontally to an external heat flux of 50 kW/m2.
The mechanical properties of film, including tensile strength, elongation at break and 100 % modulus, were determined by universal mechanical testing machine (RGT-20A, Shenzhen Reger Instrument Equipment Co., Ltd., China) and the film was made into rectangular sample according to GB/T 1040.3-2006, which size was 150 mm × 20 mm. The impact strength of the film was tested by QCJ film impact tester (Shanghai Le Ao Test Instrument Co., Ltd.).
The peel strength was determined by universal mechanical testing machine (RGT-20A, Shenzhen Reger Instrument Equipment Co., Ltd., China) according to the requirements of GB/T 2970. The waterborne polyurethane emulsion was evenly coated on the surface of two PVCs (130 mm × 30 mm), the sample was activated at 70 °C for 5 min, and then the sample was given a pressure of 1.2 MPa for 24 h. At last, the peel strength was tested. At last, the TEM (JEM-2100, JEOL Ltd., Japan) was used to investigate the surface morphology of emulsion particles.
Results and discussion
Analysis of FT-IR
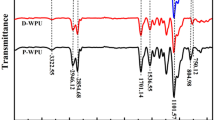
In the FT-IR spectrums in Fig. 2, the characteristic absorption peaks of –NCO which should be shown at 2200–2300 cm−1 was gone. The peaks of 1225 and 1106 cm−1 refer to C–O–C stretching vibration, and the peak of 1727 cm−1 belongs to the stretching vibration of C=O. The peak at 3299 cm−1 presents N–H stretching vibration of carbamide. All of these peaks send a massage that the samples are polyurethane. Compared with the waterborne polyurethane without fyrol-6, the samples modified with fyrol-6(PU5, PUf) showed obvious peaks at 1070 and 1226 cm−1 which belong to P–O–C and P=O, respectively, but the stretching vibration peak of P=O is covered by the peak of C–O–C, so it hard to be seen. The soaking liquids of alcohol of films of C–FWPU and FWPU were also tested by FT-IR and the characteristic absorption peaks of fyrol-6 cannot be found. These two situations shown above revealed that the fyrol-6 had already been introduced into the systems of polyurethane.
Properties of WPU dispersions
In this paper, statuses of the different WPU dispersions are shown in Tables 1 and 2. The result of Table 1 showed that the emulsions are always blue due to the increase of the mass of flame retardant. But the stability of WPU emulsions becomes worse when compared to the emulsions without flame retardant; besides, the viscosity of the emulsion is also reduced. The most possible reason was that the amount of fire-retardant fyrol-6 was too much to react with isocyanate totally and the consistency between fyrol-6 and WPU was not very good, so that too much residual fyrol-6 destroyed the stability of emulsions seriously and accelerated the separation of two phases, meanwhile affected the viscosity of the emulsion. But the stability of emulsions was pretty good when the ratio of fire retardant was not very high (<15 %).
Table 2 shows the emulsion statuses of PUa–PUf. From it, the conclusion could be drawn that the emulsions became white gradually with the increase of TMP. Simultaneously, the particle size also became larger and larger, which increases the viscosity of the emulsion. However, the emulsions of PUa–PUf had good stability.
The wettability and water absorption of the films
Contact angle is a physical quantity to measure the wettability degree of material. The larger the contact angle, the stronger the hydrophobicity of the material, the smaller the contact angle, the stronger the hydrophilicity of material. In this experiment, 3 μL of distilled water was used for detecting contact angle. The combination of contact angle and water absorption can give a very good evaluation on the water resistance of the film. The wettability and water absorption of the films are discussed in this paper.
Table 3 shows that the contact angles between films and water became smaller and smaller with the increase of the fyrol-6. The film of PU1 has a contact angle of 83.5°, while the films of PU4 and PU5 are 64.3° and 57.0°, which can be observed from the images of Fig. 3a–c. Correspondingly, the water absorption also increased, from 13.7 to 22.3 %. This phenomenon indicated that the hydrophily of the films became stronger and stronger. The most important reason was the introduction of the phosphonate. On the other words, the existence of P–O bond changed the surface energy of the films and increased the hydrophily of the films.
From Table 4, it can be found that with the increase of TMP, the contact angle increases from 57.0° to 73.2°; meanwhile, the water absorption decreases from 22.3 to 12.4 %. Besides, the particle sizes of emulsions increase with the increase of TMP content. This result may be ascribed to the crosslinking of PU molecular chains. Crosslinking changed the linear structure of the molecule to a three-dimensional network. Thereby, the segmental mobility of molecule is reduced. Moreover, the amount of polar urethane and urea group at the surface of WPU film is also reduced by the crosslinked network which restricts movement of molecular chains and this decrease produces a decline of the surface energy of WPU film.
XRD analysis
The X-ray diffractometer was used to investigate the crystallinity of WPU [26]. Figure 4 shows the X-ray diffraction (XRD) curves of pure WPU, FWPU and C-FWPU. For pure WPU, nearly amorphous diffraction peak is seen near 2θ = 20°, indicating the crystallinity of polyurethane [27]. As can be observed from Fig. 4, all the curves of the diffractogram show the similar diffraction peak around 20°, which indicates the characteristic diffraction peak of the crystalline PU [28]. But the diffraction peaks of FWPU and C-FWPU in 20° are weaker than that of pure WPU. Furthermore, the intensity of C-FWPU is a little weaker than FWPU. It implies that the introduction of fyrol-6 disturbs the crystallinity of WPU. Simultaneously, the three hydroxyl groups of TMP reacts with -NCO group to develop a structure of three-dimensional network which limits the movement of chain segments and reduces the regularity of chain segments. Thereby, the intensity of diffraction peak is further reduced. In short, the addition of fyrol-6 and TMP can influence crystallinity of WPU and hinder its crystallization.
Thermal properties analysis
TG and DTG curves of ordinary and modified WPU films under N2 atmosphere are presented in Fig. 5a, b; the corresponding detailed data are also recorded in Table 5, showing the 5 wt% weight loss temperature (T ini), the maximum decomposition temperature (T max) and the char yield at 600 °C.
As depicted in Fig. 5, there are two main stages of degradation for pure WPU resulting from urethane and urea groups in hard segment and polyether soft segment, respectively [29]. However, for the FWPU and C-WPU, TG trace presents a typical three-step decomposition process, and accordingly there are three DTG peaks in the range of 100–500 °C. These statuses of degradation correspond to the degradation of phosphate groups, urethane and urea groups in hard segment and polyether soft segment, respectively.
On one hand, in contrast to ordinary WPU, the T ini of FWPU is much lower, as evidenced in Table 5. It is plausible to assume that the “abnormal” phenomenon should be ascribed to the degradation of phosphate groups which bond energy is relatively weaker [30, 31]. However, the T max of FWPU has a great enhancement, from 340.2 °C (PU1) to 368.9 °C (PU5). Besides, the existence of fyrol-6 helps increase the mass of residual carbon from 8.9 % (PU1) to 19.8 % (PU5), making an increase of 122.4 %. It is generally known that carbon layer can cut off oxygen and heat, so the mass of residual carbon can reflect the flame resistance of the material. When all of these changes are considered, the introduction of fyrol-6 increased the flame resistance of the films at a certain extent. Compared with the previous studies of Funda Celebi et al. [16], PU5 has a higher residual carbon.
On the other hand, it is clear that the T ini of C-WPU (PUf) is a little lower than that of FWPU (PU5), while the T max and residual carbon are little higher. It shows that crosslinking modification have some good effects on thermal stability of polyurethane, but not too much.
Fire properties
The results of LOI and UL-94 tests for PU1–PU5 are listed in Table 6. From this table, the LOI value of PU1 is 17.0 % and the UL-94 test shows no rate, which implies poorer flame retardancy. However, the addition of fyrol-6 enhanced the LOI values significantly. When the ratio of fyrol-6 reaches 12 % (PU5), the LOI value meets a high value of 28.1 % and meanwhile UL-94 satisfies a rating of V-2. All of these results indicated that fyrol-6 has good effect of flame retardancy, which is better than the similar research of Celebi et al. [32] in which the LOL value is 27 %. The enhancement of flame retardancy is attributed to the increase of char residue and the condensed flame-retardant effect derived from the addition of fyrol-6. The fyrol-6 has two hydroxyl groups, which can be used as a part of chain extension agent. Hydroxyl and isocyanate groups can interact with dibutyltin dilaurate and form a multi-core bridge coordination compound, this bridge can link the hydroxyl to isocyanate group and make the isocyanate and hydroxyl groups of the system become much more close. Therefore, fyrol-6 is introduced into the system of waterborne polyurethane [33], so as the phosphate. Because of lower bond energy, the C–P breaks first and forms a kind of phosphate during film burning. Moreover, the phosphate would be decomposed into phosphoric acid. At the same time, the phosphoric acid is further dehydrated to form metaphosphoric acid and the polymerization reaction of metaphosphoric acid produces poly-metaphosphoric acid, which promoted the enhancement of residual carbon [34].
Table 7 shows that adding crosslinker (TMP) has only little effect on the value of LOI and vertical burning of the samples whose flame-retardant fyrol-6 content is 12 % (PUa–PUf) and it also sends the message that the flame resistance of the film mainly depends on the fyrol-6 content.
Cone calorimeter analysis
Cone calorimeter is one of the most ideal experimental instruments to characterize the combustion performance of materials in the laboratory scale at present, and has been widely used to study the flame-retardant properties of materials. The simulative experimental environment is very similar to the real combustion environment of the material. Therefore, the experimental data obtained from the cone calorimeter test can reflect the combustion behavior of the material more truly. In these experimental data, the heat release rate (HRR) and total heat release (THR) are the two most important evaluation indexes.
The HRR and THR curves of ordinary WPU (PU1) and flame-retardant WPU (PU5) are shown in Fig. 6a, b, respectively, and the relative data are shown in Table 8. From Fig. 6 and Table 7, it can be learned that the peak heat release rate (PHRR) reaches maximum value in a very short period of time after the sample is ignited. Compared to the blank samples (PU1), the PHRR of FWPU (PU5) is decreased by about 27.3 %, from 235.2 to 171.1 kW/m2. Besides, THR is decreased by 15.03 %, from 65.12 to 55.33 MJ/m2. This is mainly due to the introduction of fyrol-6, which made the molecular chain equip with the phosphate group. When the material is heated, the phosphate will produce metaphosphoric acid, which can accelerate the dehydration and carbonization of the material, and form a large amount of dense residual carbon layer. These carbon layers play an important role in heat insulation. In addition, compared with the ordinary WPU (PU1), there is a delay of 3 s in the ignition time of flame-retardant samples (PU5), from 7 to 10 s; at the same time, burning time is from 1058 to 1411 s.
Compared with ordinary FWPU (PU5), C-FWPU (PUf) has the same ignition time. Besides, both PHRR and THR were not significantly changed, which showed that the crosslinking modification did not have much effect on the flame-retardant properties of the blank FWPU (PU5). In summary, the flame retardant has relatively good flame-retardant effect on waterborne polyurethane.
Mechanical property analysis
Mechanical properties measurement is an important test for WPU because mechanical performance directly affects the application of the materials. The data of tensile strength, 100 % modulus, elongation, impact strength and peel strength of common WPU, FWPU and C-WPU are listed in Tables 9 and 10, respectively. From Table 9, it can be seen that the tensile strength and 100 % modulus of the WPU film gradually decreased with the increase of the flame-retardant dose. The tensile strength, 100 % modulus and impact strength decreased by 67.9, 33.7 and 33.3 %, respectively, when the amount of fyrol-6 is 12 %, while the elongation increased by 95.3 %. This change comes down to the long molecular chain of flame retardant which can increase the flexibility of the molecular chain of polyurethane. Furthermore, the residual fyrol-6 can be considered as a plasticizer. All of these reasons generate the increase of elongation and decrease of tensile strength. The peel strength of the waterborne polyurethane emulsion is reduced by 42.0 %. The peel strength is related to the degree of crystallinity of the WPU film [35]. The addition of fyrol-6 hindered the crystallization of the WPU film, and decreased the degree of crystallinity. So the peel strength of FWPU emulsion is much lower than that of pure emulsion.
Table 10 shows the mechanics of performance changes of PUa–PUf films which are modified by TMP. When compared with the ordinary FWPU (PU5) film, tensile strength, impact strength and 100 % modulus of C-FWPU (PUf) are significantly improved 103.5, 33.3 and 22.2 % respectively. What is more, the elongation decreased by 32.1 %. This is mainly due to that TMP changes the original linear molecular chain into a crosslinked network due to the existence of three hydroxyl groups. The entanglement between molecules becomes more serious and the intermolecular force increases. However, compared with the blank FWPU emulsion, the peel strength of the C-FWPU emulsion is not much enhanced. This is mainly attributed to the fact that addition of TMP did not enhance the crystallization properties of the polyurethane, so its peel strength is not greatly enhanced, which is reflected in Fig. 4.
TEM observation
By means of the TEM, the appearance and dispersity of modified WPU can be analyzed. Figure 7a, b represents the TEM images of FWPU (PU5) and C-FWPU (PUf). It can be observed that the addition of crosslinker (TMP) makes the dispersity of FWPU become poorer. At the same time, the particle becomes irregular and larger. All of these phenomenons reveal the changes which come from the crosslinking of TMP.
Conclusion
In this study, the FWPU was synthesized by introducing fire-retardant fyrol-6; moreover, the FWPU was crosslinked by TMP to enhance its water resistance and mechanical properties. The various properties were analyzed by TGA, LOI, UL-94, cone, TEM, XRD and contact angle analyzer.
The results of LOI and UL-94 test indicated that when the mass of TMP and fyrol-6 reached 1.7 and 12 %, respectively, the value of LOI of the film reached 28.1 % and UL-94 met a rating of V-2; moreover, the data of cone calorimeter showed that the PHRR and THR were significantly reduced. In addition, the main mechanical properties, including tensile strength, 100 % modulus and impact strength, of FWPU modified by TMP were also significantly improved. The analysis of XRD attested that modification causes disorder in the crystallinity of the WPU which is the main reason that leads to the decrease in the peel strength of modified WPU. The images of TEM showed that the modification changes the size and the shape of the emulsion particle and decreased the dispersity of FWPU. The introduction of flame retardant decreased contact angle gradually and crosslinking modification increased it slowly.
In general, the modification of WPU enhanced the flame retardancy of the original flammable polyurethane film; at the same time, its comprehensive performance had not been influenced so much.
References
Pérez-limiñana MA, Arán-Ais F, Torró-Palau AM (2005) Characterization of waterborne polyurethane adhesives containing different amounts of ionic groups. Int J Adhes Adhes 25:507–517
Eun HK, Woo RL, Sang WM (2010) Characterization of waterborne polyurethane for ecofriendly functional floor plate. Prog Org Coat 68:130–134
Yong SL, Richard CL (2008) Soybean-oil-based waterborne polyurethane dispersions: effects of polyol functionality and hard segment content on properties. Biomacromolecules 9:3332–3340
Xiao DC, Peter RC, Michel AH (2008) Preparation and properties of plasticized starch modified with poly (ε-caprolactone) based waterborne polyurethane. Carbohyd Polym 71:119–125
Nanda AK, Wicks DA (2006) The influence of the ionic concentration, concentration of the polymer, degree of neutralization and chain extension on aqueous polyurethane dispersions prepared by the acetone process. Polymer 47:1805–1811
Sarish S, Luqman CA, Min MA (2015) Waterborne polyurethane dispersions synthesized from jatropha oil. Ind Crop Prod 64:194–200
El-Sayed AA, El-Gabry LK, Allam OG (2010) Application of prepared waterborne polyurethane extended with chitosan to impart antibacterial properties to acrylic fabrics. J Mater Sci Mater Med 21:507–514
Hui D, Zhao YH, Li QF (2008) Synthesis and characterization of waterborne polyurethane adhesive from MDI and HDI. J Appl Polym Sci 110:1396–1402
Yoon SS, Kim SC (2005) Modification of aqueous polyurethane dispersions by polybutadiene. J Appl Polym Sci 95:1062–1068
Young KJ, Cheong IW, Jung HK (2001) Chain extension study of aqueous polyurethane dispersions. Colloids Surf A 179:71–78
Kakarla RR, Anjanapura VR, Han MJ (2008) Synthesis and characterization of novel polyurethanes based on 4,4′-{1,4-phenylenebis[methylylidenenitrilo]} diphenol. Polym Bull 60:609–616
Kakarla RR, Anjanapura VR, Han MJ (2009) Synthesis and characterization of pyridine-based polyurethanes. Des Monomers Polym 12:109–118
Orgilés-Calpena E, Arán-Aís F, Torró-Palau AM (2012) Effect of amount of carbon nanotubes in polyurethane dispersions. Macromol Symp 321:135–139
Sang HC, Dong HK, Anjanapura VR (2012) Properties of graphene/waterborne polyurethane nanocomposites cast from colloidal dispersion mixtures. J Macromol Sci Phys 51:197–207
Limin G, Ge Zhen, Huang Muhua (2015) Halogen-free flame-retardant waterborne polyurethane with a novel cyclic structure of phosphorus: nitrogen synergistic flame retardant. J Appl Polym Sci 132:41288
Funda G, Leyla A, Güngör G, Idrìs MA (2003) Synthesis and characterization of waterborne and phosphorus-containing flame retardant polyurethane coatings. J Coat Technol 75:65–71
Peikun Z, Yazhou H, Saiqi T et al (2015) Flame retardance, mechanical and thermal properties of waterborne polyurethane conjugated with phosphorous-nitrogen intumescent flame retardant. Polym Compos 30:23630
Peikun Z, Saiqi T, Haojun F (2015) Flame retardancy and hydrolysis resistance of waterborne polyurethane bearing organophosphate moieties lateral chain. Prog Org Coat 89:170–180
Zhao YP, Yan JJ, Chen DM et al (2013) Preparation and characterization of compound halogen-free flame retardant polyurethane foams. J Fun Mater 5:697–699
Shuyu L, Matthias N, Henri M et al (2012) Flame retardancy and thermal decomposition of flexible polyurethane foams: structural influence of organophosphorus compounds. Polym Degrad Stab 97:2428–2440
Wang X, Xing WY, Feng XM et al (2014) Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: synthesis, flammability and mechanism. Polym Chem 5:1145–1154
Kahraman MV, Kayaman-Apohan N, Arsu N (2004) Flame retardance of epoxy acrylate resin modified with phosphorus containing compounds. Prog Org Coat 51:213–219
Chung YJ, Kim Y, Kim S (2009) Flame retardant properties of polyurethane produced by the addition of phosphorous containing polyurethane oligomers. J Ind Eng Chem 15:888–893
Gang W, Jin QL, Yun JL (2016) Flame retardancy and thermal degradation mechanism of a novel post-chain extension flame retardant waterborne polyurethane. Polym Degrad Stab 123:36–46
Doris P, Andreas K, Hartmut K (2015) Biobased aliphatic polyesters with DOPO substituents for enhanced flame retardancy. Macromol Chem Phys 216:1447–1461
Liang L, Zheng BX, Can BO, Li Z (2015) Effects of crosslinking on adhesion behavior of waterborne polyurethane ink binder. Prog Org Coat 88:155–163
Lei W, Yiding S, Xiaojuan L, Zhongjin L (2011) Synthesis and properties of crosslinked waterborne polyurethane. J Polym Res 18:469–476
Lai XJ, Li XR, Wang L, Shen YD (2010) Synthesis and characterizations of waterborne polyurethane modified with 3-aminopropyltriethoxysilane. Polym Bull 65:45–57
Levchik SV, Weil ED (2004) Thermal decomposition, combustion and fire-retardancy of polyurethanes-a review of the recent literature. Polym Int 53:1585–1610
Velencoso MM, Ramos MJ, Klein R, De Lucas A (2014) Thermal degradation and fire behaviour of novel polyurethanes based on phosphate polyols. Polym Degrad Stab 101:40–51
Wang X, Hu Y, Song L, Xing W (2010) Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 51:2435–2445
Celebi F, Osman P, Leyla A (2004) Synthesis and characterization of water-dispersed flame retardant polyurethane resin using phosphorus containing chain extender. J Appl Polym Sci 91:1314–1321
Hu C, Wang SF (1995) Use of organotin compounds. Chem Adhes 3:160–163
Wang CC, Dai Z, Huang YP, Xu GW (2010) Waterborne polyurethane modified with organic phosphorus of flame retardant. J Fun Polym 23:285–290
Kim BS, Jeong HY, Kim BY (2005) Surface characterizations of polyurethanes having different types of soft segment. Colloids Surf A 268:60–67
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, L., Guo, J. & Zhao, S. Flame-retardant and crosslinking modification of MDI-based waterborne polyurethane. Polym. Bull. 74, 2099–2116 (2017). https://doi.org/10.1007/s00289-016-1826-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1826-9