Abstract
Poly(n-octadecyl methacrylate-co-2-hydroxyethyl methacrylate) [poly(OMA-co-HEMA)] block copolymers were synthesized by free radical polymerization from n-octadecyl methacrylate (OMA) and 2-hydroxyethyl methacrylate (HEMA) and these copolymers have been assessed as stabilizer in the preparation of polycaprolactone (PCL) microspheres using non-aqueous dispersion polymerization. The copolymer poly(OMA-co-HEMA) has been used as in situ stabilizer in the preparation of PCL microspheres at 80 °C. Spherical PCL microspheres are found to be well dispersed and uniform in size which demonstrated the efficient of OMA: HEMA copolymer as replacement of preformed stabilizer in synthesis of PCL microspheres. The factors affecting in the preparation of PCL microspheres such as polymer molecular weights and molecular weight distribution, particle size and distribution and morphology were studied. The synthesized poly(OMA-co-HEMA) copolymer used as in situ stabilizer is characterized by FT-IR, NMR, XRD and GPC techniques. The morphology and particle sizes of the prepared microspheres are observed by field emission scanning electron microscope.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last two decades, it has been seen that the importance and interest is significantly increased for a paradigm shift from biostable to biodegradable biomaterials for medical and related applications. A spectacular advance in new polyester materials and the development of therapeutic devices for tissue engineering and drug delivery systems has been promoted [1]. Tissue engineering has gained increasing attention because of its significant medical applications in restoring, maintaining or improving functions for injured tissues or organs. In general, the formulations for a drug-delivery system are based on the polymer microparticles, scaffolds, implants and fibres. Biodegradable polymeric microspheres have been developed now a days for the application in tissue engineering like bone or skin grafting and drug delivery system specially for the curing of a targeted organ that needs special dosages without harming the other parts of the body such as tumour, cancer etc., [2–4]. Non toxic polymer microspheres with uniform size, porosity and controllable degradability added a new dimension for the innovation of novel biodegradable polymeric microsphere systems that fulfill all these criteria. The formation of stable microparticles is necessary both in drug delivery application as it regulates the therapeutic effects, biological activity of the microencapsulated drug, drug release rate and most importantly the degradation time. Biodegradable polymers such as polylactide (PLA), polycaprolactone (PCL), polyglycolide (PLGA) etc., show good biocompatibility and biodegradability [5–7]. These polymers as matrix material of microparticles can be decomposed to non toxic and low molecular weight species and later on metabolized and absorbed by the body. Among them polycaprolactone (PCL) is one of the widely used biodegradable polymers due to its good drug permeability and biocompatibility. Several techniques are used to produce polymeric microparticles from polyesters, including solvent evaporation, phase separation and spray drying techniques [8–12]. The drawbacks of these techniques are the multi-step preparation processes and cannot provide the necessary narrow size distribution of biocompatible particles, resulted with a wide range of polymer particles. Non-aqueous polymerization processes such as dispersion polymerization in organic media has been developed in the synthesis of colloidal polymer microparticles. In this process, the polymerization is carried out in a medium that dissolves the monomer but not the polymer and the polymerization proceeds in the reaction medium until a critical molecular weight for the solubility of the polymer chain, and then the precipitated primary particles are stabilized by a polymeric stabilizer [13]. In a typical non-aqueous polymerization monomer, initiator and stabilizer being solubilized in an organic solvent and this type of polymerization can therefore be considered as a precipitation polymerization in which the aggregation of the disperse particles is prevented and the particle size is controlled by the presence of a steric stabilizer [14]. The most important features of an efficient stabilizer for synthesis of polymer particles in non-aqueous polymerization are a non-polar liquid soluble component capable of steric stabilization of the particles and an anchoring component compatible with the particle [14]. The choice of stabilizer should be such that it is biologically compatible or the low adsorption of the stabilizer to the microparticles formed. Therefore, the study on the well stabilized polymer microspheres formation is now attracted much attention to the chemists [15–18]. A number of materials either preformed or prepared in situ have been employed as stabilizers [19] and many were commercially available materials such as poly(vinylpyrrolidone) [20], poly(vinylalcohol) [21], polystyrene-block-(ethylene-co-propylene) [22] and methacryloxypropyl-terminated polydimethylsiloxane [23]. Relatively few polymers have been designed for the specific application as dispersion polymerization stabilizers such as polystyrene-b-polybutadiene [24].
Block or graft copolymers due to their unique amphiphilic properties in solution, act as efficient emulsifiers and stabilizers for a wide range of emulsion types including oil-in-oil emulsion [25]. These copolymers are efficient emulsifiers for stabilizing emulsions composed of two immiscible organic liquids, when each of them is a selective solvent of one of the blocks of the copolymer in the preparation of microspheres and nanospheres. [26–33]. The recent publications of Klapper et al. [34] provide clear evidence that block copolymer stabilized oil-in-oil emulsions can be considered as microreactors or nanoreactors for the polymerization of a wide range of water-sensitive monomer and catalyst systems. Paveer et al. [35] reported that the graft copolymers of variable ratio were found to show favourable low toxicity and could be electrospun when blended with PCL into very well defined nanofibres for potential tissue engineering applications. Phthaloyl protected Chitosan (CS) was esterified with maleic ester functionalized mPEG-b-PCL to give the graft block copolymer CS-g-(mPEG-b-PCL) with variable compositions was reported by Lu et al. [36] The use of polymeric stabilizer were reported by Slomkowski et al. [37] and Muranaka et al. [38] in the synthesis of poly(d,l-lactide) (PDLLA) and poly(l,l-lactide) (PLLA) microspheres with narrow size distribution by dispersion polymerization method in an oil-in-oil emulsion system. It describes the difficulty in the formation of monodisperse particles as the block copolymers adsorbed on the surface of the primary particles that disturbs the equilibrium state [38]. Rather it is more beneficial if an in situ graft copolymer stabilizer is produced during the formation of precursor polymer that contains active sites for the chain transfer of radicals in the dispersion polymerization. These types of polymeric stabilizers ignore the formation of micelles in the solution in the initial stage of polymerization and therefore reduce the chances of adsorption on the primary particles and increase the room for monodisperse particle formation [38].
A block copolymer as a dispersion stabilizer for use in the ring opening dispersion polymerization of polyester comprises at least two blocks linked by chemical valences. At least one of the blocks is hydrophilic and at least another of the said blocks is hydrophobic. The hydrophobic block can contain a plurality of similar or dissimilar pendent groups having chemically reactive functionality a well defined block copolymer is designed and synthesized. Block copolymer systems studied till now are based on non-degradable backbone structures which result mostly as a drawback for the long-term clinical applications. Therefore, to combine both a degradable and a functional segment in one block copolymer is essential for therapeutic purposes [39].
So, it is important to understand the role of dispersion stabilizer how to control the particle size and distribution in the formation of polymer particles. In the present work, a novel polymeric stabilizer is synthesized with a long chain hydrophobic and hydrophilic acrylate end group to prepare PCL microspheres by dispersion polymerization of ε-caprolactone. The present study is aiming to prepare the poly(n-octadecyl methacrylate-co-2-hydroxyethyl methacrylate) [poly(OMA-co-HEMA)] copolymer of different molecular weights and then to prepare graft copolymer, poly-(ε-caprolactone)-graft-poly(n-octadecyl methacrylate-co-2-hydroxyethyl methacrylate) [poly(OMA-co-HEMA)-g-PCL] by ring opening dispersion polymerization of ε-caprolactone (CL) in the presence of [P(OMA-co-HEMA)] where graft copolymer will act as in situ stabilizer in the formation of PCL microspheres. The effectiveness of this in situ stabilizer has been established in the formation of spherical and stable PCL microspheres against coagulation.
Experimental
Materials
ε-Caprolactone (Sigma Aldrich) was purified by treating with NaOH solution and distilled under vacuum and 2-hydroxyethyl methacrylate (Merck) was purified by passing through silica column and again distilled under vacuum. n-Octadecyl methacrylate was prepared in the laboratory by the esterification of methacrylic acid with n-octadecanol and recrystallize twice with hexane prior to use. Tin(II)2-ethylhexanoate(Sn(oct)2) (Sigma Aldrich), N, N dimethylformamide (DMF) (Merck), Xylene (Merck), Heptane (Rankem), Tetrahydrofuran (THF) are of HPLC grade and were used as received. 2, 2′-Azobisisobutyronitrile (AIBN) was recrystallized from methanol and dried in vacuo.
Synthesis of P(OMA-co-HEMA)
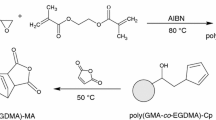
Poly(OMA-co-HEMA) was synthesized according to Scheme 1. A mixture of monomers (20 % w/v to solvent) OMA (3.6 g) and HEMA (2.4 g) and 30 mL dehydrated DMF was placed in a round bottom flask fitted with a nitrogen adaptor. Nitrogen was purged for 15 minutes to remove the oxygen and the flask was heated up to 70 °C in an oil bath. AIBN (0.6 g) dissolved in DMF was added to initiate the polymerization with constant stirring. The polymerization was conducted for 5 hours after cooling to the room temperature; the reaction mixture was poured in cold excess methanol to remove the unreacted monomer. After purification the obtained polymer was dried in vacuum to get white powdery solid (yield ≈70 %).
Preparation of PCL microspheres
Scheme 2 describes the preparation of PCL microspheres by ring opening polymerization of ε-caprolactone (CL) in presence of Sn(oct)2 catalyst stabilized by poly(OMA-co-HEMA). ε-Caprolactone (3 g, 10 % w/v) was added to 20 mL solution of poly(OMA-co-HEMA) (0.6 g, 20 % w/w) in xylene/heptane (30 ml, 1:2 v/v) in a round bottom flask with constant stirring at 1000 rpm and nitrogen was purged for 15 minues to remove air. Sn(oct)2 (0.06 g, 2 % w/w) was dissolved in 10 mL of xylene/heptane (1:2 v/v) and added to the reaction mixture and the system was heated up to 80 °C for 4 hours. After the polymerization was completed, the reaction mixture was cooled and poured in excess heptanes by stirring vigorously. PCL microspheres were separated from the solution mixture by centrifuge and washed several times with heptanes and again re-dispersed in heptanes to collect the PCL microspheres. The graft copolymer was separated from the solution as solid precipitate [38].
Characterization
The molecular weights and the polydispersity index (PDI) were measured with a Waters gel permeation chromatography (GPC) equipment of Waters Model 515 solvent delivery system at a flow rate of 1.0 mL min−1 through a combination of Waters HR1, HR3, and HR4 Styragel columns. The analysis was performed at room temperature using purified high-performance liquid chromatography (HPLC) grade THF as the eluent. A Waters differential refractometer Model 2410 was used as the detector. The sample concentration was 0.2 % (w/v), and the volume injected was 50 µL. The GPC curves were analyzed with the calibration curve obtained by nine narrow molecular weight distribution polystyrene samples (Waters). Data were recorded and processed using the Millennium 2.0 software package.
The chemical structure of the synthesized copolymer and graft copolymer was confirmed with a the 1H NMR and 13C NMR spectra that was recorded on a Bruker 300 MHz FT-NMR spectrometer in CDCl3 using tetramethylsilane (TMS) as the internal reference. IR spectra of the compounds were measured (4000–500 cm−1) using a Perkin–Elmer IR 883 spectrophotometer. The X-ray diffractogram of the polymers were recorded on a model JDX-11P3A JEOL diffractometer with a solid sample using a Ni filter with CuK∞ radiation at 35 kV and 10 mA in the wide-angle range of 2° < 2θ < 60°.
Scanning electron microscope (SEM, JSM6360 JEOL) was used to study the morphology of the PCL particles, particle sizes, and size distributions. The SEM samples were prepared with a drop of diluted dispersion on an aluminum sample stub, dried, and then sputter-coated with gold. Particle sizes were calculated from electron micrographs measuring nearly hundred particles diameter.
Results and discussion
The block copolymers synthesized by free radical copolymerization of OMA and HEMA using AIBN as initiator is summarized in Table 1. In this study, we are going to describe the main role of the block copolymer as stabilizer in the preparation of PCL microparticles. PCL microparticles are needed in the drug-delivery system to protect the active agent during its transport throughout the body. For performing this, some of the basic requirements such as ideal shape of the polymeric particles as distribution of their sizes have to be considered. A stabilizer helps to preserve the particle suspensions from agglomeration as well as its chemical nature and concentration controls the size of polymeric particles. To investigate the effect of the molecular structure of poly(OMA-co-HEMA) on the particle size and size distribution of PCL microspheres, poly(OMA-co-HEMA) of five different molecular weights were synthesized (Scheme 1). The analytical results of synthesized copolymers are given in the Table 1. The formation of copolymer is confirmed by FTIR spectra where the C=C stretching near 1800–1900 cm−1 is absent in the copolymer. The sharp alkane stretching at 2922 and 2852 and 1384 and 1486 cm−1 confirms the long chain hydrophobic end group in the copolymer (Fig. 1) [40, 41]. The 1H NMR spectra shows the peaks around 4.1 ppm (COOCH2 for HEMA unit) and 3.9 ppm (COOCH2 for OMA unit) and 1.9 ppm for -CH2 protons of HEMA unit. The peaks around 5.6 and 6.1 for double bonded CH are absent for both OMA and HEMA units (Fig. 2).
The effect of the stabilizer concentration on the dispersion polymerization was studied from 5–70 % with respect to monomer CL. Figure 3 illustrates the GPC traces of the grafted copolymer poly(OMA-co-HEMA)-g-PCL and the original copolymer poly(OMA-co-HEMA). Under the similar experimental conditions, the molecular weight of the grafted polymer increases with respect to the parent block copolymer and this clearly indicates that the grafting of CL occurred through parent copolymer. The molar ratio of OMA to HEMA in the copolymer chain as well as the molecular weight of poly(OMA-co-HEMA) are varied to achieve the proper balance of effective anchor to soluble portion for the use of such copolymers as steric stabilizers in dispersion polymerization of PCL. The carboxyl groups in polymers are supposed to be good effective anchors on to the polymer surface in non-aqueous media, so polymer that contains both the carboxyl group and the long hydrocarbon chain could be a suitable steric stabilizer in this case. Earlier studies showed that there are several parameters such as the structure and composition of the steric stabilizer, the stabilizer concentration, the monomer concentration, the reaction time and temperature play important effects on dispersion polymerization in non-aqueous media [42–46].
PCL microsphere preparation
Preliminary research reported herein is to focus on the effect of the block copolymer and to investigate the efficiency of poly(OMA-co-HEMA) as stabilizer for the dispersion polymerization of CL. Figure 4 shows SEM micrographs of the PCL particles prepared in the absence and presence of the poly(OMA-co-HEMA) stabilizer. For the PCL particles prepared in the absence of poly(OMA-co-HEMA), particles are found to be agglomerated and in irregular shape (Fig. 4a). However, addition of poly(OMA-co-HEMA), gave better results where the particles are spherical, with smooth surface in the particle size range of 2–20 micron (Fig. 4b–f). This can be explained that the block copolymer which acts as a stabilizer formed a protective layer around the suspended PCL particles which prevents them to agglomerate. Therefore, the use of the block copolymer as stabilizer is necessary to achieve spherical polymer particles as well as the particle morphology. In general, in dispersion polymerization, all reaction ingredients are dissolved in the medium, in which particles are generated from the oligomeric species and microspheres subsequently grown by the adsorption of oligomers and monomers from the medium. The process of dispersion polymerization is separated into two stages, nucleation and particle growth, the former is short but complex and sensitive, whereas the latter is relatively long, simple, and robust [47].
The in situ stabilizer plays a critical role in the formation of the PCL microparticles. So, to investigate the efficiency of poly(OMA-co-HEMA) copolymer as stabilizer for the dispersion polymerizations of CL, the molar ratio of OMA to HEMA is varied to achieve different molecular weights of the stabilizers (Table 1). In a typical set up in this study, the starting reaction medium was homogeneous and the nucleation stage is apparently detected by the observation of turbidity in the solution over a certain time. Then, the polymerization solution became turbid after reaching the reaction conditions, indicating the nucleation of small particles and white latex was seen to be formed after few minutes of the reaction and remained stable all along the particle growth regime. The particle number and particle number distribution are determined by the nucleation stage if no secondary particles or coagulum is formed during the particle growth stage [46–48]. Figure 4 clearly indicates the morphology of PCL particles prepared with different molar ratio of the poly(OMA-co-HEMA) stabilizer and in all cases spherical particles were obtained without any sign of coagulation.
Figure 5 shows that the particle size decreases with the increase of molar ratio HEMA with respect to OMA. This may be due to the production of a large number of stabilizing molecules which can induce a large surface area that reduces the surface energy. This behavior is observed in the conventional dispersion polymerization. Thus, the stabilizing ability of colloids increased with the increase segment of HEMA in the poly(OMA-co-HEMA) copolymer. It is also found that there is a significant increase of molecular weight with the increase segment of HEMA in the poly(OMA-co-HEMA) (Table 1). It could be explained by the fact that the increasing molecular weight of the polymeric stabilizer while keeping the number of chains constant in the continuous phase, may induce a reduction of the final particle size. This can be attributed to the fact that longer OMA-co-HEMA chains both create better barrier against particle aggregation and occupy a larger volume at the particle surface. Therefore more particle surface area can be stabilized with the larger polymer stabilizer, resulting in smaller particles. Shen et al. [49] have attributed the variation of particle size as a function of polymer molecular weight (MW) to both a faster adsorption of the longer chain stabilizers and an increase in viscosity of the continuous phase as MW is increased. It is also accepted that the high solubility of smaller MW stabilizers will slow down adsorption to the particle surfaces. Indeed, this has driven researchers to develop chemical anchoring mechanisms for the stabilizers to enable a more controlled process through efficient particle seed stabilization. All phenomena mentioned above are linked to larger seeds being formed in the case of the smaller MW stabilizer, which eventually leads to fewer particles synthesized. The stabilization of the particles depends on the condition of the initial micelle formation by the stabilizer in the solution and the formation of primary particle formation. High molecular weight copolymer with long hydrophobic end groups act as good stabilizing agent and forms a good emulsion in oil system. The hydrophilic segments of the block copolymers provide the colloidal stabilization of the polymer particles along with a specific surface functionality, the hydrophobic segments strongly adsorb or anchor to the polymer–solvent interface. Thus the relatively long hydrophobic lengths are expected to be more efficient. Particle size of PCL increased with the decrease in concentration of HEMA hydrophilic group. At monomer ratio 3:3 the molecular weight was significantly increased as the rate of the polymerization of HEMA is less than OMA (Mayo–Lewis equation for copolymerization). POMA being a long chain hydrophobic molecule better stabilizes the miceller system and the formation of microspheres. When the ratio of both the monomers was kept equal, the molecular weight of the block copolymer increases and it results in the formation of stable but smaller size hydrophobic PCL microspheres. The formation of spherical microspheres having smooth surface is the particle enlargement by mainly chemical bonding of tiny small particles. It means that the poly(OMA-co-HEMA) block copolymer studied herein is proved to be an efficient stabilizer at different molar ratio of OMA:HEMA. It suggests that the HEMA segments of the stabilizer favorably interact with the PCL dispersed phase, ensuring physical adsorption of the block copolymers onto the growing PCL particles, while the pendent group segments of OMA extends into the solvent phase, preventing the flocculation of the particles.
The formation of PCL grafted to poly(OMA-co-HEMA) polymer shows the broadening of the characteristic peaks for aliphatic groups at 2947 and 2865 cm−1 and splitting of degenerate transmittance peaks for carbonyl groups at 1727 cm−1 for PCL (Fig. 1). From 1H NMR, (Fig. 2) poly(OMA-co-HEMA)-g-PCL polymer formation is confirmed by the peaks around 3.7 which is attributed to the methylene protons adjacent to the hydroxyl end group of the grafting PCL chain. Splitting of the significant peaks in the grafting of PCL to poly(OMA-co-HEMA) has been shown in the Fig. 2.
The X-ray diffraction patterns of poly(OMA-co-HEMA) and poly(OMA-co-HEMA)-g-PCL during the dispersion polymerization at room temperature from is shown in Fig. 6. In the XRD pattern of the copolymer, sharp diffraction peak appear at 2θ = 22° which is consistent with the typical diffraction peak of the copolymer in the crystalline form. However, in the poly(OMA-co-HEMA)-g-PCL, the sharp crystalline structure has diminished significantly which resulted that grafting occurred through PCL to the poly(OMA-co-HEMA).
Steric stabilizer poly(OMA-co-HEMA) formed by the anchoring adsorption of its graft copolymer poly(OMA-co-HEMA)-g-PCL, acted as a true stabilizer to form PCL spherical particles against coalescence. The formation of in situ grafting of the poly(OMA-co-HEMA)-g-PCL during the dispersion polymerization was confirmed by FT-IR spectra and it is indeed clear that PCL segment is required to interact with the precipitated particles in the preparation of stable PCL microspheres. On the other hand, the rate of anchoring adsorption of the grafted stabilizer on the particle surface is also important as the particle instability may result in a flocculation due to the insufficient adsorption in situ graft stabilizer on the particle surface. In general, a polymeric stabilizer used in the dispersion polymerization is not only physically adsorbed but also chemically bound by forming a covalent bond with the monomer. Thus, it cannot be removed from the polymeric particles because of the nucleation starting on PVP molecules by the abstraction of labile hydrogen [50]. In the TEMPO mediated dispersion polymerization, the steric stabilizer PVP was reportedly located not only exclusively on the outside of the PS particles, but also inside the latex particles [51]. So, it can be concluded that the adsorption of poly(OMA-co-HEMA)-g-PCL was minimum in the polymerization of CL using poly(OMA-co-HEMA) as a stabilizer.
Conclusions
-
This work demonstrates the efficient synthesis of poly(n-octadecyl methacrylate-co-2-hydroxyethyl methacrylate) [poly(OMA-co-HEMA)] block copolymers as stabilizer in the preparation of PCL microspheres.
-
Steric stabilizer poly(OMA-co-HEMA) formed by the anchoring adsorption of its graft copolymer poly(OMA-co-HEMA)-g-PCL, acted as a true stabilizer to form PCL spherical particles against coalescence.
-
Scanning electron microscopic images of PCL microspheres are found to be well dispersed and PCL particle sizes increases with the increase of stabilizer concentration. The particle stability and size of the microspheres are found to be depended on the ratio OMA: HEMA copolymer and its molecular weight.
-
It is well established that the stabilizing ability of colloids increased with the increase segment of HEMA in the poly(OMA-co-HEMA) copolymer also the MWs of the copolymer increases with the increase segment of HEMA in the poly(OMA-co-HEMA) and particle size decrease. This work provides a guideline for the synthesis of polymer microparticles with the block or graft copolymers for their unique amphiphilic properties in solution.
References
Rezwan K, Chen QZ, Blaker JJ, Boccaccini AR (2006) Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27:3413–3431
Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, Neves NM, Reis RL (2007) Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J Royal Soc Interface 4:999–1030
Nair LS, Laurencin CT (2006) Polymers as biomaterials for tissue engineering and controlled drug delivery. Adv Biochem Eng/Biotech 102:47–90
Yang Y, Chung T, Ng NP (2001) Morphology, drug distribution, and in vitro release profiles of biodegradable polymeric microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. Biomaterials 22:231–241
Rajput MS, Agrawal P (2010) Microspheres in cancer therapy. Ind J Cancer 47:458–468
Egilmez NK, Jong YS, Sabel MS, Jacob JS, Mathiowitz E, Bankert RB (2000) In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res 60:3832–3837
Sapin A, Garcion E, Clavreul A, Lagarce F, Benoit JP, Mene P (2006) Development of new polymer-based particulate systems for anti-glioma vaccination. Int J Pharm 309:1–5
Zhu G, Mallery SR, Schwendeman SP (2000) Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide). Nat Biotechnol 18:52–57
Ishizu K, Fukutomi T (1988) Core-shell type polymer microspheres prepared from block copolymers. J Polym Sci Part C Polym Lett 26:281–286
Li Z, Wei X, Ngai T (2011) Controlled production of polymer microspheres from microgel-stabilized high internal phase emulsions. Chem Commun 47:331–333
Kang F, Jiang G, Hinderliter A, DeLuca PP, Singh J (2002) Lysozyme stability in primary emulsion for PLGA microsphere preparation: effect of recovery methods and stabilizing excipients. J Pharm Res 19:629–633
Liu M, Chen L, Zhao Y, Gan L, Zhu D, Xiong W, Lv Y, Xu Z, Hao Z, Chen L (2012) Preparation, characterization and properties of liposome-loaded polycaprolactone microspheres as a drug delivery system. Colloids Surf A Physicochem Eng Asp 395:131–136
Xu W, Wang L, Ling Y, Wei K, Zhong S (2014) Enhancement of compressive strength and cytocompatibility using apatite coated hexagonal mesoporous silica/poly(lactic acid-glycolic acid) microsphere scaffolds for bone tissue engineering. RSC Adv 4:13495–13501
Penfold HV, Holder SJ, McKenzie BE (2010) Octadecyl acrylate—Methyl methacrylate block and gradient copolymers from ATRP: comb-like stabilizers for the preparation of micro- and nano-particles of poly(methyl methacrylate) and poly(acrylonitrile) by non-aqueous dispersion polymerization. Polymer 51:1904–1913
Thomson RC, Yaszemski MJ, Powers JM, Mikos AG (1996) Fabrication of biodegradable polymer scaffolds to engineer trabecular bone. J Biomater Sci Polym Ed 7:23–38
Srivastava S, Sinha VR (2013) Stavudine loaded biodegradable polymeric microspheres as a depot system for parenteral delivery. Int J Pharm Sci Drug Res 5:1–13
Gabikian P, Tyler BM, Zhang I, Li KW, Brem H, Walter KA (2014) Radiosensitization of malignant gliomas following intracranial delivery of paclitaxel biodegradable polymer microspheres: laboratory investigation. J Neurosurg 120:1078–1085
Brem H, Gabikian P (2001) Biodegradable polymer implants to treat brain tumors. J Control Release 74:63–67
Barrett KEJ (1975) Dispersion polymerization in organic media. Wiley, New York
Song J, Winnik MA (2005) Cross-linked, monodisperse, micron-sized polystyrene particles by two-stage dispersion polymerization. Macromolecules 38:8300–8307
Jongpaiboonkit L, Ford TF, Murphy WL (2009) Growth of hydroxyapatite coatings on biodegradable polymer microspheres. ACS Appl Mater Interfaces 1:1504–1511
Hirzinger B, Helmstedt M, Stejskal J (2000) Light scattering studies on core–shell systems: determination of size parameters of sterically stabilized poly(methylmethacrylate) dispersions. Polymer 41:2883–2891
Klein SM, Manoharan VN, Pine DJ, Lange FF (2003) Preparation of monodisperse PMMA microspheres in nonpolar solvents by dispersion polymerization with a macromonomeric stabilizer. Colloid Polym Sci 282:7–13
Garcıa I, Tercjak A, Rueda L, Mondragon I (2008) Self-assembled nanomaterials using magnetic nanoparticles modified with polystyrene brushes and poly(styrene-b-butadiene-b-styrene). Macromolecules 41:9295–9298
Atanase LI, Riess G (2011) Block copolymers as polymeric stabilizers in non-aqueous emulsion polymerization. Polym Int 11:1563–1573
Reddy KR, Hassan M, Gomes VG (2015) Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis. Appl Catal A Gen 489:1–16
Yamamoto T, Kawaguchi K (2016) Relationship between surface potential and particle size in soap-free emulsion copolymerization of styrene and methyl methacrylate using a water- or oil-soluble initiator. J Colloid Polym Sci 294:281–284
Reddy KR, Sina BC, Ryua KS, Kimb JC, Chungc H, Leea Y (2009) Conducting polymer functionalized multi-walled carbon nanotubes with noble metal nanoparticles: synthesis, morphological characteristics and electrical properties. Synth Met 159:595–603
Reddy KR, Park W, Sin BC, Noh J, Lee Y (2009) Synthesis of electrically conductive and superparamagnetic monodispersed iron oxide-conjugated polymer composite nanoparticles by in situ chemical oxidative polymerization. J Colloid Interface Sci 335:34–39
Choi SH, Kim DH, Raghu AV, Reddy KR, Lee H, Yoon KS, Jeong HM, Kim BK (2012) Properties of graphene/waterborne polyurethane nanocomposites cast from colloidal dispersion mixtures. J Macromol Sci Part B Physics 51:197–207
Zhang YP, Lee SH, Reddy KR, Gopalan AI, Lee KP (2007) Synthesis and characterization of core-shell SiO2 nanoparticles/Poly(3-aminophenylboronic acid) composites. J Appl Polym Sci 104:2743–2750
Reddy KR, Lee KP, Gopalan AI (2007) Self-assembly directed synthesis of poly(ortho-toluidine)-metal (gold and palladium) composite nanospheres. J Nanosci Nanotech 7:3117–3125
Reddy KR, Lee KP, Gopalan AI, Showkat A (2007) Synthesis and properties of magnetite/poly(aniline-co-8-amino-2-naphthalenesulfonic acid) (SPAN) nanocomposites. Polym Adv Technol 18:38–43
Klapper M, Nenov S, Haschick R, Muller K, Mullen K (2008) Oil-in-oil emulsions: a unique tool for the formation of polymer nanoparticles. Acc Chem Res 41:1190–1201
Paaver U, Tamm I, Laidmae I, Lust A, Kirsimae K, Veski P, Kogermann K, Heinämäki J (2014) Soluplus graft copolymer: potential novel carrier polymer in electrospinning of nanofibrous drug delivery systems for wound therapy. BioMed Res Int 2014:1–7
Lu Y, Liu L, Guo S (2007) Novel amphiphilic ternary polysaccharide derivates chitosan-g- PCL-b-MPEG: synthesis, characterization, and aggregation in aqueous solution. Biopolymers 86:403–408
Jakubowski W, Lutz J, Slomkowski S, Matyjaszewski K (2005) Block and random copolymers as surfactants for dispersion polymerization I. Synthesis via atom transfer radical polymerization and ring-opening polymerization. J Polym Sci Part A Polym Chem 43:1498–1510
Muranaka M, Kitamura Y, Yoshizawa H (2007) Preparation of biodegradable microspheres by anionic dispersion polymerization with PLA copolymeric dispersion stabilizer. Colloid Polym Sci 285:1441–1448
Sugihara S, Blanazs A, Armes SP, Ryan AJ, Lewis AL (2011) Aqueous dispersion polymerization: a new paradigm for in situ block copolymer self-assembly in concentrated solution. J Am Chem Soc 133:15707–15713
Reddy KR, Raghu AV, Jeong HM, Siddaramaiah (2009) Synthesis and characterization of pyridine-based polyurethanes. Des Monomer Polym 12:109–118
Hassan M, Reddy KR, Haque E, Minett AI, Gomes VG (2013) High-yield aqueous phase exfoliation of graphene for facile nanocomposite synthesis via emulsion polymerization. J Colloid Interface Sci 410:43–51
Shiho H, DeSimone JM (2001) Dispersion polymerization of glycidyl methacrylate in supercritical carbon dioxide. Macromolecules 34:1198–1203
Canelas DA, Betts DE, DeSimone JM (1996) Dispersion polymerization of styrene in supercritical carbon dioxide: importance of effective surfactants. Macromolecules 29:2818–2821
Giles MR, O’Connor SJ, Nay JN, Winder RJ, Howdle SM (2000) Novel graft stabilizers for the free radical polymerization of methyl methacrylate in supercritical carbon dioxide. Macromolecules 33:1996–1999
Saikia PJ, Lee JM, Lee BH, Choe SJ (2007) Influence of a reversible addition–fragmentation chain transfer agent in the dispersion polymerization of styrene. J Polym Sci Part A Polym Chem 45:348–360
Saikia PJ, Lee JM, Lee BH, Choe SJ (2007) Reversible addition fragmentation chain transfer mediated dispersion polymerization of styrene. Macromol Symp 248:249–258
Song J, Tronc F, Winnik MA (2004) Two-stage dispersion polymerization toward monodisperse, controlled micrometer-sized copolymer particles. J Am Chem Soc 126:6562–6563
Saikia PJ, Lee JM, Lee BH, Choe SJ (2008) Reaction parameters in the RAFT mediated dispersion polymerization of styrene. J Polym Sci Part A Polym Chem 46:872–885
Shen S, Sudol ED, El-Aasser MS (1993) Control of particle size in dispersion polymerization of methyl methacrylate. J Polym Sci Part A Polym Chem 31:1393–1402
Ober CK, Lok KB, Hair ML (1985) Monodispersed, micron-sized polystyrene particles by dispersion polymerization. J Polym Sci Polym Lett 23:103–108
Gabaston LI, Jackson RA, Armes SP (1998) Living free-radical dispersion polymerization of styrene. Macromolecules 31:2883–2888
Acknowledgments
The authors wish to thank Dr D. Ramaiah, Director, CSIR-NEIST, Jorhat, Assam for permission to publish the results to the CSIR Network Project, M2D (CSC-0134) for funding.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huda, M.K., Das, P.P., Saikia, P.J. et al. Synthesis of poly(n-octadecyl methacrylate-co-2-hydroxyethyl methacrylate) copolymer and their utilization as polymeric stabilizer in the preparation of PCL microspheres. Polym. Bull. 74, 1661–1676 (2017). https://doi.org/10.1007/s00289-016-1795-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-016-1795-z