Abstract
The polymer nanocomposite films consisted of poly(ethylene oxide) (PEO) matrix with different weight percentage concentrations of silicon dioxide (SiO2) as nanofiller have been prepared by melt-pressed technique. The complex dielectric function, ac electrical conductivity, electric modulus and complex impedance spectra of the nanocomposite films have been investigated over the frequency range 20 Hz to 1 MHz, at room temperature. The real part of permittivity increases with decrease of frequency below 10 kHz, whereas these values remain almost constant in the high-frequency region. The high-frequency permittivity of these nanocomposites decreases with increase of SiO2 concentration. The relaxation peaks are observed in dielectric loss, loss tangent and loss modulus spectra of the nanocomposites which are corresponding to the PEO segmental motion (local chain dynamics). It is found that the PEO chain dynamics is much faster in the nanocomposites as compared to that in the pristine PEO film. The real part of electrical conductivity spectra of these materials has linear behaviour of two different slopes in the frequency regions of above 10 kHz and below 1 kHz. The interactions between PEO chain and SiO2 nanoparticles, and their effect on PEO spherulites in these nanocomposites are explored by X-ray diffraction measurements.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the synthetic polymers, PEO is the most promising industrial and technological material. The linear chain PEO is a polar, non-ionic, hydrophilic in nature and of semicrystalline morphology. It has high degree of solubility in water and many organic dipolar solvents. The highly flexible type PEO film can be easily made by solution-casting method and also by the melt-pressed technique. Due to these common properties of PEO, it is often considered as a model system to understand the fundamental behaviour of polymer interactions and adsorption with SiO2 nanoparticles [1–7]. Further, the SiO2 has mild reactivity and good chemical properties which recognized it as potential nanofiller in the preparation of polymer nanocomposite (PNC) materials. The presence of ether oxygen atom in PEO backbone realizes it as solid solvent for alkali metal salts, and therefore, it is frequently used as a potential matrix for the preparation of flexible type novel solid polymeric electrolyte (SPE) film for the rechargeable lithium-ion batteries [8–12]. The addition of a small amount of SiO2 in the PEO matrix has become a common practise to improve the ion transportation mechanism in the SPE materials [13–15], and also the sensing performance of such hybrid material [16].
The sol–gel and solution-casting techniques are very popular for the preparation of PNC materials [1, 3, 5–7, 17]. In addition to these techniques, recently the PEO–SiO2 nanocomposites were also prepared by direct melt blending technique, and compared their viscoelastic properties with the solution blend nanocomposites, to confirm the material processing effect on the polymer chain dynamics and the structural properties [18]. Our survey of literature revealed that, so far the PEO and SiO2 interactions and their effect on the polymer physico-chemical properties are characterized by rheological, differential scanning calorimetry (DSC), thermo gravimetric analysis (TGA), Fourier transform infrared spectroscopy (FTIR), dynamical mechanical analysis (DMA), X-ray diffraction (XRD) scanning electron microscopy (SEM) and transmission electron microscopy (TEM) measurements [1, 3–7, 19]. Besides these experiments, the dielectric relaxation spectroscopy (DRS) is also a powerful tool for the study of polymer and nanofiller interactions and their effect on polymer dynamics in the PNC materials [20–31]. Further, the investigation on dielectric properties of such PNCs can provide the in-depth discussion of nano-dielectrics, which is an emerging and fast-moving topic in electrical insulation for microelectronic devices. Survey of literature reveals that the dielectric properties of solution-cast prepared PEO–SiO2 nanocomposites are recently investigated along with their rheological and thermal properties [29, 30]. But the detailed dielectric and electrical properties, and the molecular relaxation processes in the melt compounded PEO–SiO2 nanocomposites are still unexplored. The PNC films prepared by melt-pressed technique have highly smooth surfaces and remain free from air voids formations in presence of nanofiller due to their preparation under high pressure. These physical properties are needed for suitability of such materials in fabrication of microelectronic devices. Further, the melt-pressed technique is technological appropriate due to fast preparation of PNC materials by this method as compared to longer time consuming solution-casting preparation method.
In the present paper, PEO–x wt% SiO2 nanocomposites of varying concentrations (x wt%) of SiO2 in the PEO matrix are prepared by melt-pressing technique, which is a solvent free PNC preparation method. The structural properties of these nanocomposite films are characterized by XRD measurements, whereas the dielectric properties and polymer chain dynamics are explored by DRS study in the frequency range 20 Hz to 1 MHz. The aim of this study is to search a dielectric material of required permittivity value that can work as inter-level dielectric over a broad radio frequency range to replace the traditional insulator in various electronic devices [32–35]. The dielectric characterization of such nano-dielectric materials over the frequency range of present experiment is important to confirm their potential applications in audio and radio frequencies operating devices and also in preparation of solid polymer nanocomposite electrolytes.
Experimental
Materials
The PEO (M w = 600,000 g mol−1) and SiO2 nanopowder [99.5 trace metal basis and 5–15 nm particles sizes (spherical and porous)] were purchased from Sigma-Aldrich.
Preparation of PEO–x wt% SiO2 nanocomposite films
Initially, the required amounts of PEO and the respective amounts of SiO2 for the wt% concentrations x = 0, 1, 3, 5 and 10 with respect to weight of PEO, were mechanically blended in dry state using an agate mortar and pestle. These compositions were further blended using high energy planetary ball mill agate vessel with agate balls at 200 rpm for 10 min duration to achieve the homogenous mixtures. After that the melt compounded PNC films of PEO–x wt% SiO2 mixtures were obtained using hot press polymer film making unit. Each homogenous blended material was first heated at 80 °C in 60 mm diameter stainless steel die having 1 mm spacer, and subsequently pressed the melted material under 3 ton of pressure per unit area. After that by slow cooling of the material up to room temperature, the smooth surfaces PEO–x wt% SiO2 film was obtained. The temperature for melt compounding of the material was kept above the PEO melting temperature 65 °C to achieve the required characteristics of nanocomposite formation.
Measurements
Agilent technologies 4284A precision LCR meter and Agilent 16451B solid dielectric test fixture having a four terminals nickel-plated cobalt electrodes of 38 mm active diameter were used for the dielectric and electrical characterization of the PEO–x wt% SiO2 nanocomposite films. The measurements were carried out in the frequency range 20 Hz to 1 MHz of 1 volt alternating current (ac) source at 27 ± 1 °C. The parallel mode operation frequency-dependent values of capacitance C P, resistance R p and loss tangent tan δ = ε″/ε′ (dissipation factor D) of the capacitive dielectric test fixture loaded with the nanocomposite film were measured. Prior to the sample measurements, the open circuit calibration of the dielectric test fixture was performed to eliminate the effect of stray capacitance. The equations used based on C p, R p and tan δ values for the evaluation of complex dielectric function ε*(ω) = ε′ − jε″, ac electrical conductivity σ*(ω) = σ′ + jσ″, electric modulus M*(ω) = M′ + jM″ and complex impedance Z*(ω) = Z′ − jZ″ of the nanocomposite films are described earlier [25]. These complex quantities can be transformed to each other according to the scheme 1/ε*(ω) = jωC 0 Z*(ω) = M*(ω) = jωε 0/σ*(ω).
The XRD patterns of PEO, SiO2 and PEO–x wt% SiO2 nanocomposites in the angular range 2θ from 10° to 30° were recorded in reflection mode using a PANalytical X’pert Pro MPD diffractometer of Cu Kα radiation (λ = 1.5406 Å), operated at 45 kV and 40 mA, with a scanned step size of 0.05° s−1. The d-spacing between diffractional lattice planes of PEO was determined by Bragg’s relation λ = 2d sin θ, whereas the mean crystallite length (size) L in the direction perpendicular to hkl plane was evaluated by Scherrer’s equation L = 0.94 λ/β cos θ, where β is full width at half maximum (FWHM) (the broadening of peak at half-height expressed in radians of 2θ i.e. width measured in 2θ degrees and then multiplied by π/180). The values of peak position 2θ and FWHM were obtained using X’pert pro® software. The intensity ratio IR = I PNC/I PEO was determined using the intensity values of PNC (I PNC) and pristine PEO (I PEO) of the same peak. The obtained values of 2θ, d, FWHM, L, I PNC, I PEO and IR of the nanocomposites at different concentrations of SiO2 are recorded in Table 1.
Results and discussion
Structural analysis
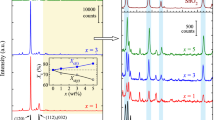
Figure 1 shows that the XRD pattern of pure PEO has major crystalline peaks at 2θ = 19.22° and 23.41°, which are corresponding to its 120 and concerted 112,032 crystalline phases, respectively [36]. The inset of Fig. 1 shows that the SiO2 has broad and diffused peak around 23° which confirms its predominantly amorphous morphology. The XRD pattern of the PEO–x wt% SiO2 nanocomposite films also have 120, and 112,032 peaks of PEO, but their intensities (height) vary significantly with SiO2 concentration as compared to that of pristine PEO. In XRD diffractogram, peak heights and peak widths are related to the crystalline phase concentrations and the crystalline size, respectively. For the PEO and its nanocomposites with SiO2, the observed 120 peaks are sharp and intense, whereas concerted 112,032 peaks have comparative less intensities and relatively their large broadening. Table 1 shows that the dispersion of SiO2 in PEO matrix results a small shift of PEO main crystalline peaks towards higher angle side, due to which there is a little decrease in d-spacing values of nanocomposites. The values of L and IR corresponding to 120 and 112,032 peaks of PNC films at different SiO2 concentrations are plotted in Fig. 2 for their comparative study. It is found that the addition of 1 wt% SiO2 in PEO matrix increases the L 120 value by 15 % and also the corresponding peak intensity by 107 %, whereas the L 112 value has an increase of 4 % and a drop in its peak intensity by 42 % (Table 1). These changes in XRD parameters reveal that a large structural rearrangement takes place in the PEO matrix in presence of only 1 wt% SiO2. At 3 wt% SiO2 concentration, the IR120 value has some decrease as compared to 1 wt% SiO2 concentration PNC film, and it approaches close to pure PEO at 5 and 10 wt% SiO2 concentrations PNC films. The L 112,032 and IR112,032 values of the PNC films show a relatively less variation with increase of SiO2 concentration above 1 wt% (Fig. 2). These results confirm the large alteration in hydrogen bonded interactions between ether oxygen of PEO chain and the silanol groups of SiO2 nanoparticles with the variation in concentration of nanofiller. In addition to these interactions, the possibility of weaker hydrophobic interactions of the ethylene –CH2–CH2– groups of PEO with Si–O–Si groups of the silica as suggested earlier [1], cannot be ruled out for the changes in L and IR values of the nanocomposites.
Earlier studies [1, 3, 7, 12] have established that the repeating monomer units in the long chain of polymers form stacks of parallel segments called lamellae, which result in chain folding back on themselves. When the semicrystalline PEO is melted and subsequently cooled, it frequently crystallizes to form a morphology composed of symmetrical super-structures called spherulites [7, 12]. The SEM images confirm that these symmetrical structures of PEO grow from a central point when several lamellae form together as a nucleus [29, 30, 37]. Further, these lamellae grow outwards at a linear rate which depends on the crystallization temperature and affects the chain mobility within the system. The present XRD study on the PEO–x wt% SiO2 nanocomposites also reveals the large increase of crystallite size L 120 when 1 and 3 wt% SiO2 are dispersed in PEO matrix, which suggests the increase in PEO spherulites growth during their crystallization. But at higher SiO2 concentrations, it seems that the enhancement in the PEO spherulites size is restricted by its interaction with SiO2 nanoparticles. The size of the SiO2 nanoparticles and relative size of PEO (chain dimension or the radius of gyration, R g) are important consideration for PEO adsorption on the SiO2 particles [1]. For the PEO of molar mass 600,000 g mol−1, its R g value is nearly 20 nm, which is high as compared to the radius of SiO2 nanoparticles used in this study. Due to this fact, there is possibility of the adsorption of 1 to 2 chains of PEO onto each SiO2 nanoparticle. For more PEO chains adsorptions, it is needed that PEO chain would adopt energetically unfavourable conformations to become more compact than its most probable conformation to continue H-bond formation [1]. Such structural behaviour can result in a large increase of PEO crystallite size at 1 and 3 wt% SiO2 concentrations in the PEO matrix when nanocomposites are prepared by the melt compounding technique as observed in the present study.
Complex dielectric spectra
Figure 3 shows the frequency-dependent real part ε′ and loss ε″ of complex dielectric function for PEO–x wt% SiO2 nanocomposites, at ambient temperature. The ε′ values have significant increase with the decrease of frequency below 10 kHz, whereas these values remain almost steady in the high-frequency region which represents their high frequency limiting permittivity ε ∞ values. The ε′ spectrum of pure PEO is also found in consistent of the earlier reported result of solution-cast prepared PEO film [29, 30, 38]. Further, inset of Fig. 3 shows that in the steady state permittivity of high-frequency region, the ε′ values of the nanocomposite films are lower than that of the pure PEO (see ε ∞ values at 1 MHz which are given in Table 2). Although pure SiO2 relative permittivity is 3.5 [39], and it is close to that of the pure PEO (~3.3 at 1 MHz, Table 2). But the decrease of ε′ values at high frequencies with the addition of SiO2 in these nanocomposites confirms that the interactions of PEO with SiO2 reduce the parallel dipolar ordering per unit volume of the composite material. The decrease of high frequency permittivity of these flexible PNC films confirms their suitability in radio frequency integrated microelectronic devices as substrate material of low permittivity [33].
The ε″ spectra of the PEO–x wt% SiO2 nanocomposite films (Fig. 3) have a relaxation peak around 1 kHz, which can be assigned to the PEO segmental dynamics (local chain motion of PEO), and it is commonly denoted by γ relaxation [38]. The values of dielectric relaxation time τ ε corresponding to this molecular dynamics of these nanocomposite films are evaluated from the value of dielectric loss peak frequency f p(ε″), using the relation τ ε = (2π f p(ε″))−1, and these values at various SiO2 concentrations are recorded in Table 2. The observed τ ε values of the studied PNC films are of the order of hundreds of microseconds.
AC conductivity spectra
Figure 4 shows that the real part σ′ of complex ac conductivity of PEO–x wt% SiO2 nanocomposite films increases with the increase of frequency. Due to semicrystalline structures of PEO, the σ′(f) plots have two straight lines of different slopes (regions I and II, as indicated in Fig. 4) in the low and high frequencies of the spectrum, respectively, and a transition occurs from one region to another in the frequency range from 1 to 10 kHz, as marked in Fig. 4. In this transition region, a relaxation peak occurs in their loss tangent (tan δ = ε″/ε′) spectra. The values of dc conductivities σ dc(I) and σ dc(II) of region I and region II, respectively, were determined by straight line fit of σ′ data of the corresponding region. The evaluated values of σ dc(I) and σ dc(II) of the nanocomposites are recorded in Table 2. It is found that in region I, the σ dc value of pure PEO is high as compared to the values of nanocomposites, whereas in region II it shows a reverse trend (Table 2). The peak intensity of tan δ plots decreases anomalously with increase of SiO2 concentration and it also has shift towards higher frequency side as compared to the peak of pristine PEO. The relaxation time τ tan δ of the nanocomposite films are determined from the tan δ peak frequency f p(tanδ) values using the relation τ tan δ = (2π f p(tan δ))−1. These τ tan δ values are also recorded in Table 2. Further, the magnitude and frequency position of tan δ peak of melt compounded pure PEO are found in agreement of the values of solution-cast pure PEO film at room temperature [29, 30]. Inset of Fig. 4 shows that the loss part σ″ of ac conductivity of pure PEO increases almost linearly with increase of frequency and it also has two distinguishable parts of different slopes in the low- and high-frequency regions of the spectra. But there is insignificant variation in σ″ values with SiO2 concentration of the PNC films (not shown in the inset) as compared to the σ″ spectrum of pure PEO.
Electric modulus spectra
The modulus spectra are commonly studied for the solid dielectrics because they are free from contribution of electrode polarization effect and also independent of the electrode material, electrode/dielectric contact and the adsorbed impurities in the dielectric sample [23–26]. Figure 5 shows the spectra of real part M′ and loss M″ of the electric modulus of PEO–x wt% SiO2 nanocomposites. It is found that the M′ have shoulders in the low- and high-frequency regions, whereas dispersion in M′ occurs in the mid-frequency region. The M″ spectra of these PNC films have the relaxation peaks where the M′ spectra have dispersion. The magnitudes of these M″ peaks up to 5 wt% SiO2 concentration are found nearly same. The values of electric modulus relaxation time τM are determined from the M″ peak frequency f p(M″) values using the relation τ M = (2π f p(M″))−1, and the evaluated τ M values at different concentrations of SiO2 are recorded in Table 2.
Impedance behaviour
Figure 6 shows the spectra of real part Z′ and imaginary part Z″ of complex impedance of the PEO–x wt% SiO2 nanocomposites. It is found that on logarithmic scale both the Z′ and Z″ have decreases with the increase of frequency. An abrupt change in the slope of Z′ values is observed, which results in two separate linear parts of different slopes in the low- and high-frequency regions, as discussed in their σ′ spectra. In the low-frequency region the Z′ values of pristine PEO is found higher than the PNC films, whereas in high-frequency region all the materials have same Z′ values. But, the Z″ values of PNC films are found lower than the pristine PEO over the entire frequency range. Further, on logarithmic scale the Z″ values of these materials decrease almost linearly with increase of frequency. The relatively high Z″ values as compared to the corresponding Z′ values confirm a highly capacitive behaviour of these nanocomposite films, which is owing to their low values of the dc conductivity.
Dielectric relaxation and PEO segmental dynamics
Figure 7 shows the variation of ε ∞, τ ε , τ tan δ , τ M , σ dc(I) and σ dc(II) values with SiO2 concentration of the PEO–x wt% SiO2 nanocomposites. It is found that the ε ∞ values have anomalous decrease with the increase of SiO2 concentration in the nanocomposites. The values of various relaxation times are found in the order τ ε > τ tan δ > τ M for these nanocomposite films, which is a common characteristic of several polymeric dielectric materials [23–26]. Further, the relaxation time values of the nanocomposites are found significantly low as compared to that of the pure PEO film. From Fig. 7, it is inferred that the dispersion of only 1 wt% SiO2 in the PEO matrix abruptly decreases the values of all the relaxation times, which confirms that the local chain dynamics of PEO is highly facilitated by its interactions with SiO2 nanoparticles. This facilitated PEO dynamics is the fact due to which the ionic conductivity of the SPEs based on PEO matrix with SiO2 nanofiller increases, because the ions’ mobility of such SPEs is mainly governed by the PEO segmental motion [8–12]. Further, the increase of SiO2 concentration above 1 wt% shows a less variation in the values of relaxation times of the nanocomposites.
The σ dc(I) values of the PEO–x wt% SiO2 nanocomposites, initially, decrease non-linearly with increase of SiO2 concentration up to 5 wt% (Fig. 7), and at 10 wt% it shows a little increase, which confirms that the dispersion of SiO2 reduces the dc electric conductivity values as obtained from their low-frequency σ′ spectra (region I). But the σ dc(II) values obtained from the high-frequency σ′ spectra (region II) have an initial increase up to 3 wt% SiO2, and above this concentration these values gradually decrease. Earlier studies revealed that the dc conductivity plateau occurs in the high-frequency region for most of the PEO-based ion conducting SPE materials [9–12, 40], and therefore, the increase of σ dc(II) at low SiO2 concentration also favours the enhancement of ionic conductivity of these nanocomposite based prepared SPE materials [13–15]. Around 5 wt% SiO2, the amount of amorphicity and the PEO segmental dynamics of the nanocomposite have optimum values in regards to their use in preparation of high ionic conductivity SPE materials.
Conclusions
The structural, dielectric and electrical properties of melt compounded PEO–x wt% SiO2 nanocomposites were reported and discussed in regards to the changes in PEO crystallinity and also its chain dynamics. The relaxation times of these nanocomposites are determined from the loss spectra of different formalism. Results confirm that the PEO chain segmental dynamics enhances abruptly with the dispersion of just 1 wt% SiO2 in the PEO matrix, and after that it remains almost same with further increase of SiO2 concentration. The ε′ values of the nanocomposite films have steady state behaviour above 100 kHz, and these values decrease non-linearly with increase of SiO2 concentration. The dc conductivity values obtained from low-frequency σ′ spectra of the PNC films are about two orders of magnitude lower than that of the values obtained from their high-frequency σ′ spectra. The intensity of 120 reflection peak of PEO at 1 and 3 wt% SiO2 concentrations in PNC films are higher than that of pristine PEO, but the concerted 112,032 peak intensity is significantly low at these SiO2 concentrations. At 5 and 10 wt% SiO2, the relative low intensities of both the PEO peaks confirm a large increase in the amount of amorphous phase of PEO in the nanocomposites. On the basis of the values of dielectric parameters and the structural properties, the importance of these PNC films as substrate/insulators in fabrication of microelectronic integrated circuit was confirmed. Further the facts which involve in the enhancement of ionic conductivity of solid state electrolytes based on these materials were also explored from this study.
References
Madathingal RR, Wunder SL (2011) Confinement effects of silica nanoparticles with radii smaller and longer than R g of absorbed poly(ethylene oxide). Macromolecules 44:2873–2882
Hong B, Panagiotopoulos AZ (2012) Molecular dynamics simulations of silica nanoparticles grafted with poly(ethylene oxide) oligomer chains. J Phys Chem B 116:2385–2395
Toskas G, Cherif C, Hund RD, Laourine E, Fahmi A, Mahltig B (2011) Inorganic/organic (SiO2)/PEO hybrid electrospun nanofibers produced from a modified sol and their surface modification possibilities. Appl Mater Interfaces 3:3673–3681
Gage RA, Currie EPK, Cohen Stuart MA (2001) Adsorption of nanocolloidal SiO2 particles on PEO brushes. Macromolecules 34:5078–5080
Waddon AJ, Petrovic ZS (2002) Spherulite crystallization in poly(ethylene oxide)-silica nanocomposites. Retardation of growth rates through reduced molecular mobility. Polym J 34:876–881
Madathingal RR, Wunder SL (2011) Thermal degradation of PEO on SiO2 nanoparticles as a function of SiO2 silanol density, hydrophobicity and size. Thermochim Acta 523:182–186
Burgaz E (2011) Poly(ethylene oxide)/clay/silica nanocomposites: morphology and thermochemical properties. Polymer 52:5118–5126
Karan NK, Pradhan DK, Thomas R, Natesan B, Katiyar RS (2008) Solid polymer electrolytes based on polyethylene oxide and lithium trifluoro-methane sulfonate (PEO–LiCF3SO3): ionic conductivity and dielectric relaxation. Solid State Ionics 179:689–696
Choudhary S, Sengwa RJ (2011) Dielectric spectroscopy and confirmation of ion conduction mechanism in direct melt compounded hot-press polymer nanocomposite electrolytes. Ionics 17:811–819
Choudhary S, Sengwa RJ (2012) Effect of different anions of lithium salt and MMT nanofiller on ion conduction in melt-compounded PEO-LiX-MMT electrolytes. Ionics 18:379–384
Karmakar A, Ghosh A (2012) Dielectric permittivity and electric modulus of polyethylene oxide (PEO)–LiClO4 composite electrolytes. Curr Appl Phys 12:539–543
Choudhary S, Sengwa RJ (2013) Effects of preparation methods on structure, ionic conductivity and dielectric relaxation of solid polymeric electrolytes. Mater Chem Phys 142:172–181
Ketabi S, Lian K (2013) Effect of SiO2 on conductivity and structural properties of PEO–EMIHSO4 polymer electrolyte and enabled solid electrochemical capacitors. Electrochim Acta 103:174–178
Liu Y, Lee JY, Hong L (2002) Functionalized SiO2 in poly(ethylene oxide)-based polymer electrolytes. J Power Sources 109:507–514
Liu S, Imanishi N, Zhang T, Hirano A, Takeda Y, Yamamoto O, Yang J (2010) Effect of nano-silica filler in polymer electrolyte on Li dendrite formation in Li/poly(ethylene oxide)–Li(CF3SO2)2N/Li. J Power Sources 195:6847–6853
Fabbri P, Pilati F, Rovati L, Mckenzie R, Mijovic J (2011) Poly(ethylene oxide)–silica hybrids entrapping sensitive dyes for biomedical optical pH sensors: molecular dynamics and optical response. Optical Mater 33:1362–1369
Koo JH. In: Manasreh O (ed) (2006) Nanoscience and technology, polymer nanocomposites: processing, characterization and applications. McGraw-Hill, New York
Capuano G, Filippone G, Romeo G, Acierno D (2012) Universal features of the melt elasticity of interacting polymer nanocomposites. Langmuir 28:5458–5463
Jiang S, Yu D, Ji X, An L, Jiang B (2000) Confined crystallization behaviour of PEO in silica networks. Polymer 41:2041–2046
Bur AJ, Lee YuH, Roth SC, Start PR (2005) Measuring the extent of exfoliation in polymer/clay nanocomposites using real-time process monitoring methods. Polymer 46:10908–10918
Kripotou S, Pissis P, Savelyev YV, Robota LP, Travinskaya TV (2010) Polymer dynamics in polyurethane/clay nanocomposites studied by dielectric and thermal techniques. J Macromol Sci Part B Phys 49:86–110
Rekik H, Ghallabi Z, Royaud I, Arous M, Seytre G, Boiteux G, Kallel A (2013) Dielectric relaxation behaviour in semi-crystalline polyvinylidene fluoride(PVDF)/TiO2 nanocomposites. Compos B 45:1199–1206
Sengwa RJ, Choudhary S (2010) Investigation of correlation between dielectric parameters and nanostructures in aqueous solution grown poly(vinyl alcohol)–montmorillonite clay nanocomposites by dielectric relaxation spectroscopy. Express Polym Lett 4:559–569
Choudhary S, Sengwa RJ (2012) Dielectric properties and structures of melt-compounded poly(ethylene oxide)–montmorillonite nanocomposites. J Appl Polym Sci 124:4847–4853
Sengwa RJ, Choudhary S, Sankhla S (2010) Dielectric properties of montmorillonite clay filled poly(vinyl alcohol)/poly(ethylene oxide) blend nanocomposites. Compos Sci Technol 70:1621–1627
Sengwa RJ, Choudhary S (2011) Dielectric relaxations and structures of nano clay in solution cast poly(ethylene oxide)–montmorillonite clay nanocomposites. J Macromol Sci Part B Phys 50:1313–1324
Galaburda MV, Klonos P, Guńko VM, Bogatyrov VM, Borysenko MV, Pissis P (2014) Dielectric properties and thermal destruction of poly(dimethyl siloxane)/Fe2O3/SiO2 nanocomposites. Appl Surf Sci 305:67–76
Iurzhenko M, Mamunya Y, Boiteux G, Seytre G, Nikaj E, Gain O, Lebedev E, Ishchenko S (2014) Relaxation processes in hybrid organic-inorganic polymer nanosystems polymerized in situ. Nanoscale Res Lett 9:217(1–9)
Reading M, Vaughan AS (2010) Comparison of rheological, thermal and electrical properties of Poly(ethylene oxide) composites with micro and nano sized silicon dioxide filler. In: IEEE conference ICSD 2010, Potsdam, pp 1–4
Reading M, Vaughan AS (2010) The effect of thermal ageing on the rheological, thermal and electrical properties of poly(ethylene oxide). In: IEEE conference CEIDP 2010, West Lafayette, pp 1–4
Pluta M, Jeszka JK, Boiteux G (2007) Polylactide/montmorillonite nanocomposites. Structure, dielectric, viscoelastic and thermal properties. Eur Polym J 43:2819–2835
Zhang YH, Lu SG, Li YQ, Dang ZM, Xin JH, Fu SY, Li GT, Guo RR, Li LI (2005) Novel silica tube/polyimide composite films with variable low dielectric constant. Adv Mater 17:1056–1059
Lee YJ, Huang JM, Kuo SW, Chang FC (2005) Low-dielectric, nanoporous polyimide films prepared from PEO–POSS nanoparticles. Polymer 46:10056–10065
Wang Q, Wang C, Wang T (2013) Controlled low dielectric porous films template by silica microspheres: microstructure, formation mechanism and properties. J Colloids Interface Sci 389:99–105
Dang JM, Yuan JK, Yao SH, Lio RJ (2013) Flexible nanodielectric materials with high permittivity for power energy storage. Adv Mater 25:6334–6365
Choudhary S, Sengwa RJ (2014) Intercalated clay structures and amorphous behaviour of solution cast and melt pressed poly(ethylene oxide)–clay nanocomposites. J Appl Polym Sci 131:39898(1–9)
Ji J, Keen J, Zhong WH (2011) Simultaneous improvement in ionic conductivity and mechanical properties of multi-functional block-copolymer modified solid polymer electrolytes for lithium ion batteries. J Power Sources 196:10163–10168
Jin X, Zhang S, Runt J (2002) Observation of a fast dielectric relaxation in semi-crystalline poly(ethylene oxide). Polymer 43:6247–6254
Dutta K, De SK (2007) Electrical conductivity and dielectric properties of SiO2 nanoparticles dispersed in conductivity polymer matrix. J Nanoparticle Research 9:631–638
Pradhan DK, Choudhary RNP, Samantaray BK (2009) Studies of dielectric and electrical properties of plasticized polymer nanocomposite electrolytes. Mater Chem Phys 115:557–561
Acknowledgments
Authors are grateful to the Department of Science and Technology (DST), New Delhi for providing the experimental facilities through research projects Nos. SR/S2/CMP-09/2002, SR/S2/CMP-0072/2010 and the DST–FIST program. One of the authors SC is thankful to the DST, New Delhi for the award of SERB Fast Track Young Scientist Research Project No. SR/FTP/PS-013/2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choudhary, S., Sengwa, R.J. Dielectric dispersion and relaxation studies of melt compounded poly(ethylene oxide)/silicon dioxide nanocomposites. Polym. Bull. 72, 2591–2604 (2015). https://doi.org/10.1007/s00289-015-1424-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-015-1424-2