Abstract
In the present study, bacterial and fungal endophytes are isolated from Calotropis procera, a drought-resistant plant and studied for their role in plant growth promotion. Among bacterial sp. Enterobacter cloacae subsp. cloacae strain CPR5B and fungus, Penicillium citrinum strain CPL1F, were identified as potent endophytes as both strains were able to produce Indole Acetic Acid (IAA) and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase and solubilize phosphate. Penicillium citrinum CPL1F also been shown to produce siderophore. The IAA production was observed to be 94.28 μg/mL and 17.1 μg/mL for bacterial and fungal sp., respectively. The phosphate solubilization was observed to be 76.41 μg/mL and 114.57 μg/mL, respectively. The in vitro plant treatment studies with bacterium and fungus under irrigated and non-irrigated conditions showed that both strains had promoted plant growth in both conditions with respect to their control. Both the strains showed significant changes in most of the growth parameters under endophyte-treated irrigated and non-irrigated conditions, suggesting their stress-dependent plant growth promotion. The present findings will contribute to exploring endophytes that enhance plant growth in adverse conditions and act as plant growth-promoting endophytes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Endophytes are microorganisms that spend their life or part of it inside the plant tissues, promoting plant growth and development. Almost every plant on earth is harbored by one or more endophytes. They reside in intercellular and intracellular spaces of tissues of plants without causing harm to the host. Colonization of endophytes brings changes in host gene expression, metabolic functions, and many physiological aspects. Some endophytes adopt molecular mechanisms that are favorable to increasing abiotic stress tolerance [1, 2]. They improve the accessibility of soil nutrients under natural as well as extreme conditions. They are also known to produce biologically active metabolites and substances. Production of these substances helps to maintain their own metabolism as well as elevate plant growth potential [3, 4].
Drought stress is one of the major abiotic stresses responsible for low crop production and widespread crop failures. In India, 68% of the country’s area is vulnerable to drought (https://nidm.gov.in/PDF/manuals/Drought_Manual.pdf). Microbe-induced drought resistance can become an important aspect in sustainable agriculture keeping the soil degradation in check due to chemical fertilizers [5, 6]. Wheat is a major agricultural crop that provides a good source of nutrition, particularly in resource-poor countries [5]. Over half of the world’s wheat fields are influenced by periodic drought stress. Therefore, improving stress tolerance in wheat is essential for maintaining productivity and food security. Increasing drought tolerance in major crops, such as wheat and rice, is an important goal, as the world’s population is increasing more rapidly than the area of agricultural land [7].
Endophytes can promote plant growth through different direct and indirect mechanisms, such as nitrogen fixation, solubilization of minerals, (phosphorus, zinc, and potassium) production of phytohormones, (auxins, gibberellic acid, cytokinin, abscisic acid) production of siderophore, and HCN. Due to these attributes, endophytes are placed on the suitable candidates list to serve their role as ‘biofertilizers.’
The phytohormone Indole Acetic Acid (IAA) has been reported to be produced by endophytic microorganisms. Microorganisms corresponding to bacterial genera Bacillus, Acinetobacter, Pseudomonas, Azospirillum, and Azotobacter have been identified to produce IAA [4]. The main biosynthetic pathways for IAA production utilized by bacterial species are (i) indole-3-pyruvic acid, (ii) indole-3-acetamide, and (iii) indole-3-acetonitrile pathways [8, 9]. Penicillium roqueforti, Trichoderma spp., Serendipita indica, and Phoma glomerata have been found to produce IAA [10]. Further investigations on the biosynthetic pathways involved in the production of fungal IAA are still needed to be done.
Production of enzymes such as 1-aminocyclopropane-1-carboxylate (ACC) deaminase lowers the ethylene level under several biotic and abiotic stresses. The ACC released from the roots and seeds of plants is assimilated by bacteria having the enzyme ACC deaminase. It influences ACC efflux, causing a decrease in root concentration of ACC and ethylene, thus stimulating root growth and development. The conversion of ACC, the immediate precursor of ethylene, to ammonia and α-ketobutyrate is catalyzed by ACC deaminase. Bacteria producing ACC deaminase cleaves the immediate precursor of ethylene, i.e., ACC in plants, to produce ammonia and α-ketobutyrate that lead to reduced ethylene level in the plants and enhance growth [11,12,13]. However, this potential needs to be explored in the context of various stresses.
Insoluble inorganic phosphate solubilization by endophytic microbes depends on their capability to produce organic acids. These organic acids lower the pH, facilitating the interchange of metal parts of phosphates and forming soluble phosphate salts. Thus, the endophytic phosphate solubilizers help in mineral acquisition to enhance plant growth. Bacterial genera corresponding to Pseudomonas and Bacillus are efficient phosphate solubilizers, whereas fungal genera of Aspergillus and Penicillium are good phosphate solubilizers [14, 15]. Bacillus subtilis, B. circulans, B. polymyxa, B. megaterium, Pseudomonas striata, and P. aeruginosa are some important phosphate solubilizers. Aspergillus tubingensis, Aspergillus foetidus, Aspergillus niger, Aspergillus flavus, and Penicillium chrysogenum are some of important fungal phosphate solubilizers [16, 17].
Microorganisms have mechanism for siderophore production which helps in uptake of iron. Siderophores are low molecular mass iron chelating compound which mainly functions to scavenge insoluble ferric iron from various environments [18]. Bacterial genera which produce siderophores are Pseudomonas, Azotobacter, Bacillus, Rhizobium, and Enterobacter [19]. Another feature of siderophore-producing endophytes is that they compete for iron and make it unavailable for phytopathogens [20]. In some studies, it has been found that endophytic bacteria are involved in effective transportation of stored iron [21, 22].
The present study is carried out on drought-resistant plant Calotropis procera which has high medicinal value. It is found abundantly along the roadsides and grows in, nutrient-poor, sandy soil [23, 24]. The presence of (Universal stress protein)-like gene in the plant helps the plant to respond to various abiotic stresses, such as salinity, drought, and toxic metals. Thus, there might be a possibility that endophytes inhabiting this plant may contribute to the promotion of plant growth under adverse conditions [25]. However, to draw overall conclusions about the positives of endophytes for plant drought stress tolerance, it is imperative to identify the suitable host–endophyte combinations that provide enhanced tolerance. In this regard, we isolated the bacterial and fungal endophytes from drought-resistant plant, further identifying a potent endophyte that has the maximum number of plant growth-promoting traits and also evaluated their ability to tolerate NaCl stress. The isolates that showed a maximum number of plant growth-promoting traits, were treated with the wheat plantlets (Wheat variety HD 2967) to examine their ability to confer resistance to water deficit in the new host. The results of the current study are important because they open up possibilities of using endophytes from drought-adapted plants to mitigate water deficit in agricultural crops.
Materials and Methods
Isolation of Endophytes
Three to four samples of healthy plants of Calotropis procera (root, stem, and leaves) were randomly chosen from the roadsides at least 15 m distance apart from Deen Dayal Upadhyaya Gorakhpur University, (26.7479° N, 83.3812° E), Uttar Pradesh, India. The plant parts were thoroughly rinsed in running tap water, followed by Tween 20 for 1 min to remove debris, dust, soil granules, and surface microorganisms. Final washing was done with sterile distilled water 3 times. The root, stem, and leaves were cut into small pieces of 1–2 cm with the help of a sterile blade and scissors. Sections of root, stem, and leaves of the plant were surface sterilized by consecutive immersion in 2% sodium hypochlorite for 2 min, 70% ethanol for 30 s, and 0.1% mercuric chloride for 2 min followed by proper washing with sterile distilled water for 5–6 times [26]. A piece of each plant sample excised longitudinally such that the inner surface was exposed to the Nutrient Agar (NA, 5.0-g peptone, 1.5-g yeast extract, 1.5-g beef extract, 5.0-g NaCl, 20-g agar, and 1-L distilled water; pH 7.2) and Potato Dextrose Agar (PDA, 4.0-g potato extract, 20.0-g dextrose, 15.0-g agar, and 1-L distilled water; pH 5.6) medium for isolation of bacterial and fungal endophytes, respectively. The surface-sterilized piece of each plant sample was aseptically transferred to a separate petri dish with medium to observe the success of surface sterilization and treated as a control. The NA plates were transferred to the incubator, at 37 °C for 72 h. The plates were observed on a daily basis for the growth of bacterial endophytes. The PDA plates were incubated at 28 ± 2 °C for 1–2 weeks and observed for fungal growth. Morphologically distinct bacterial and fungal isolates were re-streaked on a fresh medium to obtain pure culture on their same respective medium. For temporary storage and regular use, the pure cultures of isolated bacteria were preserved on plates at 4 °C and in sterile vials with 40% (v/v) glycerol for long-term storage at − 20 °C. The fungal isolates were stored at 4 °C for further investigations.
Characterization of Plant Growth-Promoting Traits
IAA (Indole Acetic Acid) Production
The estimation of IAA was done as described by Gordon and Weber, with slight modification [27]. The endophytic bacterial culture was adjusted to an OD600 nm of 1 and 100 μL of culture was inoculated into Nutrient broth supplemented with a concentration 1 g/L of l-tryptophan and incubated for 7 days at 37 °C. Two mycelium disk of fungus (8-mm cork borer) was inoculated in Potato Dextrose Broth (PDB) supplemented with a concentration 1 g/L of l-tryptophan and incubated at 28 °C, for 7 days. Five ml of each culture was collected from the incubating broth after 7 days and centrifuged at 5000 rpm for 20 min. One ml of the supernatant was mixed with 2 mL of Salkowski’s reagent (49 mL of 70% perchloric acid, 49 mL of distilled water; 2 mL of 0.5-M FeCl3) and incubated in the dark for 30 min. The development of a pink color indicated IAA production as the optical density was measured at 535 nm. The amount of IAA produced was estimated by a pure IAA standard graph. All the IAA production experiments were performed in triplicate.
ACC Deaminase Production
ACC deaminase production of bacterial and fungal isolates was analyzed by modification in methods [28]. The isolates were inoculated onto DF salts minimal medium (potassium dihydrogen phosphate 4 g/L, disodium hydrogen phosphate 6 g/L, magnesium sulfate heptahydrate 0.2 g/L, ferrous sulfate heptahydrate 0.1 g/L, boric acid 10 μg/L, manganese (II) sulfate 10 μg/L, zinc sulfate 70 μg/L, copper (II) sulfate 50 μg/L, molybdenum (VI) oxide 10 μg/L, glucose 2 g/L, gluconic acid 2 g/L, citric acid 2 g/L, agar 12 g/L) amended with 3 mM of ACC as sole source of nitrogen. The consideration of positive results depended upon the growth of bacterial endophytes on the medium after 3 days of incubation and fungal isolates after 5 days of incubation.
Phosphate Solubilization
For detection of phosphate-solubilizing bacterial endophytes, the isolates were streaked onto NBRIP (National Botanical Research Institute’s phosphate growth) medium (g/L: glucose, 10 g; tricalcium phosphate 5 g; magnesium chloride hexahydrate 5 g; magnesium sulfate heptahydrate 0.25 g; potassium chloride 0.2 g; ammonium sulfate 0.1 g; and 15 g agar). The cultures were incubated at 37 °C for 3 days. The clear zone induced by bacterial isolates were considered as positive [29]. For fungal isolates, strains were inoculated onto PKV medium (g/L: yeast extract 0.5 g, dextrose 10 g, calcium phosphate 5 g, ammonium sulfate 0.5 g, potassium chloride 0.2 g, magnesium sulfate 0.1 g, manganese sulfate 0.0001 g, ferrous sulfate 0.0001 g, and 15 g of agar). The cultures were incubated at 28 °C for 5 days. Clear halo zones surrounding the colony were considered positive for phosphate solubilization [30]. For quantitative estimation of phosphate, the endophytic bacterial culture was adjusted to an OD600 nm of 1 at and 100 μL of culture cultures were grown on NBRIP and two mycelium disks of fungal culture were inoculated into Pikovskaya broth medium and incubated for 7 days at 150 rpm on a shaker. Cultures were then centrifuged at 4000 rpm for 10 min at 4 °C to get cell-free supernatant. Solubilized phosphate in the culture supernatant was measured following molybdate blue color method at 882 nm according to Murphy and Riley [31]. To calculate the amount of phosphate, optical density against concentration (μg mL−1) of the standard solution was plotted on a graph. The standard solution was prepared by dissolving 0.2195 g of KH2PO4 in 1-L water to get 50-μg mL−1 solution. Further dilutions (1, 2, 3, 4, 5 μg mL−1) were made to get the standard curve of KH2PO4. Uninoculated sterilized broth medium was used as control. The experiment was performed in triplicate.
Siderophore Production
The isolates were observed to produce siderophores on blue agar CAS medium containing chrome azurol S (CAS) and hexadecyltrimethylammonium bromide (HDTMA) as indicators by following the modification in methods described by Schwyn and Neilands [32]. The first solution: CAS (60.5 mg) was dissolved in 50-mL water and mixed with 10-mL iron (III) solution (1-mM FeCl3, 6H2O, 10-mM HCl). The first solution was further mixed slowly in second solution containing hexadecyltrimethylammonium (HDTMA) (72.9 mg) dissolved in 40-mL water and the final solution was autoclaved. The final 100 mL of solution was added to 900 mL of autoclaved NB and PDB medium of pH 6.8 for bacterial and fungal isolates, respectively. The blue agar medium was aseptically poured onto sterile plates and solidified. The bacterial isolates were inoculated on CAS medium for 24 h and the yellow halo observed around the colonies after incubation at 28 ± 2 °C was considered positive. The mycelial tips of the fungus were cut and inoculated on chrome azurol S (CAS) agar and incubated at 28 °C for 3–5 days under a dark condition. The medium was poured on plates and observed for pink or yellow halo zone after 4–5 days of incubation at 28 ± 2 °C were considered positive.
Molecular Characterization of Plant Growth-Promoting Endophyte (PGPE)
The task for identification and sequencing was outsourced to MTCC (Microbial Type Culture Collection) Chandigarh, India. Genomic DNA extraction from bacterial and fungal cultures was carried out with Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research, USA). The concentration of each endophyte DNA was quantified using the Nanodrop Spectrophotometer (Thermo Scientific). Isolated bacterial DNA was amplified with 16S rRNA-specific primers (27F-5′-AGAGTTTGATCCTGGCTCAG 3′ and 1492-R-5′-GGTTACCTTGTTACGACTT-3′). PCR (Polymerase Chain Reaction) amplification was performed with 10× polymerase buffer, 1U of Taq polymerase, 10-mM dNTPs, 10 pmol of each 16 s RNA primer, 10-mM MgCl2, and 1.0 μL of DNA template. The amplification conditions were set as an initial 94 °C denaturation for 5 min (1 cycle), followed by 35 cycles of 95 °C for 1 min, 52 °C for 1 min, and 72 °C for 2 min. A final extension step was performed at 72 °C for 10 min (1 cycle). PCR amplicon was gel eluted and purified using QIAquick Gel Extraction Kit (Qiagen). The purified PCR product was sequenced using Sanger DNA sequencing method. 16S rRNA gene sequences were subjected to similarity search using EzBiocloud database (http://www.ezbiocloud.net/). For fungi, the ITS region is amplified consisting of the primer pairs ITS-1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS-4 (5′TCCTCCGCTTA TTGATATGC-3′) [33]. PCR (Polymerase Chain Reaction) amplification was performed with 10× polymerase buffer, 1U of Taq polymerase, 10-mM dNTPs, 50 pmol for each ITS primers, 10-mM MgCl2, and 1.0 μL of DNA template. The amplification conditions were set as an initial 94 °C denaturation for 5 min (1 cycle), followed by 35 cycles of 95 °C for 1 min, 52 °C for 1 min, and 72 °C for 2 min. A final extension step was performed at 72 °C for 10 min (1 cycle). The product of PCR was separated on 1% agarose gel electrophoresis. The PCR amplicon was purified using the Zymoclean™ Gel DNA Recovery Kit (Zymo Research, USA) for further sequencing. For ITS region sequencing, the fungal PCR product was further amplified through PCR using 10 pmol of ITS5F and ITS4R primers with initial denaturation at 96 °C of 2 min followed by 24 cycles of 10-s denaturation at 96 °C, 5-s annealing at 50 °C, and 4-min extension at 60 °C.
The sequences were further submitted to NCBI for procurement of accession number. The phylogenetic relationship of the endophytic isolates and their close neighbors was produced by Neighbor-joining method. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates). The evolutionary distance was computed by Maximum Composite Likelihood method. The evolutionary assessment and construction of phylogenetic tree were done through MEGA 11 software.
Determination of Potential PGPE Growth at Different Salt Concentrations (Tolerance to Salinity Stress)
Bacterial and fungal isolates were checked for tolerance at varying concentrations of salt with major modification in the method described by Christakis et al. [34]. Fresh culture was streaked on Nutrient Agar and Potato Dextrose medium containing 2%, 5%, 7%, 10%, 12%, and 15% (w/v) NaCl. After 24 h and 3 days of incubation for bacterial and fungus, respectively, the results were recorded as positive and negative as growth observance.
Effect of Endophyte Treatment in Wheat Plant
To analyze their ability to improve plant performance under irrigated and non-irrigated conditions, the endophytes were applied to wheat plants. The wheat variety (HD 2967) was surface sterilized with NaOCl (5%) for 3 min and HgCl2 for 2 min further thoroughly rinsed with autoclaved distilled water. The sterilized seeds were germinated for 2–3 days at 20 °C in the dark. The seedlings of same size were selected and sown in autoclaved soil (perlite (8–12%), coco-peat (78–80%), and vermiculite (10–12%) [11]. Initially, 5 seedlings were sown at a depth of 3 cm per 15 cm pot, and were planted. The pots were kept in a growth chamber at 20 °C, with 16/8-h (light/dark) photoperiod. One week of growth was allowed so as to plantlets attain a certain similar height and following the thinning process, 4 plantlets per pot, the roots of the plant were treated with 2 mL of the bacterial suspension (OD600 nm of 1) in sterilized water. Similarly, in the case of isolated fungus, cultured in PDA broth (50 ml containing 3 g of fungal mycelium), the fungal mycelium in 2 mL of sterilized water was applied to the plants [35]. Non-treated seedlings were watered with autoclaved sterilized water. After a week of endophyte treatment watering was stopped for 8 days; however, a positive control in the experiment was properly irrigated. Irrigation proceeded further for 1 day after the drought treatment. Plant health was assessed in terms of length and biomass measurements [36]. Two independent experiments were carried out containing 4 plants per treatment in each experiment.
Statistical Analysis
The quantification analysis was made based on experiments repeated three times. The in vitro treatment of wheat plants was carried out in two independent treatments. Each treatment contains four plants per endophyte treatment and control. The collected data were statistically evaluated based on Two-Sample t test assuming equal variance applied to compare the means. The cut-off for statistical significance was set as < 0.05. The analysis was carried out on MS EXCEL. The comparison was done between control irrigated with endophyte-treated irrigated, control non-irrigated with endophyte-treated non-irrigated, and, endophyte-treated irrigated and endophyte-treated non-irrigated conditions.
CPR5B identified as Enterobacter cloacae subsp. cloacae (NCBI Accession No. OK560111).
CPL1F identified as Penicillium citrinum (NCBI Accession No. OK606060).
Results
Isolation of Endophytes
A total of 20 endophytes have been obtained from the plant. Among them, 14 were bacterial (6 from roots, 4 from stem, and 4 from leaf) and 6 fungal (1 from root, 5 from stem, and 1 from leaf) isolates. The control showed no bacterial or fungal growth on the medium indicating successful surface sterilization. All the bacterial and fungal isolates were further studied for plant growth-promoting traits, like IAA, ACC deaminase, siderophore production, and phosphate solubilization (Tables 1, 2).
IAA Production
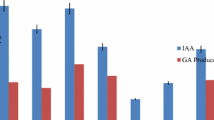
Among all endophytes, 15 isolates developed pink color after adding the Salkowski reagent to bacterial and fungal supernatant, indicating IAA production. The highest IAA production was recorded to be produced by CPR5B, 94.28 μg/mL ± 0.71, whereas the lowest CPS1F, 8.54 μg/mL ± 0.41 after 7 days of incubation. The IAA production can be categorized according to the quantity of IAA they produce low: 45% (0.00–20.00 μg/mL), moderate: 45% (21.00–50.00 μg/mL), and high level: 10% (50.00–100 μg/mL).
ACC Deaminase Production
All 17 strains except CPS3F, CPL2F, and CPS4F were able to produce the enzyme. The growth was observed on the DF salt minimal medium supplemented with ACC as the sole source of nitrogen.
Phosphate Solubilization
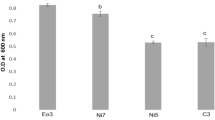
In the present study, the majority of endophytic bacterial strains solubilized phosphate compared to fungal endophytes. The formation of the clear zone on NBRIP and PKV medium was observed for phosphate solubilization. Among bacterial strains CPR1B, CPR2B, CPR3B, CPR4B, CPR5B, CPS1B, CPS2B, CPS3B, CPL1B, and CPL2B solubilized phosphate on NBRIP medium, whereas CPS1F, CPS2F, and CPL1F solubilized phosphate on PKV Agar medium. The endophytic phosphate solubilization ranges from 68.15 μg/mL ± 0.35 to 237.38 μg/mL ± 1.11. The highest phosphate solubilization was recorded in fungal strain CPS1F (Tables 1, 2).
Siderophore Production
Siderophore production was observed in a few bacterial and most of fungal endophytic species of C. procera. Among bacterial endophytes, only four strains CPR3B, CPR6B, CPS1B, and CPS2B were able to produce siderophores, while the majority of fungal strains CPS1F, CPS2F, CPS5F, and CPL1F produced siderophores on CAS medium. The production of yellow and pink zones indicated positive siderophore production.
Identification of Potential Plant Growth-Promoting Endophytes (PGPE)
Among all the endophytic bacteria, CPR5B showed high IAA production, ACC deaminase production, and phosphate solubilization; fungal endophyte CPL1F showed all growth-promoting traits positive with moderate IAA production and phosphate solubilization. Therefore, these two strains were further subjected for identification. The NCBI BLAST analysis of 16S rRNA sequence showed 99.93% identity with E. cloacae (NCBI Accession No. MN371802), whereas EZBioCloud database also showed maximum similarity; 99.79% with Enterobacter cloacae subsp. cloacae (CP001918). Thus, the bacterium was identified as Enterobacter cloacae subsp. cloacae (NCBI Accession No. OK560111) and fungus as Penicillium citrinum (NCBI Accession No. OK606060). It showed maximum similarity; 99.80% with P. griseofulvum (NCBI Accession No. MK834749) and many P. citrinum strains. Both strains evolutionary relationship was ascertained by phylogenetic tree (Figs. 1, 2).
Phylogenetic tree constructed based on 16S rRNA gene sequence of Enterobacter cloacae subsp. cloacae strain CPR5B including reference sequences by the Neighbor-Joining method. Bootstrap values are shown at each node from 500 replicates. The bar represents 0.0020 base substitution per site. This analysis involved 9 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 1470 positions in the final dataset
Phylogenetic tree constructed based on ITS region sequence of Penicillium citrinum strain CPL1F including reference sequences by the Neighbor-Joining method. Bootstrap values are shown at each node from 500 replicates. The bar represents 0.02 base substitution per site. This analysis involved 8 nucleotide sequences. Codon positions included were 1st+2nd+3rd+Noncoding. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 2186 positions in the final dataset
Salt Tolerance of PGPE
Both the potential plant growth-promoting endophytes showed tolerance to salt stress. The growth of strain CPR5B (Enterobacter cloacae subsp., cloacae) was observed up to 10% of NaCl in the medium. The CPL1F strain (Penicillium citrinum) showed a tolerance of up to 15% of NaCl (Table 3).
Effect on Plant Growth Parameters in the Presence of Enterobacter cloacae subsp. cloacae Strain CPR5B on Growth of Triticum aestivum (HD 2967) Under Irrigated/Unstressed and Non-irrigated/Stressed Conditions
In the present study, with the treatment of E. cloacae in wheat plantlets, we have found a significant increase (P < 0.0001) in root and shoot length, fresh weight (P < 0.01), and non-significant increase in dry weight by 1.36, 1.30, 1.15, and 1.06 folds, respectively, as compared to their non-treated irrigated control. Under CPR5B-treated non-irrigated conditions, there was a significant increase (P < 0.0001) in root length by 1.69 folds, a non-significant increase in shoot length and dry weight by 1.14 and 1.09 folds and 1.12 folds non-significant decrease in fresh weight was observed when compared with their non-irrigated control (Figs. 3, 4, 5).
Effect on Plant Growth Parameters in the Presence of Penicillium citrinum CPL1F on Growth of Triticum aestivum (HD 2967) Under Irrigated/Unstressed and Non-irrigated/Stressed Conditions
In vitro studies in the presence of P. citrinum in wheat plantlets showed that under treated CPL1F-irrigated conditions, shoot length was significantly increased (P < 0.0001) by 1.57 folds when compared with untreated irrigated control. A significant increase (P < 0.001) was observed in the fresh weight of plantlets by 1.20 folds. Dry weight also showed an increase of 1.24 folds (P < 0.01). However, a non-significant increase was observed in root length by 1.04 folds. Under non-irrigated conditions, CPL1F-treated wheat plantlets showed that the root length and shoot length were significantly increased (P < 0.0001) by 1.82 and 1.66 folds, respectively, as compared to untreated non-irrigated control. A significant increase (P = 0.001) was observed in fresh weight by 1.35 folds. An increase in dry weight by 1.42 folds was also recorded (P < 0.005) (Figs. 3, 4, 6).
Discussion
A total 20 endophytes were isolated from C. procera. All the endophytes were analyzed for plant growth-promoting abilities. IAA is considered a hormone of plant growth, involved in cell elongation, and helps in the formation of root and root hairs. The amount of IAA produced depends upon the type and amount of production media used, the concentration of l-tryptophan, and the incubation period. Various types of endophytic bacteria have been reported to produce IAA employing different biosynthesis pathways [4, 3637]. Both strains CPR5B and CPL1F were able to produce IAA; however, strain CPR5B showed highest IAA production among all endophytes. Enzyme ACC deaminase reduces the ethylene amount produced by the plant during drought stress and also helps in nodulation. Endophytes which produce IAA as well as ACC deaminase has significant role in mitigating the effects of ethylene that is produced by plants under most of the stressors. Both the strains demonstrated their growth on medium containing ACC as sole source of nitrogen. Inoculating crops with ACC deaminase-producing bacteria could reduce the inhibitory level of ethylene produced under drought stress [38]. An experiment previously conducted showed increased drought tolerance upon the inoculation of Pseudomonas sp. having ACC deaminase activity in wheat seedlings [39]. Phosphate solubilization helps in phosphorus acquisition and make it available to the plant. Both the endophytic strains have been able to solubilized the phosphate on their respective medium. Previous study done on phosphate-solubilizing bacterial endophytes isolated from the leaves of Pulicaria insisa (Lam) DC formed a clear zone on PKV medium. These bacterial sp. were identified as Agrobacterium fabrum, Acinetobacter radioresistant, Bacillus cereus, and Bacillus subtilis and also promoted plant growth [40]. Greater efficiency of phosphate solubilization can be achieved through inoculation by combining bacteria (Bacillus, Pseudomonas, and Burkholderia), with other beneficial bacteria (Rhizobium and Enterobacter), fungi (Arthrobotrys, Penicillium, and Aspergillus), and mycorrhizae [41]. However, only Penicillium citrinum strain CPL1F was observed for siderophore production, suggesting its role in uptake of iron. A study done previously showed that siderophore-producing fungi, Trichoderma spp. is important for plant growth promotion in sunchoke plant [42]. For the salinity test of the CPR5B and CPL1F, the strains were grown on media containing varied NaCl concentrations. CPR5B was able to tolerate up to 10% salt-stressed conditions, whereas CPL1F was able to grow efficiently on 15% salt-stressed conditions. Thus, CPR5B can be categorized in ‘borderline extreme halotolerant’ and CPL1F in ‘extremely halotolerant’ species as they can grow in 10% and 15% NaCl-stressed conditions [43]. Previous research had shown that E. cloacae showed high tolerance to 3-M NaCl concentrations, and inoculation with bacterium enhanced the growth of soybean plantlets under salt stress [44, 45]. Penicillium strains have been found to tolerate high-salt concentrations of up to 20% [46].
The in vitro treatment with Enterobacter cloacae subsp. cloacae, strain CPR5B, showed increase in the folds of root length under non-irrigated conditions with respect to irrigated conditions. The possible explanation for the increase in folds of root length is that endophyte might produce IAA as well as ACC deaminase under drought stress and enhanced root for better nutrient acquisition and absorption of water. It has also been shown that treatment with CPR5B increased root length, shoot length, and fresh weight significantly in wheat plantlets when compared to non-treated irrigated control. A study previously conducted showed that treatment with bacterial endophytes from C. procera increased root length and shoot length in rice plants when compared to untreated control. The root dry weight was also found to be increased in rice plants [47]. Previously, endophytic bacteria isolated from C. procera and Pseudomonas IUK001 showed 100 and 90% insecticidal activity against Galleria mellonella larvae. The rice plants inoculated with bacteria showed increased total lengths (root + shoot) and dry and fresh weight [48].
Through the in vitro treatment with Penicillium citrinum, strain CPL1F, an increase in the folds of all growth parameters was found under non-irrigated conditions as compared to irrigated conditions in the presence of Penicillium citrinum, suggesting its potential role in plant growth promotion under stress conditions. As the strain is producing IAA as well as ACC deaminase, it can confer better adaptations to the stress responses. However, a significant increase in shoot length and non-significant increase in the root length under treated irrigated conditions were observed in comparison to untreated irrigated controls. Similar findings, in which the shoot length was increased in the presence of P. citrinum culture filtrate, have been observed in rice plantlets [49]. Our study coincides with a study, in which, fungal endophytes from C. procera increased growth parameters in inoculated maize plants. Two Phoma sp. isolated from Tinospora cordifolia and C. procera have enhanced in maize plants. However, Phoma sp. from C. procera showed more increase in all plant growth parameters suggesting that two same strains from different hosts may have varied effects on plant growth [50].
The observations indicated that the presence of endophytes may stimulate inherent plant responses to water stress and/or provide endophytic-derived compounds that mediate the stress response. Hence, detailed future studies on the biochemical and molecular mechanism of endophyte-induced stress tolerance. The selection of these two isolates in the current study was mainly driven by their maximum number of plant growth-promoting traits. One of the limitations of the application of endophytes to non-hosts is that the effect of endophytes may not necessarily generalize to non-hosts. However, in this study, with the treatment of endophytic bacteria, the wheat variety has shown a significant increase in plant root length, shoot length, and fresh weight. Similarly, shoot length and fresh weight showed a significant increase in treatment with endophytic fungi. The current work represents potential endophytes to confer drought resistance in wheat variety HD 2967, threatened by stress. The biochemical and molecular mechanism for providing drought tolerance to new hosts needs to be addressed in future. Furthermore, future studies can be done by inoculation of these isolates in combination to impart drought tolerance in different agricultural crops.
Conclusion
This work suggests that bacterial and fungal endophytes from drought-resistant plants may promote plant growth. Both species have shown positive for the majority of plant growth-promoting traits. Furthermore, the bacterial sp. Enterobacter cloacae subsp., cloacae had shown moderate resistance to salt; however, the fungi Penicillium citrinum proved to be more efficient than bacterial sp. and had shown more tolerance under extreme salt stress. Thus, future research may be done to explore their effect on plant growth under salt stress. Penicillium citrinum CPL1F also demonstrated an increase in growth parameters under non-irrigated conditions compared to Enterobacter cloacae subsp. cloacae CPR5B. The present study shows that Enterobacter cloacae subsp. cloacae and Penicillium citrinum might act as beneficial plant growth-promoting agents under water stress conditions and hence, there is a possibility of the scientific utilization of these strains in modern as well as traditional agriculture.
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- ROS:
-
Reactive oxygen species
- NaCl:
-
Sodium chloride
- NA:
-
Nutrient agar
- PDA:
-
Potato dextrose agar
- PDB:
-
Potato dextrose broth
- NBRIP:
-
National Botanical Research Institute’s Phosphate growth medium
- PKV:
-
Pikovskaya agar medium
- CAS:
-
Chrome azurol S
- HDTMA:
-
Hexadecyltrimethylammonium bromide
- KH2PO4 :
-
Monopotassium phosphate
- FeCl3 :
-
Ferric chloride
- Pmol:
-
Picomole
- μl:
-
Microliter
- μg:
-
Microgram
- mM:
-
Millimolar
- g/L:
-
Gram per liter
- 16S rRNA:
-
16S ribosomal Ribonucleic acid
- BLAST:
-
Basic local alignment search tool
- bp:
-
Base pair
- cm:
-
Centimeter
- DNA:
-
Deoxyribonucleic acid
- g:
-
Gram
- IAA:
-
Indole-3-acetic acid
- ACC:
-
1-Aminocyclopropane-1-carboxylate (ACC)
- NCBI:
-
National Center for Biotechnology Information
- nm:
-
Nanometer
- PCR:
-
Polymerase chain reaction
- PGPE:
-
Plant growth-promoting endophytes
References
Khare E, Mishra J, Arora NK (2018) Multifaceted interactions between endophytes and plant: developments and prospects. Front Microbiol 9:2732. https://doi.org/10.3389/fmicb.2018.02732
Kaur T, Devi R, Kour D et al (2021) Plant growth promoting soil microbiomes and their potential implications for agricultural and environmental sustainability. Biologia 76:2687–2709. https://doi.org/10.1007/s11756-021-00806-w
Arnold AE, Henk DA, Eells RL, Lutzoni F, Vilgalys R (2007) Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 99:185–206. https://doi.org/10.1080/15572536.2007.11832578
Eid AM, Fouda A, Abdel-Rahman MA, Salem SS, Elsaied A, Oelmüller R, Hijri M, Bhowmik A, Elkelish A, Hassan SE (2021) Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants 10:935. https://doi.org/10.3390/plants10050935
Patel S, Jinal HN, Amaresan N (2017) Isolation and characterization of drought resistance bacteria for plant growth promoting properties and their effect on chilli (Capsicum annuum) seedling under salt stress. Biocatal Agric Biotechnol 12:85–89. https://doi.org/10.1016/j.bcab.2017.09.002
Etesami H, Maheshwari DK (2018) Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: action mechanisms and future prospects. Ecotoxicol Environ Saf 156:225–246. https://doi.org/10.1016/j.ecoenv.2018.03.013
Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, Sadia S, Nasim W, Adkins S, Saud S, Ihsan MZ (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147. https://doi.org/10.3389/fpls.2017.01147
Cassán F, Vanderleyden J, Spaepen S (2014) Physiological and agronomical aspects of phytohormone production by model plant-growth-promoting rhizobacteria (PGPR) belonging to the genus Azospirillum. J Plant Growth Regul 33:440–459. https://doi.org/10.1007/s00344-013-9362-4
Dudeja SS, Suneja-Madan P, Paul M, Maheswari R, Kothe E (2021) Bacterial endophytes: molecular interactions with their hosts. J Basic Microbiol 61:475–505. https://doi.org/10.1002/jobm.202000657
Baron NC, Rigobelo EC (2022) Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology 13:39–55. https://doi.org/10.1080/21501203.2021.1945699
Khan AL, Halo BA, Elyassi A, Ali S, Al-Hosni K, Hussain J, Al-Harrasi A, Lee IJ (2016) Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron J Biotechnol 21:58–64. https://doi.org/10.1016/j.ejbt.2016.02.001
Chandwani S, Amaresan N (2022) Role of ACC deaminase producing bacteria for abiotic stress management and sustainable agriculture production. Environ Sci Pollut Res 20:1–7. https://doi.org/10.1007/s11356-022-18745-7
Gamalero E, Glick BR (2015) Bacterial modulation of plant ethylene levels. Plant Physiol 169:13–22. https://doi.org/10.1104/pp.15.00284
Yadav LS, Kushwaha V, Jain A (2020) Isolation and screening of phosphate solubilizing fungi from okra rhizosphere soil and their effect on the growth of okra plant (Abelmoschous esculentus L.). Trop Plant Res 7:277–284. https://doi.org/10.22271/tpr.2020.v7.i2.033
Raymond NS, Gómez-Muñoz B, van der Bom FJ, Nybroe O, Jensen LS, Müller-Stöver DS, Oberson A, Richardson AE (2021) Phosphate-solubilising microorganisms for improved crop productivity: a critical assessment. New Phytol 229:1268–1277. https://doi.org/10.1111/nph.16924
Singh KP (2023) Comparative analysis: physiological and biochemical characterization of phosphate solubilization by Pseudomonas and Bacillus species from various agricultural areas of Dehradun, Uttarakhand, India. Int J Life Sci 11(3):219–227. https://doi.org/10.5281/zenodo.8395512
Ahmad A, Moin SF, Liaqat I, Saleem S, Muhammad F, Mujahid T, Zafar U (2023) Isolation, solubilization of inorganic phosphate, and production of organic acids by individual and co-inoculated microorganisms. Geomicrobiol J 40:111–121. https://doi.org/10.1080/01490451.2022.2124329
Maheshwari R, Bhutani N, Suneja P (2019) Screening and characterization of siderophore producing endophytic bacteria from Cicer arietinum and Pisum sativum plants. J Appl Biol Biotechnol 7:7–4. https://doi.org/10.7324/jabb.2019.70502
Wittenwiler M (2007) Mechanisms of iron mobilization by siderophores. Master Studies in Environmental Sciences Master, ETH Zürich
Siddiqui ZA (2005) PGPR: prospective biocontrol agents of plant pathogens. In: PGPR: biocontrol and biofertilization. Springer, Dordrecht, pp 111–142. https://doi.org/10.1007/1-4020-4152-7_4
LeTourneau MK, Marshall MJ, Grant M, Freeze PM, Strawn DG, Lai B, Thomashow LS (2019) Phenazine-1-carboxylic acid-producing bacteria enhance the reactivity of iron minerals in dryland and irrigated wheat rhizospheres. Environ Sci Technol 53(24):14273–14284. https://doi.org/10.1021/acs.est.9b03962
Hossain GM, Ghazali AH, Islam T, Mia MA (2022) Enhanced nutrient accumulation in non-leguminous crop plants by the application of endophytic bacteria Bacillus species. In: Islam MT, Rahman M, Pandey P (eds) Bacilli in agrobiotechnology. Bacilli in climate resilient agriculture and bioprospecting. Springer, Cham, pp 349–364. https://doi.org/10.1007/978-3-030-85465-2_16
Francis JK (2003) Calotropis procera. US Department of Agriculture, Forest Service, International Institute of Tropical Forestry, Puerto Rico
Hassan LM, Galal TM, Farahat EA, El-Midany MM (2015) The biology of Calotropis procera (Aiton) WT. Trees 29:311–320. https://doi.org/10.1007/s00468-015-1158-7
Shokry AM, Al-Karim S, Ramadan A, Gadallah N, Al Attas SG, Sabir JS, Bahieldin A (2014) Detection of a Usp-like gene in Calotropis procera plant from the de novo assembled genome contigs of the high-throughput sequencing dataset. C R Biol 337:86–94. https://doi.org/10.1016/j.crvi.2013.12.008
Joshi S, Singh AV, Prasad B (2018) Enzymatic activity and plant growth promoting potential of endophytic bacteria isolated from Ocimum sanctum and Aloe vera. Int J Curr Microbiol App Sci 7:2314–2326. https://doi.org/10.20546/ijcmas.2018.706.27
Gordon SA, Weber RP (1951) Colorimetric estimation of indoleacetic acid. Plant Physiol 26(1):192
Jasim B, John Jimtha C, Jyothis M, Radhakrishnan EK (2013) Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul 71:1–11. https://doi.org/10.1007/s10725-013-9802-y
Pirhadi M, Enayatizamir N, Motamedi H, Sorkheh K (2018) Impact of soil salinity on diversity and community of sugarcane endophytic plant growth promoting bacteria (Saccharum officinarum L. Var. CP48). Appl Ecol Environ 16:725–739. https://doi.org/10.15666/aeer/1601_725739
Pandey D, Kehri HK, Zoomi I, Chaturvedi S, Chaudhary KL (2022) Seasonal effect on the diversity of soil fungi and screening for arsenic tolerance and their remediation. J Appl Biol Biotechnol 10:4–6. https://doi.org/10.7324/JABB.2022.10s106
Murphy JAMES, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophore. Ann Biochem 160:47–56. https://doi.org/10.1016/0003-2697(87)90612-9
White TJ, Bruns T, Lee SJWT, Taylor JL (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc 18:315–322
Christakis CA, Daskalogiannis G, Chatzaki A, Markakis EA, Mermigka G, Sagia A, Sarris PF (2021) Endophytic bacterial isolates from halophytes demonstrate phytopathogen biocontrol and plant growth promotion under high salinity. Front Microbiol 12:681567. https://doi.org/10.3389/fmicb.2021.681567
Khan AL, Lee IJ (2013) Endophytic Penicillium funiculosum LHL06 secretes gibberellin that reprograms Glycine max L. growth during copper stress. BMC Plant Biol 13:1–14. https://doi.org/10.1186/1471-2229-13-86
Chen C, Xin K, Liu H et al (2017) Pantoea alhagi, a novel endophytic bacterium with ability to improve growth and drought tolerance in wheat. Sci Rep 7:41564. https://doi.org/10.1038/srep41564
Duca D, Lorv J, Patten CL, Rose D, Glick BR (2014) Indole-3-acetic acid in plant–microbe interactions. Antonie Van Leeuwenhoek 106:85–125. https://doi.org/10.1007/s10482-013-0095-y
Kour D, Yadav AN (2022) Bacterial mitigation of drought stress in plants: current perspectives and future challenges. Curr Microbiol 79:1–19. https://doi.org/10.1007/s00284-022-02939-w
Chandra D, Srivastava R, Sharma AK (2018) Influence of IAA and ACC deaminase producing fluorescent pseudomonads in alleviating drought stress in wheat (Triticum aestivum). Agric Res 7:290–299. https://doi.org/10.1007/s40003-018-0305-y
Fouda A, Eid AM, Elsaied A, El-Belely EF, Barghoth MG, Azab E, Hassan SED (2021) Plant growth-promoting endophytic bacterial community inhabiting the leaves of Pulicaria incisa (Lam.) DC inherent to arid regions. Plants 10(1):76. https://doi.org/10.3390/plants10010076
Jilani G, Zhang D, Chaudhry AN, Iqbal Z, Ikram M, Bashir M (2021) Role of phosphate-solubilising microorganisms in agricultural development. In: Mohamed HI, El-Beltagi HEDS, Abd-Elsalam KA (eds) Plant growth-promoting microbes for sustainable biotic and abiotic stress management. Springer, Cham. https://doi.org/10.1007/978-3-030-66587-6_17
Suebrasri T, Harada H, Jogloy S, Ekprasert J, Boonlue S (2020) Auxin-producing fungal endophytes promote growth of sunchoke. Rhizosphere 16:100271. https://doi.org/10.1016/j.rhisph.2020.100271
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:1–13. https://doi.org/10.1186/1746-1448-4-2
Ali B, Wang X, Saleem MH, Sumaira Hafeez A, Afridi MS, Khan S, Zaib-Un-Nisa Ullah I, Amaral Júnior ATd, Alatawi A, Ali S (2022) PGPR-mediated salt tolerance in maize by modulating plant physiology, antioxidant defense, compatible solutes accumulation and bio-surfactant producing genes. Plants 11:345. https://doi.org/10.3390/plants11030345
Agha MS, Abbas MA, Sofy MR, Haroun SA, Mowafy AM (2021) Dual inoculation of Bradyrhizobium and Enterobacter alleviates the adverse effect of salinity on Glycine max seedling. Notulae Botanicae. Hortic Agrobotanici Cluj-Napoca 49(3):12461. https://doi.org/10.15835/nbha49312461
Dhakar K, Sharma A, Pandey A (2014) Cold, pH and salt tolerant Penicillium spp. inhabit the high-altitude soils in Himalaya, India. World J Microbiol Biotechnol 30:1315–1324. https://doi.org/10.1007/s11274-013-1545-4
Hamayun M, Khan N, Khan MN, Qadir M, Hussain A, Iqbal A, Lee IJ (2021) Antimicrobial and plant growth-promoting activities of bacterial endophytes isolated from Calotropis procera (Ait.) WT Aiton. Biocell 45(2):363. https://doi.org/10.32604/biocell.2021.013907
Ullah I, Al-Ghamdi KM, Anwar Y, Mahyoub A, J, (2020) Exploring the Insect Control and Plant Growth Promotion Potentials of Endophytes Isolated From Calotropis procera Present in Jeddah KSA. Nat Prod Commun 15:3
Khan SA, Humayun M, Yoon H et al (2008) Plant growth promotion and Penicillium citrinum. BMC Microbiol 8:231. https://doi.org/10.1186/1471-2180-8-231
Kedar A, Rathod D, Yadav A, Agarka G, Rai M (2014) Endophytic Phoma sp. isolated from medicinal plants promote the growth of Zea mays. Nusantara Biosci 6:2. https://doi.org/10.13057/nusbiosci/n060205
Funding
Sonali Jaiswal has received Junior Research Fellowship from University Grants Commission (UGC) India [UGC Reference no: 960/ (CSIR-UGC NET JUNE 2017)] to carry out the present research work.
Author information
Authors and Affiliations
Contributions
SJ made substantial contributions to the conception or design of the work, made the analysis, and interpretation of data in the work. AO has drafted the work or revised it critically for important intellectual content. SKM has approved the version to be published, agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved, made proofreading of manuscript, and gave suggestions to improve it. All authors read and approved the final manuscript. This is a part of Sonali Jaiswal’s Ph.D. program.
Corresponding author
Ethics declarations
Conflict of interest
There was no conflict of interest among the authors.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jaiswal, S., Ojha, A. & Mishra, S.K. Assessment of Plant Growth-Promoting Parameters of Endophytes Isolated from Calotropis procera and Their Performance Under Irrigated and Non-irrigated Conditions. Curr Microbiol 81, 49 (2024). https://doi.org/10.1007/s00284-023-03570-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03570-z