Abstract
This study investigates the role of bacterial endophytes from extreme alkaline environments in alleviating alkaline stress and plant development. Stressful environmental factors, such as soil acidity and alkalinity/sodicity, frequently affect plant development. In the present study, alkaline-tolerant endophytic strains were isolated from three plant species Saccharum munja, Calotropis procera, and Chenopodium album, and 15 out of the total of 48 isolates were selected for further examination of their abiotic stress tolerance. Molecular analysis based on 16S rRNA gene sequencing revealed strains from Enterobacter, Acinetobacter, Stenotrophomonas, Bacillus, Lysinibacillus, and Mammaliicoccus genera. Out of 15 isolates based on their quantitative PGP traits and abiotic stress tolerance, 6 were finally selected for greenhouse experiments. Under alkaline conditions, results demonstrated that the strains from the genera Enterobacter, Bacillus, Stenotrophomonas, and Lysinibacillus had beneficial effects on maize growth. These findings suggest that using a combination of bacteria with multiple plant growth-promoting attributes could be a sustainable approach to enhance agricultural yield, even in a challenging alkaline environment. The study concludes that the application of bacterial endophytes from plants growing in extremely alkaline environments might provide other plants with similar stress-tolerance abilities. The outcome of the study provides a basis for future exploration of the mechanisms underlying endophyte-induced stress tolerance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agriculture faces a substantial challenge in meeting the increasing demand for food from a growing population, exacerbated by a decline in crop production resulting from a range of environmental stressors [1]. Numerous biotic and abiotic stresses have detrimental impacts on plant growth, yield, and food quality [2]. Biotic stressors encompass a variety of pathogenic infections, whereas abiotic stressors include damage from factors such as salinity, sodicity, temperature fluctuations, drought, and exposure to metals [3]. The outlook for global land degradation issues including the escalation of soil salinity and sodicity is expected to deteriorate further in the coming years, and therefore, degradation of land due to salinization/sodication is considered to be one of the most pressing environmental hazards [4, 5]. This soil salinity and sodicity will be intensified due to the increasing scarcity of good-quality irrigation water [6, 7].
Sodic soils are extensively distributed and exhibit degradation at various structural, nutritional, chemical, and microbial levels. These soils are characterized by low hydraulic conductivity and weak aggregate stability. Sodic soils are characterized by high alkaline pH (i.e., > pH8) with a high level of exchangeable sodium [4]. In excess, any form of salt is harmful to plant health and adversely affects plant growth and microbiological processes of soil [8]. Plants exposed to high pH levels experience a range of morphological, physiological, and biochemical alterations, which can result in significant modifications to their growth and development [9]. This also adversely affects soil processes like nutrient levels decomposition, nitrification, denitrification, microbial activity, and diversity [10]. In addition to this, soil alkalinity also causes damage to the root system, changed nutrient absorption, disrupted ionic balance, relative water content, photosynthetic pigments, total soluble sugar, etc. which finally causes plant mortality [11]. Soil alkalinity has a notable effect on organic matter, nitrogen, carbon, and microbial biomass. Physical and chemical methods for pH control are costly, time-consuming, and ineffective for high-pH soil, making soil alkalization a significant issue for agricultural pursuits [11].
The utilization of beneficial microbes to enhance crop productivity under stressed environmental conditions is an eco-friendly approach and is now considered a necessary aspect of agriculture to meet the growing demand for food [12, 13]. Over the past few years, substantial research work has been carried out on plant-growth-promoting bacteria (PGPB) for not only enhancing plant growth but also mitigating abiotic and biotic stresses [14]. The partnership between plants and these endophytes results in a variety of physiological and biochemical changes that produce advantageous effects on the plants, such as growth enhancer, phosphate solubilization, nitrogen fixation, and resistance to pathogen infection [15, 16]. Therefore, these endophytes could play a significant role in improving crop production and nutritional value. Aside from their overall ability to promote plant growth, alkalinity-tolerant bacteria can also stimulate the production of indole acetic acid (IAA) and 1-aminocyclopropane-1-carboxylate (ACC) deaminase, as well as facilitate phosphate solubilization in plants [17, 18]. Some PGP bacteria also possess more specific traits like heavy metal detoxifying activity, salinity tolerance, and biological control of phytopathogens [19]. Previous reports suggest the role of plant-endophytic bacteria like Pseudomonas, and Bacillus can increase plant growth and confer alkaline stress amelioration [18, 20]. Thus, the present study was conducted to isolate and screen the endophytic isolates from the native plant species grown in an alkaline-stressed environment and characterize them for their potential as an endophyte-based bioformulation for enhancing plant growth under alkaline stress conditions.
Materials and Methods
Collection of Plant Samples from Sodic Soil
Three different plant samples: Saccharum munja, Calotropis procera, and Chenopodium album were collected from sodic soil of varying pH (8–10); EC (390-400 µS cm−1) from different locations situated in and around Banthara region of Uttar Pradesh. Briefly, the pH of the soil (diluted water 1:5) was measured using a digital pH meter (7310P, WTW, Germany) [21]. S. munja was collected from CSIR-NBRI Aurawan campus (26.7098°N, 80.8226°E) whereas C. procera and C. album were collected from CSIR-NBRI Gehru campus (26.7276°N, 80.8477°E). In field conditions, the plant samples were collected along with rhizospheric soil in sterile poly bags and placed immediately on ice. In the laboratory, these samples were stored at 4 °C until further use.
Isolation and Maintenance of Endophytic Bacteria
The healthy stems, roots, and leaves of the collected plants were surface sterilized in order to isolate the endophytic bacteria. Briefly, the plant tissues were thoroughly washed with tap water for 15 min followed by three subsequent washes with sterile-distilled water and then two times washed with 70% ethanol (v/v) for 2 min and then finally sterilized with 0.1% mercuric chloride (w/v) for 3 min. Samples were then finally washed thrice using sterile-distilled water. After surface sterilization, small pieces of the plant tissues (1–3 cm) were crushed in a sterile mortar and pestle and were then mixed with sterile saline water (0.85% NaCl). They were then serially diluted up to 10–4 dilutions and then plated on nutrient agar, yeast mannitol agar, Pseudomonas isolation agar, and Hi chrome ECC (Escherichia coli and coliforms) agar plates. Further, the plates were incubated at 28 °C for 24–48 h. The plates were then observed for morphologically different colonies and further subculture onto fresh nutrient agar plates to obtain pure cultures. The glycerol stocks of pure bacterial cultures were preserved at − 80 °C for long-term storage [22, 23].
Qualitative Screening of Endophytic Bacteria for PGP Attributes and Enzymatic Activity
The ACC deaminase activity of the bacterial isolates was investigated following the procedure of Misra et al. [24], by using NFb (nitrogen-free basal media) plates with 3 mM ACC added as a nitrogen source. Estimation of IAA was carried out by the method that has been described in Bric et al. [25]. The National Botanical Research Institute's phosphate (NBRIP) medium which contains bromophenol blue dye (BPB) and tri-calcium phosphate (TCP) was used to screen the phosphate-solubilizing ability of bacterial isolates [26].
Quantitative Estimation of PGP Attributes of Endophytic Bacteria
The endophytic bacterial strains demonstrating the mentioned qualitative attributes were chosen for further quantitative evaluation including the determination of ACC deaminase activity using the method outlined by Penrose and Glick [27]. The capacity to utilize ACC (3 mM) as the only nitrogen source was tested in all the selected isolates using an M-9 minimum medium. The amount of α-ketobutyrate mg−1 protein h−1 produced as a by-product of the spectrophotometric (EVOLUTION201, Thermo Scientific, USA) reaction at 540 nm was also measured to quantify the activity of the ACC deaminase. The quantitative measurement of IAA production by the screened endophytic isolates was carried out using a tryptophan-enhanced nutrient broth medium (100 µg/mL) as mentioned in [25]. IAA was measured calorimetrically at 530 nm following the addition of orthophosphoric acid (10 mM) and Salkowaski's reagent (1 mL of 0.5 M FeCl3 in 50 mL of 35% HClO4) to the supernatant. Phosphate solubilization was measured for the selected isolates according to Nautiyal (1999) [28]. Briefly, 50 µL of bacterial culture was inoculated in test tubes with 5 mL NBRI-BPB medium and then incubated for 48 h at 28 ± 2 °C on an incubator shaker (180 rpm). Finally, the cells were centrifuged at 10,000 rpm for 10 min to extract the supernatant, which was used to determine the quantity of solubilized phosphate at 490 nm. EPS production was quantified using a slightly modified phenol–sulfuric acid technique [29]. In brief, 10 mL of the overnight grown culture of bacterial isolates was used to measure the EPS generated at 490 nm using an equal amount of 0.5 M phenol and 1 mL of 9.8 M sulphuric acid.
Abiotic Stress-Tolerance Estimation
The bacterial isolates were subjected to evaluate their abiotic stress tolerance including salt (NaCl; 1 M and 2 M), drought (PEG6000; 45% and 60%), and pH (5 and 9). The nutrient broth medium with variable conditions of stress was used to cultivate the bacterial isolates for 24 h at 30 °C with constant shaking at 180 rpm. Serial dilution plating on nutrient agar medium at regular intervals of 24 h for up to 10 days was carried out and viable cells (CFU/mL) were counted as described elsewhere [24].
Molecular Characterization of Endophytic Bacteria
Based on the 16S rRNA gene, the chosen bacterial isolates were identified. As directed by the GeneEluteTM Bacterial Genomic DNA Kit, genomic DNA was isolated. In a PCR reaction mixture consisting of 1X PCR buffer, 1.0 U Taq DNA polymerase (Thermo Scientific, USA), 200 mM of each dNTP, and 10 M of each forward and reverse primer (27F: 5′-AGAGTTTGATCCTGGCTCAG-3′ and 1492R: 5′-GGTTACCTTGTTACGACTT-3′), 20 ng of bacterial genomic DNA was amplified in a total volume of 50 µL. Molecular-grade water was used as a negative control. Thermal cycling for amplification was carried out using the GENEI-TC3000 thermocycler (GENEI, India) with the following parameters: an initial denaturation step at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and elongation at 72 °C for 60 s, with a final elongation step at 72 °C for 10 min. The resulting amplicons were size separated on 1% agarose gel and documented using the gel documentation system (Universal Hood III, Bio-Rad, USA) to examine their integrity against a 1 kb DNA ladder (Thermo Scientific, USA). PCR amplicons were purified using a QIAquick® PCR purification kit QIAGEN, Germany). The purity and concentration of the DNA were determined through 260/280 nm absorbance measures using the NanoDrop spectrophotometer 2000 (Thermo Scientific) which was found to be in the range of 1.80–2.0 ng/µL of DNA. Further purified DNA was sequenced from Applied Biosystems 3730XL DNA analyzer (Thermo Scientific, USA). The 16S rRNA gene sequences of multiple bacterial strains were aligned with sequences in the NCBI databases using ClustalW from MEGA6 software [30], and after alignment, a nucleotide substitution model selection study was conducted to determine the optimal model fit based on the lowest Akaike Information Criterion (AIC). The resulting model was used for maximum likelihood-based phylogenetic tree construction of the bacterial isolates, with the bootstrap approach chosen as the phylogenetic test and 1000 bootstrap repetitions.
Greenhouse Evaluation of Alkalotolerant Endophytes for Plant Physiology and Growth Enhancement
Selected strains of bacteria were tested for PGP capability in alkaline soil utilizing maize (Zea mays) as a model plant under greenhouse conditions with a maximum light flux of ~ 1000 μmol m−2 s−1 photosynthetic photon flux density (PPFD) during the noon, relative humidity (RH) varied between 40 and 60%, and temperature ranged from 25 to 35 °C and photoperiod of 12–14 h light and 8–10 h dark. Experiments were conducted using sterilized 2-mm sieved soils in a randomized block design with six duplicates (2.5 kg soil per pot). In summary, control soil (pH 7.4) was obtained from the CSIR-NBRI in Lucknow, whereas alkaline soil (pH 9.2) was obtained from the arable land of the CSIR-NBRI, Gheru campus, Lucknow. Each bacterial cell suspension with 108 CFU/mL was applied to surface sterilized seeds until evenly coated, whereas uninoculated control seeds were treated with water [31].
Growth Parameters and Photosynthetic Pigment Analyses
Shoot and root length were assessed right away after harvesting to determine the impact of endophytic PGP priming in sodic soil as well as normal soil, whereas samples for the dry weight (DW) were dried in a hot air oven for 72 h before being weighed. Using a UV–vis spectrophotometer (Thermo scientific evolution 201), the photosynthetic pigments (Chl a, Chl b, and carotenoids) in the leaf of plants grown in normal soil and alkaline soil were measured at wavelengths of 480, 510, 645, and 663 nm [32].
Proline and Total Soluble Sugar (TSS) Content, and Antioxidant Enzyme Activities
Proline was measured spectrophotometrically at 520 nm [33, 34], whereas total soluble sugar (TSS) was measured at 490 nm in a spectrophotometer as suggested [35]. In order to evaluate enzymatic antioxidants spectrophotometrically, a 500 mg fresh tissue sample was homogenized in extraction buffer comprising 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, and 1% (w/v) polyvinyl pyrrolidone (PVP) at temperature 4 °C. The activity of Superoxide Dismutase (SOD, EC1.15.1.1), Catalase (CAT, EC1.11.1.6), Ascorbate Peroxidase (APX, EC1.11.1.11), and guaiacol peroxidase (GPX, EC 1.11.1.9) was determined using the methods described earlier [36,37,38] respectively.
Statistical Analysis
The significance between mean values of different treatments was checked by one-way analysis of variance (ANOVA), and comparison was carried out using the Duncan Test at p ≤ 0.05. with SPSS software package version 16.0 (SPSS Inc./IBM Corp. Chicago, USA). All the results were represented graphically using Sigma Plot version 11.
Nucleotide Sequences Accession Number
The 16S rRNA gene sequences of the 15 bacterial endophytes obtained in this study have been deposited in the GenBank under the accession numbers (ON926874–ON926888) (Table S1).
Results
Endophytic Isolates
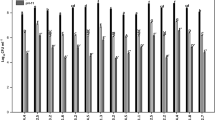
A total of 48 bacterial isolates were obtained from S. munja (roots 6; leaves 2), C. procera (roots 6; leaves 4), and C. album (roots 25; leaves 5). These isolates were evaluated for their PGP attributes such as P solubilization, ACC deaminase activity, IAA production, and enzymatic activities including chitin hydrolysis, amylase, cellulolytic production, and abiotic stress tolerance including salt (NaCl), drought (PEG6000), and alkalinity (pH) (Fig. 1). Based on initial qualitative screening results, a total of 15 isolates (10 from Chenopodium album and 5 from Calotropis procera) were selected based on their growth-promoting attributes, enzyme activities, and ability to sustain on aforementioned abiotic stresses for further characterization (Tables 1 and 2).
Plant growth-promoting traits and abiotic stress tolerance of bacterial endophytes isolated from the roots and leaves of Saccharum munja, Calotropis procera, and Chenopodium album. Results demonstrated based on the qualitative screening of bacterial endophytes for PGP attributes and abiotic stress tolerance on Y-axis, and the % of endophytes on X-axis
Molecular Characterization of the Selected Endophytic Bacteria by 16 s rRNA Sequencing
Partial 16S rRNA gene sequences of ~ 1400 nucleotides of the 15 selected isolates were compared to the type strains in the NCBI database. These 15 isolates belong to Enterobacter, Acinetobacter, Stenotrophomonas, Bacillus, Lysinibacillus, and Mammaliicoccus genera. These six bacterial genera were further classified at the species level as Enterobacter hormaechei, E. cancerogenus, Acinetobacter haemolyticus, Stenotrophomonas pavanii, Bacillus safensis, Mammalicoccus sciuri, and Lysinibacillus fusiformis based on phylogenetic analysis (Fig. 2). The 16S rRNA gene sequences were submitted to GenBank under the accession number ON926874–ON926888 (Table S1). The bacterial strains with their nearest neighbor from the NCBI database are mentioned in (Table S1).
Phylogenetic relationships of the 15 selected bacterial endophytes based on the partial 16S rRNA gene sequences. The phylogenetic analysis was run on MEGAX v. 10.1.7 using the Neighbor-Joining method and K2 + G substitution model. The sequences in bold are obtained in this study and other sequences are from the GenBank database. The numbers above each node on the tree indicate the percentages of bootstrap sampling derived from 1000 replications
Quantification of PGP Attributes
The selected 15 bacterial isolates were quantitatively characterized for PGP attributes including ACC deaminase activity, IAA production, phosphate solubilization, and EPS production (Table 1). It was observed that ACC deaminase activity ranged from 0.97 to 20.21 nmol α-ketobutyrate mg protein−1 h−1 among the selected isolates. The strain NBRI CRNA12 exhibited the highest (20.21 nmol α-ketobutyrate mg protein−1 h−1) and NBRI CRNA7 lowest (0.97 α-ketobutyrate mg protein−1 h−1) ACC deaminase activity. The IAA production ability ranged from 46.69 to 134.77 µg/mL. The highest IAA production ability was shown by NBRI CRYMA1 (134.77 µg/mL), while the lowest by NBRI CRYMA5.2 (46.69 µg/mL). The phosphate solubilization potential of the selected isolates was in the range of 33.09 to 94.14 µg/mL. The NBRI CRYMA1 strain (94.14 µg/mL) exhibited the highest and NBRI WCYMA11 (33.09 µg/mL) lowest phosphate-solubilizing potential. The EPS production ranged from 1230.45 to 380.90 µg/mL among all the selected isolates, of which NBRI CRNA6, demonstrated the highest EPS production ability with 1230.45 µg/mL while NBRI CRNA2 with minimum production potential of 380.90 µg/mL.
Abiotic Stress-Tolerance Ability of Isolates
The selected alkalotolerant endophytic strains were further subjected to evaluate abiotic stress attributes including alkalinity, drought, and pH tolerance under in vitro conditions. At 1 M NaCl, all 15 strains were able to show survival till day 10 with the most distinct at day 3 with a range of 6.98 to 9.60 Log10 CFU/mL. Whereas, when the concentration increased to 2 M NaCl, only six strains namely NBRI CRNA2, NBRI CRNA3, NBRI CRNA11, NBRI CRNA12, NBRI CRYMA1, and NBRI WCYMA9 strains exhibited luxuriant growth with a range of 6.97 to 9.26 Log10 CFU/mL. All 15 strains were able to survive at 45% and 60% PEG6000 for drought conditions with a range of 6.97–7.47 and 6.04–7.53 Log10 CFU/mL, respectively. Moreover, at pH 9 and 11, all 15 strains demonstrated their survival with 8.55–8.90 Log10 CFU/mL and 7.80–9.47 Log10 CFU/mL, respectively.
Effect of Endophyte Inoculation on Zea mays Growth in Soils with and Without Alkalinity
The 6 bacterial isolates, NBRI CRNA2 (B. safensis), NBRI CRNA3 (E. cancerogenus), NBRI CRNA11 (L. fusiformis), NBRI CRNA12 (E. hormaechei), NBRI CRYMA1 (S. pavanii), and NBRI WCYMA9 (A. haemolyticus) were chosen out of the 15 selected strains for further analysis due to their ability to withstand the assessed abiotic stresses. In greenhouse conditions, it was observed that either in normal or alkaline circumstances, the individual inoculation of selected isolates considerably improves overall Z. mays growth, as shown by the phenotypic characteristics as compared to the corresponding uninoculated control sets (Table 3; Figs. 3, 4). Inoculation of the endophytic strain led to a significant increment in root and shoot length under alkaline conditions while an increment in the fresh and dry weight was observed in normal soil conditions. The NBRI CRNA11, NBRI CRNA12, NBRI CRYMA1, and NBRI WCYMA9 strains significantly increased the root length by 76.43, 61.06, 70.16, and 51.96%, respectively under alkaline conditions (Table 3, Fig. 4). In contrast to this, NBRI CRNA2, NBRI CRNA11, and NBRI CRNA12 strains increased only shoot length by 50, 57.47, and 56.44%, respectively, under alkaline conditions (Table 3, Fig. 4). The application of NBRI CRNA3, NBRI CRNA12, and NBRI WCYMA9 strains, however, enhanced the fresh weight of maize plants by 52.60, 50.45, and 54.07%, respectively, as compared to the uninoculated control under normal conditions. While NBRI CRNA2, NBRI CRNA3, NBRI CRYMA1, and NBRI WCYMA9 enhanced the dry weight of maize plants by 66.66, 69.58, 68.61 and 78.92%, respectively, as compared to the uninoculated control under normal conditions.
Effect of six different bacterial endophytic isolates on root and shoot length of Zea mays in normal soil under greenhouse conditions. Seeds were coated with the bacterial cell suspension (108 CFU/mL) whereas the control seeds were treated with sterilized water. Results depicted higher growth of root and shoot in the endophytes-treated seeds compared to control seeds
Effect of endophytic isolates on the root and shoot length of Zea mays in alkaline soil under greenhouse conditions. Test seeds were coated with the bacterial cell suspension (108 CFU/mL) whereas the control seeds were treated with sterilized water. Results showed higher growth of root and shoot in the endophytes-treated seeds compared to the control seeds
Effect of Endophytic Strain on Total Chlorophyll, Carotenoid, Proline, and Soluble Sugar Content of Zea mays
Through the use of biochemical assays measuring chlorophyll (total), carotenoid, and soluble sugar levels, the non-enzymatic traits of the maize crop under both normal and alkaline stress conditions were evaluated by inoculation of all six bacterial endophytes following the implementation of the PGP treatment. Inoculation of endophytes accounted for significant increase in the total chlorophyll, carotenoid, and soluble sugar whereas a notable decrease in proline content was observed in alkaline soil compared to that of the uninoculated control soil (Table 3). Of the six bacterial isolates, NBRI CRNA2 (Bacillus safensis) showed the highest capacity to increase the amount of soluble sugar and overall chlorophyll in the control soil by 64.35% and 23.06%, respectively. However, NBRI WCYMA9 (Acinetobacter haemolyticus) improved the carotenoid by 37.2%, and NBRI CRYMA1 (Stenotrophomonas pavanii) and NBRI WCYMA9 (Acinetobacter haemolyticus) demonstrated a decrease in proline content by 52.0% under alkaline conditions. Interestingly, the findings also revealed a decline in total chlorophyll accumulation in plants treated with NBRI CRNA11 (Lysinibacillus fusiformis) and NBRI CRNA12 (Enterobacter hormaechei) by approximately 4% under alkaline conditions (Table 3).
Effect of Endophytes on Defense-Related Enzymes in Zea mays
The defense enzymes SOD, GPX, APX, and CAT were assayed to analyze the quenching of accumulated stress in the form of H2O2 and other oxidative stress in Zea mays plants grown in alkaline soil with and without the treatment of 6 selected endophytic strains (Fig. 5). In general, treatments significantly lowered the activity of these defense enzymes under alkaline stress as expected due to the presence of endophytic strains. However, in the case of CAT, an abnormally increased activity was observed in the strains NBRI CRYMA1 and NBRI WCYMA9. Under the alkaline stress, NBRI CRYMA1 and NBRI CRNA2 exhibited a maximum reduction of SOD by 38.64% and 24.77% in the leaves and roots, respectively. The reduction in CAT activity was best seen in the leaf samples inoculated with NBRI CRNA2 and in the roots of the plants inoculated with NBRI CRNA12 isolates.
Defense enzyme activities in maize under normal and alkaline conditions treated with endophytic isolates. SOD enzyme activity in a leaf and b root; Guaiacol peroxidase enzyme activity in c leaf and d root and Catalase enzyme activity in e leaf and f root. The values shown are the mean of three replicates in leaf and root samples. Errors bars represent standard mean errors. Different letters above the bars represent significant differences according to the ANOVA followed by the Duncan test (p ≤ 0.05) applied using the software SPSS ver. 16.0
Discussion
The growing human population necessitates higher crop yields; however, agricultural practices are hampered by abiotic stresses, i.e., acidity, alkalinity, drought, and temperature. In order to withstand the detrimental impacts of these abiotic stresses, crop plants must develop strategies for tolerating the stress. The utilization of microbial inoculants, isolated from the soil/rhizosphere/endosphere, in agricultural lands is well documented for their ability to promote plant growth and induce stress tolerance, can increase crop yields, and is well demonstrated for enhancing crop yields and protecting against pathogens [39]. It is also acknowledged that some microorganisms have this innate capacity to withstand various alkalinity levels, stimulate plant development, and regulate the plant defense system by exhibiting ACC deaminase activity, activating the plant’s anti-oxidative enzymes [40]. Reports exist on the isolation and characterization of endophytes that stimulate plant growth, but there are limited studies available that focus solely on characterizing bacterial strains from alkaline-stressed environments [10]. The present study deals with the isolation, screening, and characterization of plant-associated alkalinity-tolerant endophytic bacterial strains from three plant species, S. munja, C. procera, and C. album growing in sodic soil of varying pH. This study examined and confirmed six bacterial strains for their various plant growth-promoting attributes on maize plants under normal and alkaline conditions.
Among the selected PGP attributes, some but not all of the endophytes showed the ability to produce 1-aminocyclopropane1-carboxylate (ACC) deaminase that can be used to reduce stress due to ethylene accumulation in plants [41]. In this study, Enterobacter hormaechei NBRI CRNA12 (ON926881), showcased the best ACC deaminase activity among all other endophytic isolates. Previous reports also exemplified Enterobacter sp. as the potential alternative for promoting plant growth and development under alkaline soil [42]. Many Enterobacter sp. adapt to abiotic stress and produce a wide variety of plant growth metabolites such as exopolysaccharides, phytohormones, and stress-tolerant enzymes, results of the present study corroborate well with the findings of these previous studies [43, 44].
IAA production and P solubilization have been proposed as significant means of promoting early growth in many crops [45]. There have been several studies proving the production of IAA by bacteria isolated from respective crops [46,47,48]. In this study, among all, Stenotrophomonas pavanii NBRI CRYMA1 (ON926882) produced the highest IAA in maize. Researchers are developing techniques that use phosphate-solubilizing microorganisms to solubilize insoluble phosphates. Endophytic bacterial strains also solubilize phosphates and other nutrients to increase the availability of phosphorus for plants in soil with large amounts of precipitated phosphates [49]. The process of chemically processing insoluble phosphates is not only costly but also not environmentally friendly. During this study, some bacterial isolates were capable of solubilizing phosphate and the most efficient phosphate-solubilizing strain found belonged to the genera Stenotrophomonas which is in accordance with the earlier findings [10]. IAA production and P solubilization by bacterial isolates suggest that they have the potential to be employed as growth regulators. One of the other important PGP attributes is the production of exopolysaccharides (EPS) by endophytic microbes. As per previous reports, EPS production in some strains of Bacillus, Enterobacter, Pseudomonas, and Streptococcus has been observed [50]. By controlling the amount of nutrients and water that reach the roots and assisting in the permanent adherence of microorganisms to the root surfaces, EPS is a key factor in shaping the soil structure. In this study, it was observed that Enterobacter cancerogenus NBRI CRNA6, (ON926878), has ability to produce EPS in maize plants both in the control and alkaline soil.
In this study, the 15 alkalotolerant endophytic strains were investigated for their abiotic stress-tolerance abilities under in vitro conditions. Plant growth-promoting bacteria are well known for their ability to reduce the harmful effects of environmental stresses. The results depicted that all 15 isolates were able to grow in high pH (9 and 11), drought (45% and 60%), and salt 1 M, except on a 2 M salt concentration. Out of 15 isolates, six isolates namely Bacillus safensis NBRI CRNA2 (ON926874), Enterobacter cancerogenus NBRI CRNA3 (ON926875), Lysinibacillus fusiformis NBRI CRNA11 (ON926880), Enterobacter hormaechei NBRI CRNA12 (ON926881), Stenotrophomonas pavanii NBRI CRYMA1 (ON926882)] and Acinetobacter haemolyticus NBRI WCYMA9 (ON926887) were able to grow in all the above-mentioned abiotic stress conditions. Consequently, according to these findings, the isolates have the potential to evolve into a bioinoculant package for enhancing plant development because of their strong tolerance to high salt, drought, and alkalinity. One of the main reasons that restricting the occurrence of microorganisms in soil might be extreme pH, dryness, and salt conditions. This suggests that the chosen isolates would make excellent candidates for enhancing plant development in highly alkaline soil. The length of the shoots and roots, as well as the fresh and dry weight of the maize plant, were all considerably increased by all six bacterial isolates. The synthesis of plant growth hormones, nitrogen fixation, and P solubilization are just a few of the advantageous actions that applied bacterial isolates can carry out that may enhance plant growth.
The total chlorophyll, carotenoids, soluble sugar, amino acid, and proline contents are suitable indicators for plant health. These parameters are also known to be associated with the plant responses to abiotic stress, like alkalinity. The reduced levels of total chlorophyll, carotenoids, and sugar occur mostly because of the chloroplast impairments under alkaline stress [3, 10, 24, 50]. In the present study, the increased levels of total chlorophyll, carotenoids, and soluble sugar were recorded on the application of six selected endophytic isolates whereas the proline content did demonstrate a significant decrease under alkaline stress conditions in comparison to uninoculated control. Among the six selected alkali-tolerant plant growth-promoting endophytic isolates, Bacillus safensis NBRI CRNA2 (ON926874) significantly increased the photosynthetic pigment, soluble sugar whereas endophytic isolate Stenotrophomonas pavanii NBRI CRYMA1 (ON926882) and Acinetobacter haemolyticus NBRI WCYMA9 (ON926887) were able to decrease the proline content under high pH, drought, and alkalinity stress conditions. The findings that PGP-mediates decreased proline accumulation in treated plants under certain abiotic stress agree with various other research outputs [10, 18, 50]. Excess Na+ infusion into roots is constrained and rendered unavailable to plants in saline and alkaline circumstances by the exopolysaccharides (EPS) secreted by the endophytic PGP, which may also stimulate the development of biofilm on plant root surfaces. This can be one of the possible explanations for this kind of result. A slightly different observation was made regarding proline accumulation in the presence of endophytic PGP, it was seen that in the normal conditions with endophytic PGP inoculation, proline accumulation was slightly higher with respect to the control plant, but this observation has also been previously monitored in other similar publications maize [18, 48].
Plants employ antioxidant defense mechanisms to combat diverse abiotic stress, which limit oxidative damage caused by different abiotic stress and prevent ROS formation [24, 32, 34]. Superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GR) are the major enzymatic components that scavenge ROS. The current results exhibited that during alkaline stress, the number of anti-oxidative enzymes in bacteria-inoculated maize plants was dramatically reduced. It is speculated that bacterial application could compensate for the high-pH effects and improve water status in plants. Reduced antioxidant enzyme activity SOD offered protection against alkaline stress in maize plants inoculated with Stenotrophomonas pavanii NBRI CRYMA1 (ON926882) and Bacillus safensis NBRI CRNA2 (ON926874) isolates. In maize plants inoculated with Enterobacter, decreased activity of the CAT and GPX enzymes has also been reported, giving plants the ability to endure stress [10, 49, 50].
The present findings demonstrate that inoculation of the screened bacterial endophytes enhanced the non-enzymatic properties (chlorophyll, carotenoid, proline, and sugar) under alkaline stress conditions. The results corroborate with earlier studies confirming the presence of plant growth-promoting endophytes in different host crops. Exploiting plant–microbe interaction can be the best approach to increase food production in the current scenario of climate change. More research is required to develop efficient microbial formulations for improving plant performance under various abiotic stresses. The research should focus on the isolation of indigenous plant-growth-promoting bacteria from the stress-affected soils that could be used as biostimulants.
Conclusion
The presented research work demonstrated the role of endophytic isolates from the native plant species growing under highly alkaline soil. The putative alkalotolerant endophytic isolates were characterized for multiple plant growth-promoting attributes under normal and alkaline stress conditions. The endophytic bacterial isolates belong to Enterobacter, Bacillus, Stenotrophomonas, and Lysinibacillus genera and demonstrated phyto-beneficial effects on maize in terms of enhanced, photosynthetic pigments, and vegetative growth in normal and alkaline soil. Alkaline stress-ameliorating abilities of endophytic strains, Bacillus safensis NBRI CRNA2, Lysinibacillus fusiformis NBRI CRNA11, Stenotrophomonas pavanii NBRI CRYMA1, and Acinetobacter haemolyticus NBRI WCYMA9 were further evident by modulating the biochemical parameters and antioxidant enzyme activities. Based on the results and to complement the PGP attributes, consortia of selected endophytic bacterial strains can be developed in the future as a sustainable way to increase agricultural yield in challenging situations. These promising initial results of plant tests can be further exploited to understand the underlying mechanism of endophytes-elicited tolerance that could be further used as stress-buster biostimulants.
Data Availability
Data will be available on request.
Code Availability
Not applicable.
References
Kopittke PM, Menzies NW, Wang P, McKenna BA, Lombi E (2019) Soil and the intensification of agriculture for global food security. Environ Int 132:105078. https://doi.org/10.1016/j.envint.2019.105078
Khoshru B, Mitra D, Khoshmanzar E, Myo EM, Uniyal N, Mahakur B, Mohapatra PKD, Panneerselvam P, Boutaj H, Alizadeh M, Cely MVT, Senapati A, Rani A (2020) Current scenario and future prospects of plant growth-promoting rhizobacteria: an economic valuable resource for the agriculture revival under stressful conditions. J Plant Nutr 43(20):3062–3092. https://doi.org/10.1080/01904167.2020.1799004
Misra S, Dixit VK, Mishra SK, Chauhan PS (2019) Demonstrating the potential of abiotic stress-tolerant Jeotgalicoccus huakuii NBRI 13E for plant growth promotion and salt stress amelioration. Ann Microbiol 69(4):419–434. https://doi.org/10.1007/s13213-018-1428-x
Singh K (2016) Microbial and enzyme activities of saline and sodic soils. Land Deg Dev 27(3):706–718. https://doi.org/10.1002/ldr.2385
Dahlawi S, Naeem A, Rengel Z, Naidu R (2018) Biochar application for the remediation of salt-affected soils: challenges and opportunities. Sci Total Environ 625:320–335. https://doi.org/10.1016/j.scitotenv.2017.12.257
Wong VN, Dalal RC, Greene RS (2008) Salinity and sodicity effects on respiration and microbial biomass of soil. Biol Fert Soils 44(7):943–953. https://doi.org/10.1007/s00374-008-0279-1
Wong VN, Greene RSB, Dalal RC, Murphy BW (2010) Soil carbon dynamics in saline and sodic soils: a review. Soil Use Man 26(1):2–11. https://doi.org/10.1111/j.1475-2743.2009.00251.x
Hassan TU, Bano A, Naz I (2017) Alleviation of heavy metals toxicity by the application of plant growth promoting rhizobacteria and effects on wheat grown in saline sodic field. Int J Phytoremed 19(6):522–529. https://doi.org/10.1080/15226514.2016.1267696
Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S (2020) Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol Biochem 156:64–77. https://doi.org/10.1016/j.plaphy.2020.08.042
Dixit VK, Misra S, Mishra SK, Tewari SK, Joshi N, Chauhan PS (2020) Characterization of plant growth-promoting alkalotolerant Alcaligenes and Bacillus strains for mitigating the alkaline stress in Zea mays. Antonie Van Leeuwenhoek 113(7):889–905. https://doi.org/10.1007/s10482-020-01399-1
Hasanuzzaman M, Nahar K, Fujita M (2013) Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P, Azooz M, Prasad M (eds) Ecophysiology and Responses of Plants under Salt Stress. Springer, New York
Mishra J, Prakash J, Arora NK (2016) Role of beneficial soil microbes in sustainable agriculture and environmental management. Clim Chang Environ Sustain 4(2):137–149. https://doi.org/10.5958/2320-642X.2016.00015.6
Ojuederie OB, Babalola OO (2017) Microbial and plant-assisted bioremediation of heavy metal polluted environments: a review. Int J Environ Res Public Health 14(12):1504. https://doi.org/10.3390/ijerph14121504
Santoyo G, Moreno-Hagelsieb G, del Carmen O-M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Hayat R, Ali S, Amara U, Khalid R, Ahmed I (2010) Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol 60(4):579–598. https://doi.org/10.1007/s13213-010-0117-1
Yadav AN, Verma P, Kour D, Rana KL, Kumar V, Singh B, Dhaliwal HS (2017) Plant microbiomes and its beneficial multifunctional plant growth promoting attributes. Int J Environ Sci Nat Resour 3(1):1–8. https://doi.org/10.19080/IJESNR.2017.03.555601
Gupta S, Pandey S (2019) ACC deaminase producing bacteria with multifarious plant growth promoting traits alleviates salinity stress in French bean (Phaseolus vulgaris) plants. Front Microbiol 10:1506. https://doi.org/10.3389/fmicb.2019.01506
Misra S, Chauhan PS (2020) ACC deaminase-producing rhizosphere competent Bacillus spp mitigate salt stress and promote Zea mays growth by modulating ethylene metabolism. 3 Biotech 10(3):1–14. https://doi.org/10.1007/s13205-020-2104-y
Cherif-Silini H, Silini A, Chenari Bouket A, Alenezi FN, Luptakova L, Bouremani N, Belbahri L (2021) Tailoring next generation plant growth promoting microorganisms as versatile tools beyond soil desalinization: a road map towards field application. Sustainability 13(8):4422. https://doi.org/10.3390/su13084422
Srivastava S, Bist V, Srivastava S, Singh PC, Trivedi PK, Asif MH, Nautiyal CS (2016) Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front Plant Sci 7:587. https://doi.org/10.3389/fpls.2016.00587
Alef K, Nannipieri P (1995) Methods in applied soil microbiology and biochemistry. Academic Press, London. https://doi.org/10.1016/B978-0-12-513840-6.X5014-9
Zin NM, Sarmin NI, Ghadin N, Basri DF, Sidik NM, Hess WM, Strobel GA (2007) Bioactive endophytic streptomycetes from the Malay Peninsula. FEMS Microbiol Lett 274(1):83–88. https://doi.org/10.1111/j.1574-6968.2007.00819.x
Mowafy AM, Fawzy MM, Gebreil A, Elsayed A (2021) Endophytic Bacillus, Enterobacter, and Klebsiella enhance the growth and yield of maize. Acta Agric Scand B Soil Plant Sci 71(4):237–246. https://doi.org/10.1080/09064710.2021.1880621
Misra S, Dixit VK, Khan MH, Mishra SK, Dviwedi G, Yadav S, Lehri A, Chauhan PS (2017) Exploitation of agro-climatic environment for selection of 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing salt tolerant indigenous plant growth promoting rhizobacteria. Microbiol Res 205:25–34. https://doi.org/10.1016/j.micres.2017.08.007
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57(2):535–538. https://doi.org/10.1128/aem.57.2.535-538.1991
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43(1):51–56. https://doi.org/10.1007/s002840010259
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118(1):10–15. https://doi.org/10.1034/j.1399-3054.2003.00086.x
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170(1):265–270. https://doi.org/10.1016/S0378-1097(98)00555-2
Titus S, Gaonkar S, Srivastava RB, Karande AA (1995) Exopolymer production by a fouling marine bacterium Pseudomonas alcaligenes. Indian J Mar Sci 24:45–48
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. https://doi.org/10.1093/molbev/mst197
Nautiyal CS (1997) A method for selection and characterization of rhizosphere-competent bacteria of chickpea. Curr Microbiol 34(1):12–17. https://doi.org/10.1007/s002849900136
Joshi H, Bisht N, Mishra SK, Prasad V, Chauhan PS (2023) Bacillus amyloliquefaciens modulate carbohydrate metabolism in host-PGPR cross-talk under abiotic stress and phytohormone treatments. J Plant Growth Reg 42:4466–4483. https://doi.org/10.1007/s00344-023-10913-4
Bates LS, Waldren RA, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bisht N, Tiwari S, Singh PC, Niranjan A, Chauhan PS (2019) A multifaceted rhizobacterium Paenibacillus lentimorbus alleviates nutrient deficiency-induced stress in Cicer arietinum L. Microbiol Res 223:110–119. https://doi.org/10.1016/j.micres.2019.04.007
Dubios MK, Gilles JK, Robers PA, Smith F (1951) Calorimetric determination of sugar and related substance. Analyt Chem 26:351–356. https://doi.org/10.1021/ac60111a017
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161(2):559–566. https://doi.org/10.1016/0003-2697(87)90489-1
Aebi H (1984) Catalase in vitro. Methods in enzymology. Academic press, London, pp 121–126
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Glick BR, Cheng Z, Czarny J, Duan J (2007) Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur J Plant Pathol 119:329–339. https://doi.org/10.1007/s10658-007-9162-4
Hashem A, Abd Allah EF, Alqarawi AA, Al-Huqail AA, Wirth S, Egamberdieva D (2016) The interaction between arbuscular mycorrhizal fungi and endophytic bacteria enhances plant growth of Acacia gerrardii under salt stress. Front Microbiol 7:1089. https://doi.org/10.3389/fmicb.2016.01089
Hall JA, Peirson D, Ghosh S, Glick B (1996) Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12–2. Isr J Plant Sci 44(1):37–42. https://doi.org/10.1080/07929978.1996.10676631
Sagar A, Sayyed RZ, Ramteke PW, Sharma S, Marraiki N, Elgorban AM, Syed A (2020) ACC deaminase and antioxidant enzymes producing halophilic Enterobacter sp. PR14 promotes the growth of rice and millets under salinity stress. Physiol Mol Biol Plants 26(9):1847–1854. https://doi.org/10.1007/s12298-020-00852-9
Singh RP, Pandey DM, Jha PN, Ma Y (2022) ACC deaminase producing rhizobacterium Enterobacter cloacae ZNP-4 enhance abiotic stress tolerance in wheat plant. PLoS ONE 17(5):e0267127. https://doi.org/10.1371/journal.pone.0267127
Reshma P, Naik MK, Aiyaz M, Niranjana SR, Chennappa G, Shaikh SS, Sayyed RZ (2018) Induced systemic resistance by 2, 4-diacetylphloroglucinol positive fluorescent Pseudomonas strains against rice sheath blight. IJEB 56(3):207–212
Khalid A, Arshad M, Zahir ZA (2004) Screening plant growth-promoting rhizobacteria for improving growth and yield of wheat. J Appl Microbiol 96(3):473–480. https://doi.org/10.1046/j.1365-2672.2003.02161.x
Dey RKKP, Pal KK, Bhatt DM, Chauhan SM (2004) Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol Res 159(4):371–394. https://doi.org/10.1016/j.micres.2004.08.004
Çakmakçı R, Erat M, Erdoğan Ü, Dönmez MF (2007) The influence of plant growth–promoting rhizobacteria on growth and enzyme activities in wheat and spinach plants. J Plant Nutr Soil Sci 170(2):288–295. https://doi.org/10.1002/jpln.200625105
Mehnaz S, Kowalik T, Reynolds B, Lazarovits G (2010) Growth promoting effects of corn (Zea mays) bacterial isolates under greenhouse and field conditions. Soil Biol Biochem 42(10):1848–1856. https://doi.org/10.1016/j.soilbio.2010.07.003
Kumar NP, Audipudi V (2015) Exploration of a novel plant growth promoting bacteria Stenotrophomonas maltophilia AVP27 isolated from the chilli rhizosphere soil. Int J Eng Res Generic Sci 3(1):265–273. https://doi.org/10.20546/ijcmas.2017.611.111
Sarkar A, Pramanik K, Mitra S, Soren T, Maiti TK (2018) Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J Plant Physiol 231:434–442. https://doi.org/10.1016/j.jplph.2018.10.010
Acknowledgements
The authors acknowledge the Director, CSIR-National Botanical Research Institute for providing facilities and support during the study. CSIR-NBRI allotted the manuscript number CSIR-NBRI_MS/2023/11/09.
Funding
This work is supported by the CSIR-Network (MLP0048) and an in-house project (OLP116) funded by the Council of Scientific and Industrial Research, New Delhi, India.
Author information
Authors and Affiliations
Contributions
PSC: conceived and co-ordinated the research. SK, SKM, SM, RA, and SK: conducted experiments and analyzed the data. PSC and SK: wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
Not required.
Consent for Publication
The author confirms publication has been approved by all co-authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kar, S., Mishra, S.K., Misra, S. et al. Endophytic Alkalotolerant Plant Growth-Promoting Bacteria Render Maize (Zea mays L.) Growth Under Alkaline Stress. Curr Microbiol 81, 43 (2024). https://doi.org/10.1007/s00284-023-03557-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03557-w