Abstract
Lysobacter capsici X2-3, a plant growth-promoting rhizobacteria (PGPR), was isolated from wheat rhizosphere and has inhibitory effects against a wide range of pathogens. One important characteristic of L. capsici is its ability to produce diverse antibiotics and lytic enzymes. The GntR family of transcription factors is a common transcription factor superfamily in bacteria that has fundamental roles in bacterial metabolism regulation. However, the GntR family transcription factor in Lysobacter has not been identified. In this study, to obtain an understanding of the GntR/HutC gene function in L. capsici X2-3, a random Tn5-insertion mutant library of X2-3 was constructed to select genes showing pleiotropic effects on phenotype. We identified a Tn5 mutant with an insertion in LC4356 that showed reduced biofilm levels, and sequence analysis indicated that the inserted gene encodes a GntR/HutC family transcription regulator. Furthermore, the LC4356 mutant showed reduced extracellular polysaccharide (EPS) production, diminished twitching motility and decreased survival under UV radiation and high-temperature. The RT‒qPCR results indicated that the pentose phosphate pathway-related genes G6PDH, 6PGL and PGDH were upregulated in the LC4356 mutant. Thus, since L. capsici is an efficient biocontrol agent for crop protection, our findings provide fundamental insights into GntR/HutC and will be worthwhile to improve PGPR biocontrol efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lysobacter spp. are gram-negative bacteria that are frequently present in soil and freshwater. One of the most striking characteristics of Lysobacter spp. are their high lytic activity against various fungi, bacteria, and nematodes [1,2,3]. Several Lysobacter species, such as L. enzymogenes, L. antibioticus and L. capsici, have been reported to have antimicrobial activity and are potential sources of novel antibiotics [4, 5]. Among them, L. capsici has great potential as a plant pathogen biocontrol agent [2, 6]. For example, L. capsici AZ78 produces a 2,5-diketopiperazine with notable inhibitory activity against Phytophthora infestans and Plasmopara viticola [7]. In addition, L. capsici AZ78 and PG4 have been reported to act as effective antifungal agents [8]. The β-lytic protease BlpLc isolated from L. capsici VKM B-2533 T is able to lyse a variety of Staphylococcus spp. [1].
The GntR family of transcription factors is one of the most widespread families in bacteria, and these proteins regulate diverse biological processes, including motility, growth and pathogenicity in pathogenic species [9, 10]. The GntR protein typically contains a highly conserved N-terminal helix-turn-helix (HTH) DNA-binding domain and a diverse globular domain at the C-terminus [11, 12]. As the second largest GntR subfamily, HutC exhibits pleiotropic functions in prokaryotes and plays an important role in histidine metabolism, motility and bacterial virulence [13,14,15]. Disruption of HutC significantly reduced the cytotoxicity of Pseudomonas aeruginosa [16]. In the biopesticide Bacillus thuringiensis, HutC plays an important role in N-acetylglucosamine (GlcNAc) catabolism [17]. In addition, HutC serves as a pleiotropic regulator and participates in morphological development, antibiotic production and pigment biosynthesis in Saccharopolyspora erythraea, which is used industrially to produce erythromycin A for medicine [18]. In the plant growth-promoting bacterium (PGPB) P. fluorescens SBW25, HutC is functionally required for histidine catabolism and bacterial colonization in planta [15]. GntR/HutC plays an important role in some bacteria, but no research has been conducted on the biocontrol L. capsici strain X2-3.
Wheat rhizospheric L. capsici X2-3 showed broad antifungal activities against several phytopathogenic fungi and oomycetes, including Rhizoctonia cerealis, Pythium myriotylum, Bipolaris sorokiniana, B. subtilis, and R. cerealis [19, 20]. The genome size of L. capsici strain X2-3 is 6,126,365 bp, and a total of 5,117 genes were predicted in its genome [19]. The functions of the GntR family of transcription factors in some bacteria have been studied; however, their function has been less studied in PGPB, in which biofilm formation is essential for biocontrol. In this study, more than 4800 mutants were obtained by transposon mutagenesis of X2-3 using Tn5 random insertion, and seven mutant strains with phenotypic changes were selected. In mutant MT4, the insertion gene LC4356 was identified as a GntR/HutC family transcription regulator. This study showed that GntR/HutC is involved in biofilm formation, extracellular polysaccharide production, twitching motility and the stress response to UV radiation and high-temperature in L. capsici X2-3.
Materials and Methods
Strains Used in This Study
Table S1 lists the bacterial strains, plasmids and media used in the present study. The L. capsici strain X2-3 was deposited in the China General Microbiological Culture Collection Center and assigned accession number CGMCC1.61449. The whole genome shotgun sequencing project for this strain has been registered at DDBJ/EMBL/GenBank with accession number LBMI00000000. All L. capsici strains were cultivated at 28 °C in nutrient broth (NB) medium or on nutrient agar (NA) medium unless stated otherwise. Escherichia coli strain TransforMax™ EC100DTMpir+ (Epicentre Biotechnologies, Madison, WI) was grown at 37 °C in Luria–Bertani (LB) medium for plasmid rescue. In the case of mutant selection, the following concentrations of antibiotics were added to the media: E. coli 100 μg/mL kanamycin and 50 μg/mL gentamycin; L. capsici X2-3 mutants: 300 μg/mL kanamycin; and complemented strains: 200 μg/mL gentamycin.
Random Mutagenesis Analysis
To identify the genes associated with biofilms, the EZ-Tn5™ < R6Kcori/KAN-2 > Tnp Transposome Kit (Epicentre Biotechnologies, Madison, WI) was used to randomly mutate strain X2-3. Transposon mutants were selected on NA plates with 300 μg/mL kanamycin, on which the wild-type strain X2-3 was unable to grow. Bacterial colony morphology was evaluated after three days of incubation. PCR amplification of the Tn5 transposon sequence using the primers R6kF1 and R6kR1 (Table S2) was performed to confirm transposon insertion. Positive clones were selected for further studies.
Identification of the Target Genes
After extracting the genomic DNA of the mutants using CTAB, L. capsici genomic DNA was digested with EcoRI. A DNA Ligation Kit AG11801 (Accurate Biotechnology (Hunan) Co., Ltd) was used to ligate digestion products, which were then transformed into E. coli TransforMax™ EC100DTMpir+. Then, the primers KAN-2 FP-1 and R6KAN-2 RP-1 were used to sequence and analyze the mutants. Homologous sequences were identified using the BLAST and BLASTP programs (BLAST: Basic Local Alignment Search Tool (nih.gov)).
Colony Morphology Assay
The strains X2-3, MT4, and MCS4356 were grown to obtain an OD600 of 1.0 in NB medium. After this each strain (3 μL of each bacterial suspension) was overlaid on NA plates separately, and the strains X2-3, MT4, and MCS4356 were cultivated for three days. The bacterial colonies were observed under a Leica M165FC stereomicroscope. This experiment was repeated with three replicates.
Construction of Complemented Strains and Plasmids
The broad-host vector pBBR1-MCS5 [21] containing the T7 promoter was used as the vector for constructing the complemented strains. The PCR-amplified fragment containing the complete LC4356 gene was ligated to pBBR1-MCS5 with EcoRI and BamHI sites to obtain plasmid pBBR1-LC4356, and then the plasmid was transformed into the LC4356 mutant by electroporation. To confirm that the complemented strain contained both the recombinant plasmid pBBR1-LC4356 and the Tn5 transposon, the sequences were amplified by PCR with the primers 4356F and 4357R (Table S2).
Growth Curve Assay
The strains X2-3, MT4, and MCS4356 were cultivated for 24 h and grown in NB medium to OD600 = 1.0. Then, one hundred microliters of culture was diluted with 10 mL of NB medium. Strains X2-3, MT4, and MCS4356 were shaken at 200 rpm for 48 h, and their growth was examined every 4 h [22]. The growth of the bacterial cells was detected by measuring the OD600 and serial dilutions followed by plate counting to construct bacterial growth curves. We repeated the experiment four times, each with three replicates.
Biofilm Assay
Crystal violet staining was used to analyze the cells attached to the abiotic surface [23]. The strains X2-3, MT4, and MCS4356 were cultivated overnight and then grown to an OD600 of 1.0 in NB medium. One hundred microliters of culture was diluted with 10 mL of NB medium. A shaken incubation of 72 h was then performed on the strains. After removing the bacteria, the glass tubes were washed three times. Finally, 0.2% crystal violet was used to stain the glass tubes. The unbound crystal violet was removed with sterile distilled water. The crystal violet absorbance extracted from the glass tube was measured at 575 nm in 95% ethanol. Three independent experiments were performed with three replicates each.
Extracellular Polysaccharide Production Assay
Extracellular polysaccharide (EPS) was isolated from the strains by acetone precipitation [24]. To measure the quantity of EPSs in the culture supernatants, 1 mL of bacterial suspension was added to 100 mL of NB medium containing 4% glucose for three days of incubation with shaking. After high-speed centrifugation of the bacterial culture, the supernatant was precipitated with two volumes of ice-cold acetone for 24 h. Centrifugation, drying, and weighing were then performed on the precipitates. The experiments were repeated three times.
Motility Assay
The strains X2-3, MT4, and MCS4356 were cultivated for 24 h, and 3 μL of each bacterial suspension with OD600 = 1.0 was added to NYGB medium with 0.3% (w/v) agar for measure swimming motility. The bacteria were then incubated for 3 days. For twitching motility, 3 μL of each bacterial with OD600 = 1 was injected into the bottom of the medium with 1% agar after the stabbing. The agar was carefully removed and air-dried after three days of incubation. Then the cells attached to the petri dish were stained with crystal violet to assess bacterial twitching motility. Measurements of the bacteria occupied diameter were used to quantify bacterial motility [25, 26]. In total, three experiments were conducted.
Stress Tolerance Assay
The strains X2-3, MT4, and MCS4356 at the same final concentration were diluted 100-fold and grown in NB medium. After 3 days of incubation, their viability was tested in four stressful environments. For saline stress, NaCl was added to the NB medium at concentrations of 0.15, 0.25, 0.35, and 0.5 mol/L. For pH stress, sodium acetate buffer was added to the bacterial culture to create pH values of 6.2, 6.6 and 7.0 [27, 28]. For UV radiation, strains were exposed to 245 nm UV radiation at a distance of 60 cm for 45 min, followed by incubation at 28 °C [29]. For temperature stress, the strains were grown at 28, 37, 39 and 42 °C for 72 h. After 72 h of incubation to the stationary phase, the bacterial growth was calculated by measuring the OD600 and serial dilutions followed by plate counting [30]. We repeated the experiment 3 times.
Real-Time Quantitative PCR (RT‒qPCR)
The transcription level of the disrupted LC4356 gene in the strains was measured by RT‒qPCR. In addition, genes related to glucose metabolism were selected to determine whether they were affected by LC4356. All of these genes encode critical enzymes in the pentose phosphate pathway. Table S2 lists the gene-specific primers used in this assay. According to the manufacturer’s instructions, AG RNAex Pro Reagent (Accurate Biotechnology (Hunan) Co., Ltd) was used to extract total RNA from the cultures of the L. capsici strains. Both reverse transcription and cDNA amplification were performed using an Evo M-MLV RT Kit II AG11711 (Accurate Biotechnology (Hunan) Co., Ltd). The constitutively expressed 16S rRNA gene was used as the reference gene, and the mRNA levels were quantified using the 2–ΔΔCT relative quantification method [31].
Statistical Analysis
All data are the mean of at least three replicates. All data points across independent experiments were used for statistical evaluation. SPSS statistical software (version 18.0) was used to analyze the standard deviation and significant differences in the data. The means were compared using Duncan’s multiple range test (single-factor ANOVA) at the 5% probability level (P < 0.05, n = 3).
Results
Identification of the Genes Involved in L. capsici Phenotypic Alterations
To discern the genes involved in phenotypic changes, a random insertion library of L. capsici X2-3 containing more than 4800 mutants was obtained using the EZ-Tn5 transposon. Seven mutants named MT1-MT7, which showed colonies that were phenotypically different from the wild-type X2-3 colonies, were selected for subsequent study. Under a Leica stereomicroscope, the shapes and surfaces of these mutants showed differences from these of X2-3 (Fig. 1a). Therefore, the flanking sequences of the EZ-Tn5-insertion were analyzed and identified by plasmid rescue. The results from the seven mutant insertion genes and insertion sites are shown in Table 1, and five genes associated with biofilms were identified.
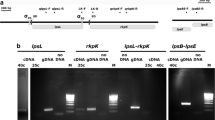
Screening and confirmation of the L. capsici X2-3 mutants. a Colony morphology of the wild-type X2-3 (a), the mutants MT1-MT7 (b–h), and the complemented strain MCS4356 (i). Two microliters of bacterial cultures were added to the NA plate after three days of incubation at 28 °C and observed by Leica stereomicroscopy. Scale bar, 2000 μm. b Schematic diagram illustrating the insertion of the Ez-Tn5 transposon into the LC4356 gene. The arrow indicates the location of the transposon insertion. c Phylogenetic analysis of GntR/HutC in L. capsici X2-3 and other bacteria. The neighbor-joining method was used to construct the phylogenetic tree in MEGA version 7.0. Numbers on the nodes of the trees indicate bootstrap percentages. GenBank accession numbers are displayed after the species name
Characterization of the MT4 Mutant
Moreover, the phenotype of the mutant strain MT4 was different from the wild-type and other mutants. Sequence analysis identified that the insertion gene LC4356 in MT4 as a GntR/HutC family transcription factor. This gene contains 753 base pairs coding for a transcription factor of 251 amino acids. BLAST analyses showed that the sequence of the GntR/HutC gene shares 41.20% identity with the HutC gene of E. coli (GenBank accession no. WP_112922747.1). The GntR/HutC protein of X2-3 was predicted by GenBank to have two important domains, including an HTH DNA-binding domain at the N-terminus, and a UTRA/ligand binding domain at the C-terminus, which is conserved in the HutC subfamily (Fig. S1). As shown in Fig. 1b, a pattern diagram was drawn to display the position and structure of GntR/HutC, and the phylogenetic tree of GntR/HutC is shown in Fig. 1c.
Mutant MT4 Has an Altered Bacterial Phenotype But No Growth Alteration Is Observed
To confirm the functionality of the predicted LC4356 gene in L. capsici X2-3, full-length LC4356 was inserted into pBBR1-MCS5, and then the resultant plasmid was transfected into MT4 to obtain the complemented mutant MCS4356 (Fig. S2). Under a Leica stereomicroscope, the shape and surface of the mutant MT4 were different than those of X2-3 and MCS4356 (Fig. 2a). The growth of X2-3, mutant MT4 and complemented strain MCS4356 was analyzed by measuring OD600 values every 4 for 48 h. As shown in Fig. 2b, bacterial growth showed no significant changes. MT4 and MCS4356 exhibited a growth trend that was consistent with that of the wild-type in the logarithmic phase as well as the stationary phase. This result was consistent with the CFU data using serial dilutions followed by plate counting (Fig. 2c). These data revealed that LC4356 is involved in phenotype alterations in L. capsici X2-3, but does not influence bacterial growth in the tested medium.
Colony morphology and growth curves of X2-3, MT4 and MCS4356. a Colony morphology of X2-3, MT4 and MCS4365. Two microliters of bacterial cultures were added to the NA plate after three days of incubation at 28 °C. Scale bar, 2000 μm. b X2-3, MT4 and MCS4356 growth curves. The strains X2-3, MT4 and MCS4356 were grown in NB medium, adjusting the OD600 value to 1.0, and then cultivated for 48 h in fresh NB. The OD600 values were measured every 4 h during culture. There were no significant differences between strains X2-3, MT4 and MCS4356. c The growth curves of X2-3, MT4 and MCS4356 for CFU data measured by serial dilution and plate counting. No significant differences between strains X2-3, MT4 and MCS4356. The average of three independent experiments was used to calculate the means, and Duncan’s multiple range test was used to compare the means at the 5% probability level
GntR/HutC Positively Influences Biofilm Formation and EPSs
To determine the differences between mutant MT4 and wild-type X2-3 in terms of biofilm formation, crystal violet staining was used to quantify the biofilms. Compared to X2-3, the mutant MT4 displayed decreased biofilm formation and a reduction in biofilm matrix adhered to the glass surface (Fig. 3a). After three days of incubation, the biofilms of the mutant MT4 and wild-type strain showed significant differences as shown by the 570/600 nm index (Fig. 3b). The growth of the mutant and wild-type strains remained similar. Therefore, their differences mainly involved changes in biofilm formation, as indicated by crystal violet staining. The biofilm of MT4 was significantly decreased by 43.08% compared to that of X2-3, while biofilm formation was partially or completely recovered in the complemented strain MCS4356. The quantity of biofilm formation by the X2-3 and MCS4356 strains was greater than that of MT4. The biofilm formation of X2-3 was very similar to that of the resultant strain X2-3(EVP) transformed into wild-type with the plasmid vector pBBR1MCS5 (Fig. S3). This result suggested that disruption of the LC4356 gene in MT4 reduced biofilm formation; however, the complemented strain recovered the biofilm formation ability of the wild-type.
Biofilms and extracellular polysaccharide production of X2-3, MT4 and MCS4356. a After three days of incubation in NB medium, biofilm formation by strains X2-3, the mutant strain MT4 and the complemented strain MCS4356 on glass bottle surfaces and glass tubes was stained with crystal violet solution. b Crystal violet staining examined biofilm formation by measuring the absorbance by the 575/600 nm index. c The quantity of extracellular polysaccharides. The strains X2-3, MT4 and MCS4356 were grown for three days in NB medium supplemented with 4% glucose at 28 °C. Then, the secreted EPSs were precipitated with two volumes of ice-cold acetone. The precipitated EPSs were pelleted by centrifugation, dried, and weighed. d Four differentially expressed genes were detected by RT‒qPCR. The X2-3, MT4 and complemented strain MSC4356 were cultivated to an OD600 = 1. Four genes, GntR/HutC family transcription regulators, glucose-6-phosphate 1-dehydrogenase (G6PDH), 6-phosphogluconolactonase (6PGL) and phosphor gluconate dehydratase (PGDH), were tested by RT‒qPCR. The actual P-values are shown in figure. Standard errors are shown in vertical bars. a represents not significantly different compared with X2-3; b represents a significant difference compared with X2-3. The average of three independent experiments was used to calculate the means, and Duncan’s multiple range test was used to compare the means at the 5% probability level (P < 0.05)
EPSs are involved in the formation of biofilms. Therefore, EPSs were isolated and their amounts estimated by precipitation with ice-cold acetone. X2-3 typically produces EPS at 3.24 g/L, whereas the mutant MT4 produced only 1.39 g for the same volume by a 57.10% decrease (Fig. 3c). Moreover, strain MCS4356 was restored EPS production to the wild-type level, giving 3.13 g/L. These results indicated that the LC4356 gene contributed to biofilm formation and the production of EPSs in L. capsici.
The Disruption of LC4356 Attenuates X2-3 Twitching Motility
Most Lysobacter species are deficient in producing surface-attached flagellum, and type IV pilus-dependent twitching motility that is a typical mobile behavior expressed by Lysobacter [32]. The motility of X2-3, MT4, and MCS4356 was quantified by measuring the migration of the strains from the center to the edge of a plate through agar. For swimming motility, the diameter of MCS4356 was 2.37 cm, which was similar to that of X2-3 (2.51 cm), while MT4 (1.59 cm) had decreased motility on the medium (Fig. 4a). The twitching motility zones for X2-3 was 2.03 cm, very similar to MCS4356 (1.93 cm), while the MT4 decreased by 34.48% in twitching motility to 1.33 cm (Fig. 4b). These results indicated that the LC4356 gene was involved in the motility of L. capsici.
Motility of X2-3, MT4 and MCS4356. a The swimming motility tests were conducted using three microlitres of each cell sample dropped onto 0.3% agar NA plates after strains X2-3, MT4, and MCS4356 were grown in NB medium for 24 h at 28 °C. After three days of incubation, the diameter of each colony was measured, and the results were taken as an indicator of bacterial swimming motility. b Three microlitres of the strains X2-3, MT4, and MCS4356 was injected into the bottom of the medium with 1% agar and after three days of incubation, the twitching motility of the bacteria was assessed by crystal violet staining. The actual P-values are shown in figure. a represents not significantly different compared with X2-3; b represents a significant difference compared with X2-3. The average of three independent experiments was used to calculate the means, and Duncan’s multiple range test was used to compare the means at the 5% probability level (P < 0.05)
The LC4356 Mutation Impairs Bacterial Resistance to UV Radiation and High-Temperature But Has No Significant Effect on Salt or pH Stress
The growth of strains X2-3, MT4 and MCS4356 was compared under different temperature, salt, pH, and UV radiation conditions to investigate the functions of LC4356 in stress tolerance. After UV radiation and high-temperature incubation conditions, the number of surviving mutant MT4 bacteria was significantly lower than that of the X2-3, and the number of surviving bacteria was reduced by 18.41 and 94.25%, respectively. (Fig. 5a and b). In contrast, the survival of mutant MT4 and strain X2-3 showed no significant differences under low pH (Fig. 5c) or high osmotic pressure conditions (Fig. 5d). The results of the absorbance measurements at 600 nm are shown in Fig. S4. These results revealed that MT4 was more susceptible to UV radiation and high-temperature.
Survival of strains X2-3, MT4 and MCS4356 under different stress conditions. After 72 h of incubation, the growth of X2-3, MT4 and MCS4356 was examined under four environmental stresses: UV radiation a, temperature b, pH c, and salt d. The surviving population relative to untreated controls was estimated using serial dilutions followed by plate counting. The actual p-values are shown in figure. The error bars represent the standard deviations of three independent experiments performed in duplicate. a, b, and c represent significant differences, and Duncan’s multiple range test was used to compare the means at the 5% probability level (P < 0.05)
Regulation of Glucose Metabolism Gene Expression by LC4356
To clarify the function of LC4356 in glucose metabolism, the transcriptional levels of the genes adjacent to LC4356 in the whole genome were analyzed by RT‒qPCR. Three genes downstream of LC4356 were predicted to be related to glucose metabolism, including genes coding for glucose-6-phosphate dehydrogenase (G6PDH), 6-phosphogluconolactonase (6PGL) and phosphogluconate dehydratase (PGDH). The results of the RT‒qPCR assays showed that the transcriptional levels of G6PDH, 6PGL and PGDH in MT4 were significantly higher than those in X2-3, and the levels of G6PDH, 6PGL and PGDH increased by 20.47-, 30.86-, and 8.18-fold in mutant MT4, respectively (Fig. 3d). These results indicated that LC4356 is involved in regulating the pentose phosphate pathway.
Discussion
GntR has been reported to play essential roles in the regulation of various biological processes, including bacterial virulence, antibiotic production, resistance, motility, and primary metabolism [33, 34]. Nevertheless, the exact role of GntR in the biocontrol of L. capsici still needs to be clarified. In this study, mutants with different colony morphologies were obtained using the EZ-Tn5 transposon. The disrupted gene in mutant MT4 was identified as a GntR/HutC family transcription factor. Our results demonstrated that the mutant MT4 significantly reduced biofilm formation, EPS production, twitching motility and stress responses but had no significant effect on bacterial growth.
In this study, the inserted flanking sequences were identified, and LC4356 was identified as a GntR/HutC family transcription factor. By comparison, we found that the amino acid sequence of L. capsici X2-3 GntR/HutC shares 96 and 100% identity with those of L. capsici (GenBank accession no. ALN83455) and L. enzymogenes (GenBank accession no. WP_057949624) strains, respectively (Fig. S5). The phylogenetic tree showed that the GntR/HutC family of transcription factors is highly conserved in Lysobacter spp. (Fig. 1a). The results of homology searches showed that the amino acid sequence of the LC4356 gene exhibits 67.95 and 64.80% identity with the amino acid sequence of Pseudoxanthomonas sp. GM95 (GenBank accession no. WP_093143266.1) and Bacillus sp. NP157 (GenBank accession no. WP_216157940.1), respectively (Fig. S5). The phylogenetic tree of GntR/HutC proteins from L. capsici and other bacteria indicated that these GntR/HutC proteins could be divided into distinct clades (Fig. 1c). This result suggests that GntR/HutC family of transcription factors may act in different ways in L. capsici X2-3.
A biofilm is generally considered to be a self-protecting mechanism, as it forms a barrier to resist stresses [35]. Such protection is due to the biofilm extracellular matrix, which is typically made of EPSs, proteins and sometimes nucleic acids [36]. Bacterial colony morphology can reflect biofilm formation. Colony morphology and biofilm formation regulation are complex processes that often involve multiple interrelated pathways [37]. For example, historical studies of polysaccharides have shown that changes in their production frequently alter colony morphology [38]. Our study on GntR/HutC showed that insertion of LC4356 altered the bacterial phenotype and resulted in a roughly half reduction in biofilm formation and the production of EPSs in L. capsici X2-3 (Figs. 2 and 3). Previous studies have found that the absence of yidC can change the structure and physical properties of the EPS matrix and reduce the stability of Streptococcus spp. biofilms [39]. In Sinorhizobium meliloti, biofilm formation was reduced, and the biofilm architecture was altered in the lpsB mutant which is defective in EPSs [40]. Our study found similar results, in that EPS production and biofilm formation were positively correlated. These findings indicated that biofilm formation and EPSs are positively coregulated by GntR/HutC family of transcription factor in L. capsici X2-3. Three genes related to glucose metabolism were analyzed by RT‒qPCR to better understand the regulatory effect of GntR/HutC. All three genes were upregulated in the MT4 mutant (Fig. 3d). This result is consistent with previous reports that GntR not only regulates glucose metabolism in P. aeruginosa but also controls biofilm formation and sugar transporter gene expression in S. mutans [10, 34]. Additionally, the overexpression of glucose-6-phosphate dehydrogenase (G6PDH) in genetically modified strains of Actinobacillus succinogenes has proven that upregulation of the oxidative pentose phosphate pathway (OPPP) can increase the yield of succinic acid and affect its biofilms [41]. Therefore, we further speculate that GntR/HutC reduced biofilm formation and EPS production by regulating the pentose phosphate pathway.
EPSs are structurally important in biofilms and is act an extracellular network that organizes individual bacteria into multicellular organisms [42]. Previous research showed that EPSs play an essential role in motility by supporting the function of type IV pili in Myxococcus xanthus [43]. In our study, twitching motility was decreased by 34.48% in mutant MT4 compared to X2-3, and the motility of complemented strain MCS4356 was restored to the wild-type level (Fig. 4). EPSs promote social motility in P. aeruginosa [44]. Our findings confirmed that the deletion of twitching motility was due to the decrease in EPS secretion in L. capsici X2-3.
The survival of many bacteria that have adapted to harsh environments has largely been attributed to biofilm formation [45, 46]. As shown in Fig. 5, the disruption of LC4356 significantly decreased mutant growth under high-temperature and UV radiation stress. Similarly, the motility and tolerance to phenol, D-sorbitol, SDS and heavy metal salts of HpaR1 mutants were significantly reduced in Xanthomonas campestris pv. campestris [28]. In addition, EPSs form a hydrated anionic matrix that protects against environmental stresses by surrounding the cell [47]. EPSs from M. xanthus play numerous roles in cell survival, including motility, biofilm formation, stress responses, and signal transduction [36, 48]. Thus, our study implied that the decreases in biofilm formation, twitching motility and stress responses are due to a decrease in GntR/HutC-regulated EPS secretion. Furthermore, deletion of GntR caused reduction of the bacterial growth rate in P. fluorescens SBW25 [15]. However, our results showed no significant differences in growth rate among the wild-type X2-3, mutant MT4 and complemented strain MCS4356 (Fig. 2b and c). This is probably due to the distinct metabolic regulation mechanisms between different strains [49], despite the GntR/HutC family regulators being widely distributed in bacteria and playing critical roles in metabolic processes.
In summary, our study demonstrated that GntR/HutC is involved in twitching motility, biofilm formation, EPS production, and stress resistance in L. capsici X2-3. EPSs have been reported to be related to antimicrobial activity because they include various metabolites, such as lactic acid bacteria (LAB) [50]. A wide variety of pathogenic microbes (bacteria, viruses, and fungi) have been reported to be inhibited by EPSs in numerous studies [44, 48]. However, comparison of the mutant MT4 with the wild-type strain showed no noticeable changes in antimicrobial activity (Fig. S6).
Conclusions
In this study, we demonstrated that inactivation of GntR/HutC significantly reduced biofilm formation, extracellular polysaccharide (EPS) production, twitching motility and stress responses in L. capsici X2-3, but had no significant effect on bacterial growth. From the analysis, we conclude that GntR/HutC reduced biofilm formation and EPS production by regulating the pentose phosphate pathway. Furthermore, we consider that the decreases in twitching motility and stress responses is due to the decreased EPS secretion regulated by GntR/HutC. L. capsici X2-3 possesses broad antimicrobial activity against both fungi and oomycetes as well as gram-positive bacteria, and is an agriculturally important biological control agent. Biofilms and motility play an important role in its biological control. This work expands our current understanding of the function of GntR/HutC in L. capsici X2-3, but the effect of GntR/HutC on the biocontrol exhibited by L. capsici remains to be further investigated.
Data Availability
If reasonable request is made, the corresponding author will provide the datasets used or analyzed during the study.
Code Availability
Not applicable.
References
Afoshin AS, Kudryakova IV, Borovikova AO, Suzina NE, Toropygin IY, Shishkova NA et al (2020) Lytic potential of Lysobacter capsici VKM B-2533(T): bacteriolytic enzymes and outer membrane vesicles. Sci Rep 10:9944. https://doi.org/10.1038/s41598-020-67122-2
Fang BZ, Xie YG, Zhou XK, Zhang XT, Liu L, Jiao JY et al (2020) Lysobacter prati sp. nov., isolated from a plateau meadow sample. Antonie Van Leeuwenhoek 113:763–772. https://doi.org/10.1007/s10482-020-01386-6
Yu L, Su W, Fey PD, Liu F, Du L (2018) Yield improvement of the anti-MRSA antibiotics WAP-8294A by CRISPR/dCas9 combined with refactoring self-protection genes in Lysobacter enzymogenes OH11. ACS Synth Biol 7:258–266. https://doi.org/10.1021/acssynbio.7b00293
Park JH, Kim R, Aslam Z, Jeon CO, Chung YR (2008) Lysobacter capsici sp. nov., with antimicrobial activity, isolated from the rhizosphere of pepper, and emended description of the genus Lysobacter. Int J Syst Evol Microbiol 58:387–392. https://doi.org/10.1099/ijs.0.65290-0
Zhao Y, Qian G, Ye Y, Wright S, Chen H, Shen Y et al (2016) Heterocyclic aromatic N-Oxidation in the biosynthesis of phenazine antibiotics from Lysobacter antibioticus. Org Lett 18:2495–2498. https://doi.org/10.1021/acs.orglett.6b01089
Puopolo G, Raio A, Zoina A (2010) Identification and characterization of Lysobacter capsici strain PG4: a new plant health-promoting rhizobacterium. J Plant Pathol 92:157–164. https://doi.org/10.4454/jpp.v92i1.25
Puopolo G, Cimmino A, Palmieri MC, Giovannini O, Evidente A, Pertot I (2014) Lysobacter capsici AZ78 produces cyclo(L-Pro-L-Tyr), a 2,5-diketopiperazine with toxic activity against sporangia of Phytophthora infestans and Plasmopara viticola. J Appl Microbiol 117:1168–1180. https://doi.org/10.1111/jam.12611
Brescia F, Marchetti-Deschmann M, Musetti R, Perazzolli M, Pertot I, Puopolo G (2020) The rhizosphere signature on the cell motility, biofilm formation and secondary metabolite production of a plant-associated Lysobacter strain. Microbiol Res 234:126424. https://doi.org/10.1016/j.micres.2020.126424
Arya G, Pal M, Sharma M, Singh B, Singh S, Agrawal V et al (2021) Molecular insights into effector binding by DgoR, a GntR/FadR family transcriptional repressor of D-galactonate metabolism in Escherichia coli. Mol Microbiol 115(4):591–609. https://doi.org/10.1111/mmi.14625
Li Z, Xiang Z, Zeng J, Li Y, Li J (2018) A GntR family transcription factor in Streptococcus mutans regulates biofilm formation and expression of multiple sugar transporter genes. Front Microbiol 9:3224. https://doi.org/10.3389/fmicb.2018.03224
Vindal V, Suma K, Ranjan A (2007) GntR family of regulators in Mycobacterium smegmatis: a sequence and structure based characterization. BMC Genom 8:289. https://doi.org/10.1186/1471-2164-8-289
Zhou X, Yan Q, Wang N (2017) Deciphering the regulon of a GntR family regulator via transcriptome and ChIP-exo analyses and its contribution to virulence in Xanthomonas citri. Mol Plant Pathol 18:249–262. https://doi.org/10.1111/mpp.12397
Abeywickrama TD, Perera IC (2021) In silico characterization and virtual screening of GntR/HutC family transcriptional regulator MoyR: a potential monooxygenase regulator in Mycobacterium tuberculosis. Biology 10:1241. https://doi.org/10.3390/biology10121241
Aravind L, Anantharaman V (2003) HutC/FarR-like bacterial transcription factors of the GntR family contain a small molecule-binding domain of the chorismate lyase fold. FEMS Microbiol Lett 222:17–23. https://doi.org/10.1016/s0378-1097(03)00242-8
Naren N, Zhang XX (2020) Global regulatory roles of the histidine-responsive transcriptional repressor HutC in Pseudomonas fluorescens SBW25. J Bacteriol 202:e00792-e819. https://doi.org/10.1128/jb.00792-19
Rietsch A, Wolfgang MC, Mekalanos JJ (2004) Effect of metabolic imbalance on expression of type III secretion genes in Pseudomonas aeruginosa. Infect Immun 72:1383–1390. https://doi.org/10.1128/iai.72.3.1383-1390.2004
Cao ZL, Tan TT, Zhang YL, Han L, Hou XY, Ma HY et al (2018) NagR(Bt) is a pleiotropic and dual transcriptional regulator in Bacillus thuringiensis. Front Microbiol 9:1899. https://doi.org/10.3389/fmicb.2018.01899
Liao CH, Xu Y, Rigali S, Ye BC (2015) DasR is a pleiotropic regulator required for antibiotic production, pigment biosynthesis, and morphological development in Saccharopolyspora erythraea. Appl Microbiol Biotechnol 99:10215–10224. https://doi.org/10.1007/s00253-015-6892-7
Yi JL, Wang J, Li Q, Liu ZX, Zhang L, Liu AX et al (2015) Draft genome sequence of the bacterium Lysobacter capsici X2–3, with a broad spectrum of antimicrobial activity against multiple plant-pathogenic microbes. Genome Announc 3:e00589-e615. https://doi.org/10.1128/genomeA.00589-15
Zhao D, Wang H, Li Z, Han S, Han C, Liu A (2022) LC_glucose-inhibited division protein is required for motility, biofilm formation, and stress response in Lysobacter capsici X2–3. Front Microbiol 13:840792. https://doi.org/10.3389/fmicb.2022.840792
Obranić S, Babić F, Maravić-Vlahoviček G (2013) Improvement of pBBR1MCS plasmids, a very useful series of broad-host-range cloning vectors. Plasmid 70:263–267. https://doi.org/10.1016/j.plasmid.2013.04.001
Rehl JM, Shippy DC, Eakley NM, Brevik MD, Sand JM, Cook ME et al (2013) GidA expression in Salmonella is modulated under certain environmental conditions. Curr Microbiol 67:279–285. https://doi.org/10.1007/s00284-013-0361-2
Zhang Y, Gao J, Wang L, Liu S, Bai Z, Zhuang X et al (2018) Environmental adaptability and quorum sensing: iron uptake regulation during biofilm formation by Paracoccus denitrificans. Appl Environ Microbiol 84:00865–00918. https://doi.org/10.1128/aem.00865-18
Javvadi S, Pandey SS, Mishra A, Pradhan BB, Chatterjee S (2018) Bacterial cyclic β-(1,2)-glucans sequester iron to protect against iron-induced toxicity. EMBO Rep 19:172–186. https://doi.org/10.15252/embr.201744650
Hosseinidoust Z, Tufenkji N, van de Ven TG (2013) Predation in homogeneous and heterogeneous phage environments affects virulence determinants of Pseudomonas aeruginosa. Appl Environ Microbiol 79:2862–2871. https://doi.org/10.1128/AEM.03817-12
Dye KJ, Vogelaar NJ, O’Hara M, Sobrado P, Santos W, Carlier PR et al (2022) Discovery of two inhibitors of the type IV pilus assembly ATPase PilB as potential antivirulence compounds. Microbiol Spectr 10:e0387722. https://doi.org/10.1128/spectrum.03877-22
Li D, Shibata Y, Takeshita T, Yamashita Y (2014) A novel gene involved in the survival of Streptococcus mutans under stress conditions. Appl Environ Microbiol 80:97–103. https://doi.org/10.1128/aem.02549-13
Su HZ, Wu L, Qi YH, Liu GF, Lu GT, Tang JL (2016) Characterization of the GntR family regulator HpaR1 of the crucifer black rot pathogen Xanthomonas campestris pathovar campestris. Sci Rep 6:19862. https://doi.org/10.1038/srep19862
Li J, Wang N (2011) The wxacO gene of Xanthomonas citri ssp. citri encodes a protein with a role in lipopolysaccharide biosynthesis, biofilm formation, stress tolerance and virulence. Mol Plant Pathol 12:381–396. https://doi.org/10.1111/j.1364-3703.2010.00681.x
Antar A, Lee MA, Yoo Y, Cho MH, Lee SW (2020) PXO_RS20535, encoding a novel response regulator, is required for chemotactic motility, biofilm formation, and tolerance to oxidative stress in Xanthomonas oryzae pv. oryzae. Pathogens 9:956. https://doi.org/10.3390/pathogens9110956
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Tomada S, Puopolo G, Perazzolli M, Musetti R, Loi N, Pertot I (2016) Pea broth enhances the biocontrol efficacy of Lysobacter capsici AZ78 by triggering cell motility associated with biogenesis of type IV pilus. Front Microbiol 7:1136. https://doi.org/10.3389/fmicb.2016.01136
Pawlik KJ, Zelkowski M, Biernacki M, Litwinska K, Jaworski P, Kotowska M (2021) GntR-like SCO3932 protein provides a link between actinomycete integrative and conjugative elements and secondary metabolism. Int J Mol Sci 22:11867. https://doi.org/10.3390/ijms222111867
Daddaoua A, Corral-Lugo A, Ramos JL, Krell T (2017) Identification of GntR as regulator of the glucose metabolism in Pseudomonas aeruginosa. Environ Microbiol 19:3721–3733. https://doi.org/10.1111/1462-2920.13871
Mielich-Süss B, Lopez D (2015) Molecular mechanisms involved in Bacillus subtilis biofilm formation. Environ Microbiol 17:555–565. https://doi.org/10.1111/1462-2920.12527
Zhou T, Nan B (2017) Exopolysaccharides promote Myxococcus xanthus social motility by inhibiting cellular reversals. Mol Microbiol 103:729–743. https://doi.org/10.1111/mmi.13585
Martín-Rodríguez AJ, Villion K, Yilmaz-Turan S, Vilaplana F, Sjöling A, Römling U (2021) Regulation of colony morphology and biofilm formation in Shewanella algae. Microb Biotechnol 14:1183–1200. https://doi.org/10.1111/1751-7915.13788
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.MB-0011-2014
Palmer SR, Ren Z, Hwang G, Liu Y, Combs A, Söderström B et al (2019) Streptococcus mutans yidC1 and yidC2 impact cell envelope biogenesis, the biofilm matrix, and biofilm biophysical properties. J Bacteriol 201:e00396-e418. https://doi.org/10.1128/jb.00396-18
Sorroche F, Bogino P, Russo DM, Zorreguieta A, Nievas F, Morales GM et al (2018) Cell Autoaggregation, biofilm formation, and plant attachment in a Sinorhizobium meliloti lpsB mutant. Mol Plant Microbe Interact 31:1075–1082. https://doi.org/10.1094/mpmi-01-18-0004-r
Bradfield MF, Nicol W (2016) The pentose phosphate pathway leads to enhanced succinic acid flux in biofilms of wild-type Actinobacillus succinogenes. Appl Microbiol Biotechnol 100:9641–9652. https://doi.org/10.1007/s00253-016-7763-6
Li C, Hurley A, Hu W, Warrick JW, Lozano GL, Ayuso JM et al (2021) Social motility of biofilm-like microcolonies in a gliding bacterium. Nat Commun 12:5700. https://doi.org/10.1038/s41467-021-25408-7
Patra P, Kissoon K, Cornejo I, Kaplan HB, Igoshin OA (2016) Colony expansion of socially motile Myxococcus xanthus cells is driven by growth, motility, and exopolysaccharide production. PLoS Comput Biol 12:e1005010. https://doi.org/10.1371/journal.pcbi.1005010
Wang S, Yu S, Zhang Z, Wei Q, Yan L, Ai G et al (2014) Coordination of swarming motility, biosurfactant synthesis, and biofilm matrix exopolysaccharide production in Pseudomonas aeruginosa. Appl Environ Microbiol 80:6724–6732. https://doi.org/10.1128/aem.01237-14
Gao T, Ding M, Yang CH, Fan H, Chai Y, Li Y (2019) The phosphotransferase system gene ptsH plays an important role in MnSOD production, biofilm formation, swarming motility, and root colonization in Bacillus cereus 905. Res Microbiol 170:86–96. https://doi.org/10.1016/j.resmic.2018.10.002
Masmoudi F, Abdelmalek N, Tounsi S, Dunlap CA, Trigui M (2019) Abiotic stress resistance, plant growth promotion and antifungal potential of halotolerant bacteria from a Tunisian solar saltern. Microbiol Res 229:126331. https://doi.org/10.1016/j.micres.2019.126331
Morcillo RJL, Manzanera M (2021) The effects of plant-associated bacterial exopolysaccharides on plant abiotic stress tolerance. Metabolites 11:337. https://doi.org/10.3390/metabo11060337
Pérez-Burgos M, Søgaard-Andersen L (2020) Biosynthesis and function of cell-surface polysaccharides in the social bacterium Myxococcus xanthus. Biol Chem 401:1375–1387. https://doi.org/10.1515/hsz-2020-0217
Liu GF, Wang XX, Su HZ, Lu GT (2021) Progress on the GntR family transcription regulators in bacteria. Yi Chuan 43:66–73. https://doi.org/10.16288/j.yczz.20-245
Abdalla AK, Ayyash MM, Olaimat AN, Osaili TM, Al-Nabulsi AA, Shah NP et al (2021) Exopolysaccharides as antimicrobial agents: mechanism and spectrum of activity. Front Microbiol 12:664395. https://doi.org/10.3389/fmicb.2021.664395
Funding
This work was supported by Outstanding Youth Foundation of Shandong Province [Grant Number ZR2021YQ20], the Shandong Modern Agricultural Industry Technology System [Grant Number SDAIT-04-08], Key Research and Development Program of Shandong Province [Grant Number 2019JZZY020608], Weifang Tobacco Company Science and Technology Project [Grant Number 2022-36].
Author information
Authors and Affiliations
Contributions
The study was conceived by JW, the majority of the experiments were conducted by JW and DZ, and some were assisted by HW, the data were analyzed by XZ, DZ, CH and AL drafted the manuscript. Before submission, all authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
There are no human subjects or animals used in this article.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, D., Wang, J., Wang, H. et al. The Transcription Regulator GntR/HutC Regulates Biofilm Formation, Motility and Stress Tolerance in Lysobacter capsici X2-3. Curr Microbiol 80, 281 (2023). https://doi.org/10.1007/s00284-023-03390-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03390-1