Abstract
This study aimed to investigate the prevalence of Methicillin- and Vancomycin-Resistant Staphylococcus aureus (MRSA, VRSA) and Vancomycin-Resistant Enterococcus (VRE) of hospital food samples in Mashhad, Iran. A total of 357 hospital food samples were collected from 13 hospitals. Enterococcus spp. and Staphylococcus aureus were identified using conventional cultural techniques following genotypic confirmation by PCR. The antibiotic resistance patterns of MRSA, VRSA, and VRE strains were analyzed using the disk diffusion methods. The prevalence of S. aureus and MRSA were 24.37% (87/357) and 22.98% (20.87), respectively. In addition, the vanB gene involved in vancomycin resistance was detected in 1.14% of the S. aureus strains. Enterococci and VRE had a prevalence of 15.4% (55/357) and 21.81% (12/55), respectively. Meat, chicken barbecues, and salad were the most commonly contaminated samples with S. aureus, MRSA, Enterococci, and VRE. PCR detected two vancomycin resistance genes, including vanA (1.81%, 1.55) and vanC2 (20%, 11.55) genes. MRSA strains revealed the highest resistance against penicillin, erythromycin, clindamycin, azithromycin, tetracycline, and gentamicin. The VRSA isolates were resistant to penicillin, ampicillin, oxacillin, cefoxitin, clindamycin, erythromycin, gentamicin, and trimethoprim–sulfamethoxazole. Furthermore, VRE isolates exhibited the highest resistance against quinupristin-dalfopristin, erythromycin, and tetracycline. The results of this study indicated that hospital foods might act as a reservoir of Enterococci spp. and S. aureus strains, which can transfer antibiotic resistance. Moreover, multidrug resistance (MDR) in some MRSA, VRSA, and VRE isolates represents a serious threat to susceptible persons in hospitals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preparing and providing healthy, nutritious meals for patients is essential in healthcare settings [1,2,3]. The majority of hospitalized patients suffer from a suppressed immune system are more vulnerable to infection and complications. Thus, hospital foods should have excellent microbial quality [2,3,4]. Bacteria are significantly responsible for food-borne diseases in hospitals among all pathogenic agents [3]. In this regard, Staphylococcus aureus (S. aureus) is considered the leading prevalent cause of food-borne outbreaks in hospitals [3, 4]. S. aureus is a gram-positive coccus commonly colonized on the skin and in the respiratory system of healthy humans [3,4,5]. Moreover, Enterococci is another Gram-positive bacterium, which causes food-borne diseases in hospital settings [6], and its species can typically be found in the human intestines [5]. The above-mentioned two microorganisms are potentially opportunistic and problematic pathogens, which have been emerged as the prime causes of healthcare-associated infections, particularly bloodstream infections and associated with the emergence of drug-resistant strains [5]. Their antibiotic-resistant strains have higher pathogenicity and cause more severe diseases than their antibiotic-susceptible strains [3, 4]. The methicillin and vancomycin resistance strains of these two bacteria have attracted worldwide concern as major phenotypes of resistant bacteria [7]. S. aureus isolates recovered from food samples and clinical infections harbored a high prevalence of resistance against routinely applied antibiotics, especially methicillin [3, 4]. Furthermore, documented data have shown that around 50–70% of S. aureus strains obtained from the hospital environment were methicillin resistant [4]. Methicillin-resistant S. aureus (MRSA) strains are typically resistant to aminoglycosides, macrolides, lincosamides, and all classical β-lactam antimicrobial drugs [8]. In addition, recent reports about vancomycin-resistant Enterococci (VRE) have represented the high incidence of resistance to commonly used groups of antimicrobial agents, especially glycopeptides, β-lactams, lincosamides, streptogramins, and aminoglycosides [9]. Meanwhile, vancomycin-resistant S. aureus (VRSA) has emerged as a food safety and medical concern [10]. The morbidity and mortality rates of antibiotic-resistant organisms have recently increased among hospitalized patients [11].
Accordingly, consuming contaminated food with multidrug-resistant pathogens poses a high risk for patients, who are more vulnerable to infection and its complications [12]. Given the lack of food epidemiological surveys on the prevalence of these microorganisms in hospital foods, this study aimed to assess the prevalence rate and antibiogram-resistant patterns of MRSA, VRSA, and VRE isolates recovered from various types of cooked and ready-to-eat food samples from hospitals in Mashhad, Iran.
Materials and Methods
Sample Collection
A total of 357 hospital food samples (321 cooked foods and 36 ready-to-eat salads) were aseptically collected from 13 hospitals in Mashhad city, Iran, from August 2019 to January 2020. The collected samples were immediately transferred to the laboratory of the Nutrition Department, Faculty of Medicine, University of Medical Sciences, Mashhad, Iran, in a cooler with ice packs and examined within 1–2 h. The samples included meat barbecue (n = 36), chicken barbecue (n = 36), cooked meat (n = 36), cooked chicken (n = 36), fried fish (n = 33), cooked rice (n = 36), dill rice (n = 36), mixed or bean rice (n = 72, mixed rice cooked with either red meat, poultry, vegetables, and/or beans), soup (n = 36), and salad (n = 36).
Enumeration, Isolation, and Identification of S. aureus and Enterococci
About 10 g of each sample was placed into sterile plastic bags. Then, 90 mL of sterile buffered peptone water 0.1% (w/v) (BPW, Merck, Germany) was added to the samples, and the mixture was thoroughly homogenized. Then, ten-fold serial dilutions of the homogenates were prepared, and the spread plate technique was employed to enumerate S. aureus and Enterococci. A total of 0.1 mL of each dilution (10−1 to 10−4) was inoculated onto the Baird-Parker agar (BPA, Merck, Germany) plates, supplemented with 1% potassium tellurite solution and 5% egg yolk emulsion in terms of S. aureus enumeration. The inoculums were evenly smeared using a sterile L-shaped glass rod. The inoculated plates were then kept in an incubator at 37 °C for 24 h. The obtained colonies were enumerated, and S. aureus was verified by a DNase test agar and a tube coagulase test. Then, 0.1 mL of each dilution (10−1 to 10−4) was inoculated onto KF-Streptococcus agar (KF, Conda, Spain) media, supplemented with 0.1 g/L 2,3,5-triphenyl tetrazolium chloride (TTC, Merck, Germany), and then incubated at 37 °C for 24–48 h in terms of Enterococci spp. Enumeration. Typical colonies of Enterococci spp. were carefully counted on KF plates after incubation, and Enterococci colonies were verified using catalase and esculin hydrolysis tests.
At this stage, 10 g of each sample was homogenized for isolation of S. aureus. Then, 90 mL of Trypticase Soy Broth (TSB, Merck, Germany) and 10% w/v sodium chloride (NaCl, Merck, Germany) were added to the homogenized samples, and the mixture was incubated for 18–24 h at 37° C. Following enrichment, a loopful of these broth cultures were streaked onto selective agar media (BP agar containing egg yolk tellurite emulsion (50 mL/L)) and incubated at 37 °C for 24 h.
About 1 g of homogenized food samples was added to test tubes containing 9 mL of Azide Dextrose broth and 6.5% w/v sodium chloride (NaCl, Merck, Germany) for isolation of Enterococci spp. Then, the mixture was mixed in a vortex mixer for one minute. In the next stage, the tubes were incubated for 24 h at 37 °C. One loopful of each enriched sample in broth was streaked onto KF agar containing triphenyl tetrazolium chloride (0.1 g/L) after incubation, and the plates were then incubated aerobically at 37 °C for 24–48 h.
Typical morphology of this bacteria (i.e., small, circular, convex, gray to black colonies, and with or without opaque halo) was used to identify S. aureus, colonies based on gram staining, catalase reaction (using 0.3% hydrogen peroxide), coagulase test (rabbit plasma), oxidase test, mannitol fermentation on Mannitol salt agar (Conda, Spain), deoxyribonuclease (DNase, Conda, Spain) test, and carbohydrate (xylose, sucrose, maltose, trehalose, and lactose) fermentation tests.
Gram staining, catalase activity, esculin hydrolysis on bile esculin azide agar (Quelab, Canada), and carbohydrate (arabinose, lactose, sorbose, mannitol, and sorbitol) fermentation tests were conducted on suspected colonies to identify Enterococci spp. These suspected colonies are observed in red, pink, or dark red and with variations in diameter from 0.3 to 2 mm. All confirmed pure colonies were kept frozen at − 20 °C in Brain Heart Infusion (BHI, Merck, Germany) broth containing 15% (v/v) glycerol (Merck, Germany) until molecular and antibiotic susceptibility testing. S. aureus ATCC 25923 and Enterococcus faecalis ATCC 29,212 strains were used as control organisms.
Phenotypic Identification of MRSA, VRSA, and VRE Isolates
MRSA strains were detected by cefoxitin (30 μg) and oxacillin (1 μg) susceptibility tests after isolation. Isolates with inhibition zone size ≤ 21 mm and cefoxitin ≤ 10 mm with oxacillin were considered resistant. Moreover, isolates with inhibition zone size ≤ 9 mm around a 30 μg disk of vancomycin were classified as VRSA. VRE strains were detected by testing with vancomycin disk with an inhibition zone ≤ 14 mm. All tests were carried out on these isolates using the Kirby–Bauer disk diffusion technique and interpreted based on the Clinical Laboratories Standards Institutes guidelines (CLSI).
DNA Extraction
A genomic DNA isolation kit (DENAzist, Iran) was used to extract chromosomal DNA from the bacterial cells. Then, the quality (A260/A280) and concentration of extracted DNA were quantified using a NanoDrop 2000 spectrophotometer (NanoDrop, Thermo Scientific, USA).
Detection of Antibiotic Resistance Genes by PCR Assay
All phenotypically identified bacterial isolates were genotyped using the PCR-based detection of their specified genes (MRSA, VRSA, and VRE strains) with specific oligonucleotide primers. Supplementary Table 1 displays the PCR primers and products used in this study (Gen Bank Accession Number NC_003923M, 293 bp). The primer sets and PCR cycling protocols used in the PCRs were selected from earlier reports (Supplementary Table 1). All PCR reactions were applied in a thermocycler (T100, Bio-Rad, USA). The PCR Thermal program used in this study is represented in Supplementary Table 2. Oligonucleotides were manufactured by Pishgam Company (Tehran, Iran), and the amplified products were visualized under UV light in a gel documentation imaging system (Quantum ST4, Vilber Louma, Germany). In this molecular assay, we used the positive controls (ATCC 25923 and ATCC 29212) and positive clinical cultures (MRSA- or VRE-positive clinical cultures) of specimens obtained from hospitalized patients at Imam Reza hospital, Mashhad, Iran for positive controls. Moreover, each run included a negative control, containing reaction mixtures lacking DNA templates.
Antimicrobial Susceptibility Pattern of MRSA, VRSA, and VRE Isolates
Antimicrobial susceptibility patterns of all isolates, identified as resistant, were studied by the simple disk diffusion technique (Kirby–Bauer) on Mueller–Hinton agar (MHA, Quelab, Canada) using disk containing antibiotics (Cyprus, Belgium, and Padtan Teb, Iran), recommended by CLSI. Suspensions equivalent to 0.5 McFarlands were inoculated on the surface of MHA plates and then incubated for up to 24 h at 37 °C. Following this period, the growth inhibition (halo) diameters around each disk were measured and interpreted according to the CLSI criteria. Enterococci faecalis ATCC 29212 served as a quality control strain for all susceptibility tests. All MRSA isolates were examined for susceptibility to penicillin (10 U), gentamicin (10 μg/disk), amikacin (30 μg/disk), kanamycin (30 μg/disk), azithromycin (15 μg/disk), erythromycin (15 μg/disk), tetracycline (30 μg/disk), doxycycline (30 μg/disk), ciprofloxacin (5 μg/disk), levofloxacin (5 μg/disk), clindamycin (2 μg/disk), trimethoprim–sulfamethoxazole (25 μg/disk), chloramphenicol (30 μg/disk), rifampin (5 μg/disk), and vancomycin (30 μg).
In addition, cefoxitin (30 μg/disk), oxacillin (1 μg/disk), penicillin (10 U), ampicillin (10 μg/disk), gentamicin (10 μg/disk), erythromycin (15 μg/disk), clindamycin (2 μg/disk), trimethoprim–sulfamethoxazole (25 μg/disk), chloramphenicol (30 μg/disk), quinupristin–dalfopristin (15 μg/disk), and teicoplanin (30 μg/disk) were tested for VRSA isolates.
Moreover, the susceptibility of VRE isolates to penicillin (10 U), ampicillin (10 μg/disk), erythromycin (15 μg/disk), tetracycline (30 μg/disk), doxycycline (30 μg/disk), ciprofloxacin (5 μg/disk), levofloxacin (5 μg/disk), rifampin (5 μg/disk), quinupristin-dalfopristin (15 μg/disk), and teicoplanin (30 μg/disk) was assessed.
Statistical Analysis
The data were analyzed based on descriptive statistical analysis with Excel software (version 2019) and SPSS software (Version 18.0; IBM, Armonk, USA). The data are expressed as means ± standard deviation (SD) with two repetitions.
Results
Enumeration of S. aureus and Enterococci
Table 1 shows the frequency of food samples contaminated above Iranian standards (> 102). As shown in Table 1, 8.12% (29/357) and 9.52% (34/357) of food samples had an unacceptable level of S. aureus and Enterococci, respectively, compared to the Iranian standard level. Moreover, the salad had the highest frequency of over-contamination for S. aureus with 19.44% (7/36) and Enterococci with 63.88% (23/36).
Prevalence of S. aureus, MRSA, and VRSA Strains
Based on our findings, S. aureus was isolated from 24.37% of the total analyzed samples (87 out of 357). The percentage of S. aureus, MRSA, and VRSA is shown in Supplementary Fig. 1 for various hospital food samples. In this figure, the meat barbecue showed the highest frequency of S. aureus (33.33%, 12/36), followed by the chicken barbecue (30.55%, 11/36) and salad (30.55%, 11.36). In contrast, dill rice and cooked meat samples presented the lowest frequency (16.66%) after cooked rice (19.44%).
The antibiotic susceptibility test (phenotypic assay) showed 20 isolates (22.98%) resistant to methicillin classified as MSRA from 87 S. aureus isolates recovered from various types of hospital food samples. The PCR assay confirmed these 20 isolates as MRSA by detecting the mecA gene. As shown in Supplementary Fig. 1, the frequencies of MRSA-positive samples were 2.77 (1/36), 2.77 (1/36), 2.77 (1/36), 2.77 (1/36), 5.55 (2/36), 6.06 (2/33), 8.33 (3/36), 11.11 (4/36), and 13.88% (5/36) for dill rice, cooked rice, cooked meat, cooked chicken, soup, fried fish, salad, meat barbecue, and chicken barbecue, respectively. The highest percentage of MRSA contamination was found in chicken and meat barbecues. In comparison, cooked chicken samples had the lowest prevalence of MRSA. Furthermore, the mecA gene was not detected in mixed rice (bean rice) samples.
According to the results of the vancomycin susceptibility test, all 87 isolates of S. aureus were susceptible to vancomycin. However, PCR results found that one isolate of S. aureus (1.14%) carried the vanB gene, obtained from meat barbecue and classified as VRSA, regardless of the vancomycin disk diffusion test results. Generally, 98.85% of the samples were negative for VRSA test.
Prevalence of Enterococci and VRE Strains
According to the results, Enterococci spp. were recovered from 15.40% of the total collected samples (55/357). The percentage of Enterococci and VRE is represented in Supplementary Fig. 2 for various hospital food samples. As shown in this figure, RTE salad exhibited the highest prevalence of Enterococci spp. (80.55%, 29/36) after chicken barbecue (30.55%, 11/36). In contrast, the lowest frequency was fried fish samples (3.03%, 1/36), and the cooked rice and cooked chicken samples were all negative.
According to the vancomycin disk diffusion method, 7.27% (4/55) of Enterococci isolates were VRE, including 1.81% of Enterococci isolates (1/55) exhibited complete resistance to vancomycin (zone diameter ≤ 14 mm). In comparison, 5.45% of Enterococci isolates (3/55) showed an intermediate resistance pattern to vancomycin (zone diameter 15–16 mm). Complete resistance to vancomycin was detected in 2.77% (1 of 36) of isolates obtained from chicken barbecue samples. In addition, the intermediate resistance to vancomycin was observed in 2.77% (1 of 36) and 5.55% (2 of 36) of isolates obtained from soup and RTE salad samples. The results of PCR assays indicated 21.81% (12/55) positive isolates for van genes (vanA and vanC2/3), which are classified as VRE. VanC2/3 (91.66%, 11/12) was the most frequently detected resistance gene among detected resistance genes. The vanA gene had the lowest prevalence (8.33%, 1/12) among detected resistance genes. In addition, there were no positive results for vanB and vanC1 genes, and the same isolate, which showed complete resistance to vancomycin, was positive for the vanA gene. Moreover, all 3 VRE isolates, which exhibited intermediate resistance to vancomycin, were positive for the vanC2/3 gene. According to Supplementary Fig. 2, RTE salad samples presented the highest prevalence of VRE-positive samples (22.22%, 8/36) after chicken barbecue (5.55%, 2/36), meat barbecue (2.77%, 1/36), and soup (2.77%, 1/36).
Antibiotic Resistance Patterns of MRSA
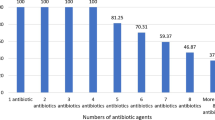
Supplementary Fig. 3 displays the antibiotic resistance patterns of MRSA isolated from food samples. The results indicated that MRSA strains harbored the highest prevalence of resistance toward antibiotic agents, including penicillin (100%), erythromycin (90%), clindamycin (90%), azithromycin (90%), tetracycline (75%), gentamicin (75%), doxycycline (65%), and trimethoprim–sulfamethoxazole (50%). On the contrary, MRSA strains harbored the lowest prevalence of resistance toward antibiotics, such as levofloxacin (5%), kanamycin (10%), chloramphenicol (10%), ciprofloxacin (40%), and amikacin (40%) antibiotic. On the other hand, all of the MRSA isolates were sensitive to rifampin (100%) (Supplementary Fig. 3).
Antibiotic Resistance Patterns of VRSA
Supplementary Fig. 4 illustrates the patterns of antibiotic resistance of VRSA isolated from food samples. Antibiogram results revealed that the isolated VRSAs were 100% resistant to ampicillin, oxacillin, cefoxitin, erythromycin, clindamycin, gentamicin, and trimethoprim– sulfamethoxazole and 100% sensitive to teicoplanin and quinupristin-dalfopristin (Supplementary Fig. 4).
Antibiotic Resistance Patterns of VRE
Supplementary Fig. 5 shows the patterns of antibiotic resistance of VRE isolated from food samples. According to these findings, VRE strains showed a high prevalence of resistance to quinupristin–dalfopristin (100%), erythromycin (83.33%), tetracycline (83.33%), doxycycline (66.66%), and rifampin (66.66%) antibiotic agents. Meanwhile, a low prevalence of resistance was observed against ampicillin (8.33%), teicoplanin (8.33%), levofloxacin (8.33%), penicillin (25%), and ciprofloxacin (33.33%) antibiotic agents.
Discussion
Antibiotic resistance is now a global concern due to the indiscriminate use of antibiotics in agriculture, the environment, veterinary medicine, and medicine. Antibiotic-resistant isolates may be able to transmit resistance genes to bacteria in the human body [13]. Therefore, hospital meals should have an acceptable level of health quality due to the weak immune system of hospitalized patients [2, 3, 14]. Thus, this study dictated the prevalence and antibiotic susceptibility pattern of S. aureus and its resistant isolates (MRSA and VRSA), as well as Enterococci spp., and its resistant isolate (namely VRE) among hospital food samples.
The results of enumeration of S. aureus in hospital food samples showed that 8.12% (29/357) of the food samples had contamination above the allowable level according to Iranian national standards. The contamination between 102 to 103 and 103 to 104 CFU/g was observed in 6.54% (21/321) and 0.31% (1/321) of all cooked food samples. In addition, 19.44% (7/36) of salad samples had contamination between 102 and 103 CFU/g. The microbial count results of a study [15] in turkey showed that 2.4% (13/530) out of 530 hospital food samples were infected with S. aureus, above the Turkish national standard. Moreover, 2.1% of cooked food samples had contamination between 102 and 103 CFU/g, and 11.4% of salad samples were contaminated with 103 to 104 CFU/g. No contamination of this bacterium was observed in soup and cooked rice samples. However, cooked rice and soup samples were positive in terms of count.
Our results showed that S. aureus prevalence in different hospital foods was 24.37% (87/357). In a study performed in Isfahan in 2018, 47 out of 457 hospital food samples (10.28%) were contaminated with S. aureus, higher than our results [3]. Another survey carried out in Tehran in 2013 showed that two samples out of 44 hospital food samples (4.54%) were contaminated with S. aureus, which was lower than our results [16]. The prevalence of this bacterium was reported from 20 to 25% in Iran in food samples of places, such as restaurants and fast foods [17, 18]. These differences could be due to sample size and type, as well as hygienic conditions in the hospital kitchen, personal staff hygiene, diagnostic methods, and geographical area [19]. There are several reasons for the high prevalence of S. aureus in hospital food samples, such as nurses and hospital staff given that S. aureus is one of the most common microorganisms, which is colonized in the nasal cavity and on the body surface, approximately 50% of the human population carries S. aureus [20]. Moreover, other factors, including low quality and inaccurate washing of raw materials, insufficient time and temperature for cooking and storing food (especially meat), use of contaminated equipment for cooking, and lack of personal hygiene, reduce hospital food quality [2, 3].
In the present study, the meat barbecue sample had the highest contamination rate (13.79%, 12.87), followed by a chicken barbecue and salad samples (12.64% for each, 11.87). In a study conducted in Esfahan in 2017 showed that the highest infection of S. aureus was observed in meat barbecue, followed by a chicken barbecue and salad samples, which was consistent with our result [4]. Similarly, Ranjbar et al. (2017b) found that the most contamination rate was reported in chicken and meat barbecue samples [17]. According to the study, beef and poultry meat are the primary sources of S. aureus pathogens in these samples [3, 21]. In addition, low cooking temperature and time for meat would be the second reason [17].
The findings of the present study showed that the lowest level of contamination was in the samples of vegetable dill rice (6.89%, 6/87), cooked meat (6.89%, 6/87), and cooked rice (8.04%, 7/87), which could be due to their preparation conditions at higher temperatures and times to kill the pathogenic bacteria [2, 3].
Moreover, 22.98% (20/87) of S. aureus isolates were classified as MRSA, harboring the mecA gene. In addition, the MRSA prevalence in the tested food samples was 5.60% (20.357). In a study performed in Tehran in 2013, 1 sample out of 44 hospital food samples (2.27%) was MRSA positive [16]. Another author in 2017 in the United States demonstrated that 29 out of 910 hospital food samples (3.2%) were MRSA positive [22]. Unlike the present study, the prevalence of MRSA strains in these two mentioned surveys was lower than our results. According to recent study about the prevalence of MRSA in food products in other settings, five out of the 120 utensil samples collected from hotels and non-hotel establishments were positive for the mecA gene. At the same time, no positive mecC gene were found [23]. In another study in Isfahan, Iran, 83 out of 119 (14.31%) restaurant food samples were positive for the mecA gene. The prevalence of MRSA strains in this study was higher than our study [17]. In addition, a higher prevalence of MRSA has been reported in various studies in other parts of Iran as much as 22.65% [18] and 37.4% [24]. The differences between their results and ours could be explained by the fact that in their studies, MRSA strains were isolated from processed (packaged hamburgers) [18] as well as raw (beef, chicken, and turkey) [24] food samples. In the present study, chicken barbecue samples (25%, 5/20), meat barbecue (20%, 4/20), and salad (15%, 3/20) samples had the highest presence of MRSA strains, respectively. Contrary to our results, a study conducted on hospital foods in Brazil and reported the highest rates of MRSA contamination in fish foods [21]. Moreover, an investigation which performed in 2017 reported that all isolates of S. aureus obtained from hospital food samples were resistant to methicillin, and the highest prevalence of MRSA strains was in meat barbecue samples (16.12%, 5/31), chicken barbecue (8.53%, 7/82), and salad (7.14%, 4/56), which were lower than our findings [4]. The high prevalence of MRSA in chicken barbecue samples may be due to the high use of human-based antibiotics (especially methicillin) in Iranian poultry husbandry as a therapeutic solution, as well as growth stimulants, which kill other bacterial competitors. Thus, S. aureus can grow and multiply more efficiently [17].
The results of the disk diffusion method showed that all MRSA isolates were resistant to penicillin antibiotics, which was consistent with Dehkordi et al. (2017) [4], Rahimi et al. (2019) [24], and Jackson et al. (2013) [25]. The high frequency of penicillin resistance in food samples may indicate the indiscriminate use of ampicillin in treating livestock and poultry infections [24]. 90% of MRSA isolates were resistant to erythromycin, which is similar to the results of Dehkordi et al. (2017) [4] and Rahimi et al. (2019) [24]. The high frequency of erythromycin resistance may reflect the widespread use of erythromycin and spiramycin antibiotics as a growth promoter in livestock and poultry feed in Iran [24]. In our findings, the resistance to clindamycin was higher than other studies (90%). In Rahimi et al. (2019) [24], 73% of MRSA isolates were resistant to clindamycin. MRSA strains isolated from the food samples of our study had high resistance to human antibiotics, such as trimethoprim–sulfamethoxazole, ciprofloxacin, and azithromycin, which can indirectly confirm the human origin of these resistant bacteria [17]. Conversely, another study indicated that all S. aureus isolates from 120 hotel and non-hotel samples (i.e., serving utensils) were sensitive to ciprofloxacin [23]. The resistance to trimethoprim–sulfamethoxazole in our study was 50%, which was consistent to studies which were conducted in Iran [3, 24]. MRSA strains were resistant to azithromycin, which was in line with Ranjbar et al. (2017b) [17], and Dehkordi et al. (2017) [4] reported that 71.8% and 59.45% of isolates were resistant to this antibiotic. Azithromycin and ciprofloxacin are human antibiotics and are not common in veterinary medicine. The high prevalence of resistance to these antibiotics may indicate cross-contamination through infected individuals and staff [3].
According to the present study, 75% of MRSA isolates were resistant to tetracycline. These results are consistent with those of Ranjbar et al. (2017b) [17] and Rahimi et al. (2019) [24] in Iran, who found that MRSA strains isolated from food samples were 92.77% and 65% resistant to tetracycline, respectively. A significant amount of antibiotics, such as tetracyclines and quinolones, are found in the soil due to the use of organic fertilizers in farms and irrigation with wastewater or treated water, which can increase the presence of these antibiotics in the food chain [12]. In our study, the resistance of MRSA strains to chloramphenicol was 10%, which was consistent with previous study conducted in China [26]. Additionally, all S. aureus isolates (100%) from 120 hotel and non-hotel samples (e.g., serving utensils) were chloramphenicol resistant [23]. The use of chloramphenicol in veterinary medicine is prohibited, and the high prevalence of resistance to chloramphenicol has been attributed to its illegal use for treatment, especially in poultry farms [17].
None of the S. aureus isolates were categorized as VRSA based on the disk diffusion method, and none of the S. aureus isolates were categorized as VRSA. The PCR results elucidated that one isolate of S. aureus (1.15%, 1.87) was classified as VRSA due to having the vanB gene. Furthermore, this isolate was also resistant to methicillin (MRSA). Another investigator in Shiraz in 2014 [27] showed that six strains of S. aureus, which had the vanA gene, did not show resistance in phenotypic tests (vancomycin disk diffusion test, vancomycin agar screen, and minimum inhibitory concentration of vancomycin). However, another study identified 2 of 85 isolates of S. aureus isolated from minced meat as MRSA strains using the vancomycin-resistant disk diffusion method. However, PCR results found no vanA and vanB genes in these isolates [19], indicating that phenotypic methods, such as disk diffusion, and screen agar may be subject to errors and in vitro mutations. Therefore, the use of more standard phenotypic methods, E-test antibiotic strips, and molecular methods, such as PCR for resistance gene tracing, is necessary to confirm 100% of VRSA strains [28]. Moreover, a recent author revealed that one isolate of S. aureus (7.69%) contained VRSA genes, such as vanA, vanB, and vanC2/3, but not vanC1. The vanB gene was observed in VRSA isolates, which was in accordance with our results. Moreover, isolates, identified as MRSA, were also VRSA [29]. Additionally, another author has shown that four of the five vanC-positive isolates were positive for the mecA gene, and MRSA and VRSA are a threat to public health in food processing environments [23].
In the present study, VRSA isolates were resistant to the antibiotics, like penicillin, ampicillin, clindamycin, erythromycin, trimethoprim, sulfamethoxazole, gentamicin, cefoxitin, and oxacillin. Additionally, they were sensitive to teicoplanin, quinupristin–dalfopristin, and vancomycin. Similar to our study, in a study carried out in Brazil [29] reported VRSA isolates resistant to the antibiotics, like penicillin, oxacillin, and clindamycin. However, unlike our study, all isolates were sensitive to gentamicin and resistant to teicoplanin. Saadat et al. [27] showed that VRSA isolates were sensitive to the antibiotic quinupristin–dalfopristin and resistant to the ampicillin antibiotic, which was consistent with our study.
According to Iranian national standards, the Enterococci count showed that 9.52% (34/357) of the hospital food samples had contamination above the permissible level. Moreover, 63.88% (23/36) of salad samples had more than 102 bacteria per gram, which was in line with the result of another study [30].
The presence of Enterococci in food is a controversial and worrying issue because this bacterium is an indicator of fecal contamination in food [31]. This study showed that 15.40% (55/357) of all hospital food samples were infected with Enterococci. Unlike the present study, in another survey performed in Tehran in 2013 none of the 44 food samples were infected with Enterococci [16]. In our study, salad samples (55.72%, 29/55) had the highest contamination rate with Enterococci. In addition, the prevalence of Enterococci in salad samples in the present study was 80.55% (29/36). In a recent study in Tehran in 2012 [32], salads had the highest prevalence of Enterococci infection (29.17%, 7/24), which was lower than our results. Vegetables and salads can be a source of contamination of intestinal bacteria, such as Enterococci because of lack of proper washing and raw consumption [30, 33]. Secondary food contamination is another most important cause of infection due to improper food preparation [34]. On the other hand, Enterococci can grow under a wide range of environmental conditions (temperature, salt, and pH) considering survival factors in cooked foods [33, 35].
In the present study, PCRs showed that 21.81% (12/55) of Enterococci species isolated from hospital food samples had van genes, which were identified as VRE. In addition, the prevalence of VRE in all hospital food samples was 3.36% (12/357). Unlike our study, in an investigation which was performed in the United States in 2017, the prevalence of VRE isolates was 2.4% (22/910) in hospital food samples, which was lower than ours [22]. A higher prevalence of VRE was reported by Novise et al. (2005), who found that 57.31% of Enterococci isolated from poultry meat (47/82) were vancomycin resistant [36].
The emergence of antibiotic-resistant Enterococci in food samples is due to the widespread use of antibiotics in agriculture and veterinary medicine [37]. Crops may also be contaminated by environmental sources, such as human and animal feces [38]. Enterococci can adapt to the environment, which are resistant to widely used antibiotics, such as tetracycline, lincomycin, erythromycin, and compounds used as growth stimulants (AGPs) in veterinary medicine [39, 40]. In the present study, all VRE isolates were resistant to quinupristin–dalfopristin. According to Raafat et al. (2016) [9], 100% of VRE isolates were resistant to quinupristin–dalfopristin, which was similar to our result, as well as some studied in Portugal, Egypt, and Italy [9, 36, 41]. Quinupristin–dalfopristin resistance may be due to the use of virginiamycin in normal animal weight gaining. Virginiamycin is a streptogramin antibiotic (quinupristin–dalfopristin) used as a growth stimulant in many countries, including Iran [9]. This antibiotic causes cross-resistance to quinupristin–dalfopristin, which has been banned in the EU since 1999 [9]. Our results also indicated the high frequency of resistance to erythromycin (83.33%) in most VRE isolates, which was consistent with the results of other researchers, including Jung et al. (2007) [42] and Fracalanzza et al. (2007) [34]. The high frequency of erythromycin resistance in food isolates may be due to the presence and proliferation of erythromycin-resistant plasmids and transposons among enterococcal species [43]. Furthermore, resistance to tetracycline, doxycycline, and ciprofloxacin in our study was 83.33%, 66.66%, and 33%, which could be due to the widespread use of tetracyclines and quinolones in animal husbandry [36]. Moreover, 66.66% of VRE isolates were resistant to rifampin, similar to Kim et al. (2017) [38] and Jahansepas et al. (2020) [37]. In addition, the sensitivity of VRE isolates to penicillin was reported in many studies [40] so that 75% of the isolates were sensitive to this antibiotic in our findings. The differences in antibiotic resistance patterns are due to factors, such as genetic changes in isolates, rate of antibiotic use, and availability of each antibiotic. This finding could indicate the most widely used antibiotic in an area [44].
Conclusion
The results showed a significant prevalence of S. aureus and Enterococci and their antibiotic-resistant strains in hospital food samples. Therefore, careful studies are required in education, observance of hygienic principles during food processing and storage, taking more severe control measures, and adherence to good veterinary practices regarding antibiotics administration. Complete cooking of food and prevention of cross-contamination seems to be the most effective way to prevent the occurrence of antibiotic-resistant strains in hospital food.
Limitation of the Study
This study had some limitations, including the lack of using MIC tests to identify resistant strains of S. aureus and Enterococcus. Furthermore, molecular identification of bacterial isolates was not performed, although the phenotypical tests in addition to the positive controls (ATCC 25923 and ATCC 29212) and positive clinical cultures (MRSA- or VRE-positive clinical cultures) of specimens obtained from hospitalized patients at Imam Reza hospital, Mashhad, Iran, were used as positive control in this study.
Data Availability
The dataset used is available from the corresponding author (email: saramohamadi12@yahoo.com), upon judicious request.
Code Availability
Not applicable.
References
Lund BM, O’Brien SJ (2009) Microbiological safety of food in hospitals and other healthcare settings. J Hosp Infect 73(2):109–120. https://doi.org/10.1016/j.jhin.2009.05.017
Ranjbar R, Masoudimanesh M, Dehkordi FS, Jonaidi-Jafari N, Rahimi E (2017) Shiga (Vero)-toxin producing Escherichia coli isolated from the hospital foods; virulence factors, o-serogroups and antimicrobial resistance properties. Antimicrob Resist Infect Control 6(1):1–11. https://doi.org/10.1186/s13756-016-0163-y
Safarpoor Dehkordi F, Basti AA, Gandomi H, Misaghi A, Rahimi E (2018) Retracted: Pathogenic Staphylococcus aureus in hospital food samples; prevalence and antimicrobial resistance properties. J Food Saf 38(6):e12501. https://doi.org/10.1111/jfs.12501
Dehkordi FS, Gandomi H, Basti AA, Misaghi A, Rahimi E (2017) Phenotypic and genotypic characterization of antibiotic resistance of methicillin-resistant Staphylococcus aureus isolated from hospital food. Antimicrob Resist Infect Control 6(1):1–11. https://doi.org/10.1186/s13756-017-0257-1
Sievert DM, Rudrik JT, Patel JB, McDonald LC, Wilkins MJ, Hageman JC (2008) Vancomycin-resistant Staphylococcus aureus in the United States, 2002–2006. Clin Infect Dis 46(5):668–674. https://doi.org/10.1086/527392
Banwo K, Sanni A, Tan H, Tian Y (2012) Phenotypic and genotypic characterization of lactic acid bacteria isolated from some Nigerian traditional fermented foods. Food Biotechnol 26(2):124–142. https://doi.org/10.1080/08905436.2012.670831
El-Zamkan MA, Mubarak AG, Ali AO (2019) Prevalence and phylogenetic relationship among methicillin-and vancomycin-resistant Staphylococci isolated from hospital’s dairy food, food handlers, and patients. J Adv Vet Anim Res 6(4):463. https://doi.org/10.5455/javar.2019.f369
Tang Y-T, Cao R, Xiao N, Li Z-S, Wang R, Zou J-M, Pei J (2018) Molecular epidemiology and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus isolates in Xiangyang, China. J Glob Antimicrob Resist 12:31–36. https://doi.org/10.1016/j.jgar.2017.08.016
Raafat SA, Elmagd EKA, Awad RA, Hassan EM, Alrasheedy ZE (2016) Prevalence of vancomycin resistant enterococci in different food samples, Egypt. J Med Microbiol 25(4):47–55
Mahajan S, Gupta V (2017) Other bacterial infections: vancomycin-resistant enterococcus (VRE), methicillin-resistant Staphylococcus aureus (MRSA). Emerging infectious uveitis. Springer, Cham, pp 87–91
Moussally M, Zahreddine N, Kazma J, Ahmadieh R, Kan SS, Kanafan ZA (2021) Prevalence of antibiotic-resistant organisms among hospitalized patients at a tertiary care center in Lebanon, 2010–2018. J Infect Public Health 14(1):12–16. https://doi.org/10.1016/j.jiph.2020.11.006
Benjelloun Touimi G, Bennani L, Berrada S, Moussa B, Bennani B (2020) Prevalence and antibiotic resistance profiles of Staphylococcus sp. isolated from food, food contact surfaces and food handlers in a Moroccan hospital kitchen. Lett Appl Microbiol 70(4):241–251. https://doi.org/10.1111/lam.13278
Caniça M, Manageiro V, Abriouel H, Moran-Gilad J, Franz CM (2019) Antibiotic resistance in foodborne bacteria. Trends Food Sci Technol 84:41–44. https://doi.org/10.1016/j.tifs.2018.08.001
Konecka-Matyjek E, Mackiw E, Krygier B, Tomczuk K, Stos K, Jarosz M (2012) National monitoring study on microbial contamination of food-contact surfaces in hospital kitchens in Poland. Ann Agric Environ Med 19(3):1
Ayçıçek H, Sarimehmetoǧlu B, Çakiroǧlu S (2004) Assessment of the microbiological quality of meals sampled at the meal serving units of a military hospital in Ankara, Turkey. Food Control 15(5):379–384. https://doi.org/10.1016/S0956-7135(03)00101-4
Gholammostafaei F, Alebouyeh M, Jabari F, Asadzadehaghdaei H, Zali M, Solaimannejad K (2014) Prevalence of antibiotic resistant bacteria isolated from foodstuff in kitchen of a hospital in Tehran. J Ilam Univ Med Sci 22(2):1–9
Ranjbar R, Shahreza MHS, Rahimi E, Jonaidi-Jafari N (2017) Methicillin-resistant Staphylococcus aureus isolates from Iranian restaurant food samples: panton-valentine leukocidin, SCCmec phenotypes and antimicrobial resistance. Trop J Pharm Res 16(8):1939–1949. https://doi.org/10.4314/tjpr.v16i8.26
Shahraz F, Dadkhah H, Khaksar R, Mahmoudzadeh M, Hosseini H, Kamran M, Bourke PJMs, (2012) Analysis of antibiotic resistance patterns and detection of mecA gene in Staphylococcus aureus isolated from packaged hamburger. Meat sci 90(3):759–763. https://doi.org/10.1016/j.meatsci.2011.11.009
Guran HS, Kahya S (2015) Species diversity and pheno-and genotypic antibiotic resistance patterns of staphylococci isolated from retail ground meats. J Food Sci 80(6):M1291–M1298. https://doi.org/10.1111/1750-3841.12893
Al-Amery K, Elhariri M, Elsayed A, El-Moghazy G, Elhelw R, El-Mahallawy H, El Hariri M, Hamza D (2019) Vancomycin-resistant Staphylococcus aureus isolated from camel meat and slaughterhouse workers in Egypt. Antimicrob Resist Infect Control 8(1):1–8. https://doi.org/10.1186/s13756-019-0585-4
Costa WLR, Ferreira JdS, Carvalho JS, Cerqueira ES, Oliveira LC, Almeida RCdC (2015) Methicillin-resistant Staphylococcus aureus in raw meats and prepared foods in public hospitals in Salvador, Bahia, Brazil. J Food Sci 80(1):M147–M150. https://doi.org/10.4236/fns.2015.614138
Kwon JH, Reske KA, Hink T, Seiler SM, Wallace MA, Bommarito KM, Burnham C-AD, Dubberke ER (2017) An evaluation of the prevalence of VRE and MRSA in hospital food. Infect Control Hosp Epidemiol 38(11):1373. https://doi.org/10.1017/ice.2017.207
Shahid AH, Nazir KNH, El Zowalaty ME, Kabir A, Sarker SA, Siddique MP, Ashour HMJOH (2021) Molecular detection of vancomycin and methicillin resistance in Staphylococcus aureus isolated from food processing environments. One Health 1(13):100276. https://doi.org/10.1016/j.onehlt.2021.100276
Rahimi F, Shafiei R (2019) Characteristics of enterotoxin-producing methicillin-resistant Staphylococcus aureus strains isolated from meat in Tehran, Iran. J Consum Prot Food Safety 14(4):389–398. https://doi.org/10.1007/s00003-019-01239-z
Jackson CR, Davis JA, Barrett JB (2013) Prevalence and characterization of methicillin-resistant Staphylococcus aureus isolates from retail meat and humans in Georgia. J Clin Microbiol 51(4):1199–1207. https://doi.org/10.1128/JCM.03166-12
Rong D, Wu Q, Xu M, Zhang J, Yu S (2017) Prevalence, virulence genes, antimicrobial susceptibility, and genetic diversity of Staphylococcus aureus from retail aquatic products in China. Front Microbiol 8:714. https://doi.org/10.3389/fmicb.2017.00714
Saadat S, Solhjoo K, Norooz-Nejad M-J, Kazemi A (2014) VanA and vanB positive vancomycin-resistant Staphylococcus aureus among clinical isolates in Shiraz, South of Iran. Oman Med J 29(5):335. https://doi.org/10.5001/omj.2014.90
Rahimipour F, Roudbari F, Azimian A, Youssefi M, Ghazvini K (2015) Prevalence of Staphylococcus aureus with reduced susceptibility against vancomycin in clinical samples isolate from Mashhad hospitals during 2014. J North Khorasan Univ Med Sci 7(2):309–318. https://doi.org/10.29252/jnkums.7.2.309
Martins PD, de Almeida TT, Basso AP, de Moura TM, Frazzon J, Tondo EC, Frazzon APG (2013) Coagulase-positive staphylococci isolated from chicken meat: pathogenic potential and vancomycin resistance. Foodborne Pathog Dis 10(9):771–776. https://doi.org/10.1089/fpd.2013.1492
Soltan Dallal MM, Shojaei Zinjanab M, Vahedi S, Mahmoudi H, Ghanbarzadeh S, Hedayati Rad F (2016) A survey of Escherichia coli, Enterococcus and total microbial count of packaged and non-packaged fresh vegetables in Tehran. J Payavard Salamat 10(3):220–229
Franz CM, Holzapfel WH, Stiles ME (1999) Enterococci at the crossroads of food safety. Inter J Food Microbiol 47(1–2):1–24. https://doi.org/10.1016/S0168-1605(99)00007-0
Faramarzi T, JonidiJafari A, Dehghani S, Mirzabeygi M, Naseh M, RahbarArasteh H (2012) A survey on bacterial contamination of food supply in the west of Tehran. J Fasa Uni Med Sci 2(1):11–18
Mazaheri Nezhad Fard R, Soltan Dallal MM, Abbaspour M, Rajabi Z (2019) Study of VanA, B, C, D, E genes in vancomycin resistant enterococci isolated from retailed dried vegetables in Tehran, Iran. Int J Enteric Pathog 7(1):9–14. https://doi.org/10.15171/ijep.2019.03
Fracalanzza SAP, Scheidegger EMD, Santos PFd, Leite PC, Teixeira LM (2007) Antimicrobial resistance profiles of enterococci isolated from poultry meat and pasteurized milk in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz 102(7):853–859. https://doi.org/10.1590/s0074-02762007005000120
Serio A, Chaves-López C, Paparella A, Suzzi G (2010) Evaluation of metabolic activities of enterococci isolated from Pecorino Abruzzese cheese. Inter Dairy J 20(7):459–464. https://doi.org/10.1016/j.idairyj.2010.02.005
Novais C, Coque T, Costa M, Sousa J, Baquero F, Peixe L (2005) High occurrence and persistence of antibiotic-resistant enterococci in poultry food samples in Portugal. J Antimicrob Chemother 56(6):1139–1143. https://doi.org/10.1093/jac/dki360
Jahansepas A, Sharifi Y, Aghazadeh M, Ahangarzadeh Rezaee M (2020) Comparative analysis of Enterococcus faecalis and Enterococcus faecium strains isolated from clinical samples and traditional cheese types in the Northwest of Iran: antimicrobial susceptibility and virulence traits. Arch Microbiol 202(4):765–772. https://doi.org/10.1007/s00203-019-01792-z
Kim M-C, Cha M-H, Ryu J-G, Woo G-J (2017) Characterization of vancomycin-resistant Enterococcus faecalis and Enterococcus faecium isolated from fresh produces and human fecal samples. Foodborne Pathog Dis 14(4):195–201. https://doi.org/10.1089/fpd.2016.2188
Gousia P, Economou V, Bozidis P, Papadopoulou C (2015) Vancomycin-resistance phenotypes, vancomycin-resistance genes, and resistance to antibiotics of enterococci isolated from food of animal origin. Foodborne Pathog Dis 12(3):214–220. https://doi.org/10.1089/fpd.2014.1832
Torre I, Pennino F, Diana MV, De Marco G, Trotta A, Borriello T, Troiano E (2012) Antimicrobial susceptibility and glycopeptide-resistance of enterococci in vegetables. Ital J Pub Health. https://doi.org/10.2427/5746
Gaglio R, Couto N, Marques C, Lopes MdFS, Moschetti G, Pomba C, Settanni L (2016) Evaluation of antimicrobial resistance and virulence of enterococci from equipment surfaces, raw materials, and traditional cheeses. Int J Food Microbiol 236:107–114. https://doi.org/10.1016/j.ijfoodmicro.2016.07.020
Jung WK, Lim JY, Kwon NH, Kim JM, Hong SK, Koo HC, Kim SH, Park YH (2007) Vancomycin-resistant enterococci from animal sources in Korea. Int J Food Microbiol 113(1):102–107. https://doi.org/10.1016/j.ijfoodmicro.2006.07.023
Ristori CA, Rowlands REG, Bergamini AMM, Lopes GISL, Paula AMRd, Oliveira MAd, Lima MdJdC, Tegani LS, Watanabe AH, Jakabi M (2012) Prevalence and antimicrobial susceptibility profile of Enterococcus spp. isolated from frozen chicken carcasses. Rev Inst Adolfo Lutz (Impresso) 71(2):237–243
Madanipour E, Mehrabi MR, Mirzaee M (2017) The antibiotic susceptibility pattern and prevalence of vanA, vanB, and vanC Genes among Enterococcus faecalis strains isolated from consumed meat. Infect Epidemiol Microbiol 3(4):117–121
Acknowledgements
The authors sincerely appreciate all those who participated in this study.
Funding
The financial support acquired from Faculty of Medicine, Mashhad University of Medical Sciences (Grant No: A-1581).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows: study conception and design, AA and MH; data collection, ST; analysis and interpretation of results, AA, ST, AN, SM, and MN; writing the manuscripts, SM.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have any conflict of interest.
Ethical Approval
Not applicable.
Consent for Participate and Publication
The authors declared their total consent for participation and publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Afshari, A., Taheri, S., Hashemi, M. et al. Methicillin- and Vancomycin-Resistant Staphylococcus aureus and Vancomycin-Resistant Enterococci Isolated from Hospital Foods: Prevalence and Antimicrobial Resistance Patterns. Curr Microbiol 79, 326 (2022). https://doi.org/10.1007/s00284-022-03022-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-03022-0