Abstract
The discovery of the potential of paraprobiotics to exert different immunological benefits suggests that further studies should be carried out to determine their potential and mechanisms of action in modulating the immune system. The objective of this study was to investigate the immune response of several microbial-associated molecular patterns (MAMPS) used at different doses in macrophage cell lines RAW-264.7 stimulated with lipopolysaccharide (LPS). Two experiments were conducted. The first was performed to determine a dose response curve for each paraprobiotic (Bifidobacterium lactis, Lactobacillus casei, Lactobacillus gasseri, Lactobacillus paracasei, and Streptococcus thermophilus). Further experiments were carried using only two doses (0.01 g/ml and 0.1 g/ml). RAW-264.7 cells were cultivated in Dubelcco’s Modified Eagle’s medium supplemented with fetal bovine serum and penicillin/streptomycin. Cells were incubated with LPS (1 μg/ml) and six concentrations of MAMPs were added. RAW-264.7 viability, myeloperoxidase activity, nitrite/nitrate concentration, reactive oxygen species production, oxidative damage, and inflammatory parameters were measured. In the LPS group, there was a significant reduction in cell viability. Myeloperoxidase and nitrite/nitrate concentrations demonstrated a better effect at 0.01 and 0.1 g/ml doses. There was a significant reduction in interleukin-6 (IL-6) levels at 0.1 g/ml dose in all paraprobiotics. IL-10 levels decreased in the LPS group and increased at 0.1 g/ml dose in all paraprobiotics. The dichlorofluorescin diacetate results were reinforced by the observed in oxidative damage. Paraprobiotics are likely to contribute to the improvement of intestinal homeostasis, immunomodulation, and host metabolism.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The health-promoting effects of probiotics have already been verified through a series of studies [1]. However, although less studied, a considerable amount of data has revealed the beneficial effects of paraprobiotics, indicating that non-viable microbial cells, microbial fractions, or cell lysates can also immunomodulate human and animal health [2].
Paraprobiotics are defined as “inactivated (non-viable) microbial cells or cellular fractions that, when consumed, confer benefits to the consumer’s health” [2]. A number of benefits associated with the consumption of paraprobiotics have already been verified, such as: immune system modulation [3], treatment of liver disease [4], reduction of diarrhea symptoms [5, 6], atopic dermatitis [7], and colitis [8], inhibition of pathogens [9, 10], prevention of dental caries [11], modulation of the intestinal microbiota; maintenance of intestinal integrity [4], and cholesterol reduction [12] in addition to reducing flatulence and food allergy risks [13, 14]. It is important to note that paraprobiotics are different from postbiotics since postbiotics refer to soluble factors (products or metabolic byproducts) secreted by live bacteria or released after bacterial lysis, such as enzymes, peptides, teichoic acids, peptidoglycan-derived muropeptides, polysaccharides, cell surface proteins, and organic acids [4].
Communication with the host can be mediated by bacterial cells and is based on the activation of the innate immune response through interaction with Toll-like receptors (TLRs) [2]. Toll-like receptors form a family of receptors that recognize molecular patterns often associated with infectious agents [15]. Among the different receptors is TLR4, which recognizes bacterial components, such as lipopolysaccharide (LPS), of gram-negative bacteria [16, 17].
In general, after the interaction among paraprobiotics, also known as microbe-associated molecular patterns (MAMPs), and pattern recognition receptors (PPRs), the innate immune response is activated to coordinate a response involving humoral and cellular components. In this context, after MAMPs-PPRs interaction, resident cells play a key role, releasing a wide variety of signaling molecules, such as prostaglandins, leukotrienes, cytokines, and chemokines that trigger the inflammatory response, representing one of the most important functions of innate immunity [18, 19].
Inflammation and oxidative stress are important mechanisms of this response, as they consist of a variety of physiological and pathological events, acting as a permissive mechanism for the action of cells and components of the immune system [20]. The intensity of the immune response and the harmful potential of the stimulating agent are directly proportional to the severity of the effects observed in the affected individuals [21].
The discovery of the potential of paraprobiotics to exert different immunological benefits suggests that further studies should be carried out to determine the potential and mechanisms of action of these compounds in modulating the immune system. Thus, the objective of this study was to investigate the response of several MAMPs (Bifidobacterium lactis, Lactobacillus casei, Lactobacillus gasseri, Lactobacillus paracasei and Streptococcus thermophilus) at different doses in a lineage of RAW-264.7 macrophages stimulated with LPS to determine its anti-inflammatory effect that could be used to mitigate inflammation in different diseases.
Material and Methods
Reagents
Heat-inactivated MAMPs of Bifidobacterium animalis subsp. lactis CCT 7858, Lactobacillus casei CCT 7859, Lactobacillus gasseri CCT 7860, Lactobacillus paracasei subsp. paracasei CCT 7861, and Streptococcus thermophilus ATCC 19258 were provided by Gabbia Biotechnology. For the production of MAMPs, probiotic microorganisms were grown in specific culture media. After confirming their growth, 5 mL tubes containing cell suspension of probiotic microorganisms were inactivated through a thermal process (time and temperature defined in the protocol of company Gabbia Biotecnologia). When inactivation was confirmed, MAMPs were used in the “in vitro” tests.
Lipopolysaccharide (LPS from Escherichia coli 026:B6) was obtained from Sigma Chemical Co (St. Louis, MO, EUA) and was used at 1 μg/ml [22]. The LPS was reconstituted in endotoxin-free water. A murine strain of RAW-264.7 macrophages was obtained from Sigma Chemical Co (St. Louis, MO, EUA, RAW-264.7 Cell Line murine: 91062702). Dulbecco’s Modified Eagle’s medium (DMEM) supplemented with L-glutamine (2 mM), streptomycin (0.1 mg/ml) and penicillin (100 U/ml) was obtained from Sigma Chemical Co (St. Louis, MO, EUA). Fetal Bovine Serum (FBS) was obtained from Gibco BRL—Life Technologies (Rockville, MO, EUA).
Cell Culture
RAW-264.7 cells were cultivated in DMEM supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin under 5% CO2 humidified conditions at 37 °C [23]. The cells were seeded at 1 × 104 cells/well in a 96-well plate and maintained for 24 h. After this period, the cells were incubated with LPS (1 μg/ml) for an additional 24 h, after which the cells were treated with different MAMPs for 24 h.
Dose Response Experiments
First, a dose response curve for each paraprobiotic was determined. Paraprobiotics at six concentrations (0.0001 g/ml; 0.001 g/ml; 0.01 g/ml; 0.1 g/ml; 1 g/ml; 2 g/ml) were added after LPS incubation as follows:
-
(1)
RAW-264.7 + DMEM (control group).
-
(2)
RAW-264.7 + LPS.
-
(3)
RAW-264.7 + LPS + MAMPs of Bifidobacterium lactis (6 different doses).
-
(4)
RAW-264.7 + LPS + MAMPs of Lactobacillus casei (6 different doses).
-
(5)
RAW-264.7 + LPS + MAMPs of Lactobacillus gasseri (6 different doses).
-
(6)
RAW-264.7 + LPS + MAMPs of Lactobacillus paracasei (6 different doses).
-
(7)
RAW-264.7 + LPS + MAMPs of Streptococcus thermophilus (6 different doses).
All experiments were performed in triplicate and four wells were used for each condition. RAW-264.7 viability, nitrite/nitrate concentration, and myeloperoxidase (MPO) activity were evaluated as described below (“Effects of paraprobiotics in ROS generation, oxidative damage, and inflammatory parameters”, “Oxidative Damage”, and “Nitrosative damage”, respectively). After determining the best dose–response for each paraprobiotic, further experiments were carried out as described below.
Effects of Paraprobiotics in ROS Generation, Oxidative Damage, and Inflammatory Parameters
RAW-264.7 cells were plated in a 96-well plate and stimulated with LPS (1 μg/ml), as described in experimental design 1. Then, MAMPs were added at 0.01 g/ml and 0.1 g/ml for 24 h (LPS have been maintained in culture). Since, maltodextrin was used during the production of MAMPs, there is a possibility that its traces could be present in used MAMPs. Thus, it was included as an additional control group, just to ensure that it did not have any substantial effect in our model.
-
(1)
RAW-264.7 + DMEM (control group).
-
(2)
RAW-264.7 + LPS.
-
(3)
RAW-264.7 + Maltodextrin (additional control—see text).
-
(4)
RAW-264.7 + LPS + MAMPs of Bifidobacterium lactis (2 different doses).
-
(5)
RAW-264.7 + LPS + MAMPs of Lactobacillus casei (2 different doses).
-
(6)
RAW-264.7 + LPS + MAMPs of Lactobacillus gasseri (2 different doses).
-
(7)
RAW-264.7 + LPS + MAMPs of Lactobacillus paracasei (2 different doses).
-
(8)
RAW-264.7 + LPS + MAMPs of Streptococcus thermophilus (2 different doses).
All experiments were performed in triplicate and four wells were used for each condition. The reactive oxygen species (ROS) production, oxidative damage, and inflammatory parameters were measured as described below (“Cell viability: MTT assay”, “ROS production”, and “Myeloperoxidase activity”, respectively).
Cell Viability: MTT Assay
An MTT cell viability assay was performed in RAW-264.7 cells [24]. 100 μL of MTT (0.5 μg/ml) were added in each well and the cells were incubated for 3 h. After the incubation period, the MTT was removed and 150 μL of isopropyl alcohol was added to dissolve the formazan crystals. The absorbance was measured at 570 nm using a microplate reader. The experiments were performed in triplicate with four wells for each condition. The results were expressed as the percentage of viable cells in comparison to the control group (DMEM—untreated cells).
Cell viability was expressed as a percentage (%), assuming that the DMEM group had 100% viable cells. Note that the DMEM group bar was considered to be 100% for all the analysis; the other groups were calculated in relation to this.
ROS Production
Samples were incubated with the carboxy-2′,7′-dichloro-dihydro-fluorescein diacetate (DCFH-DA) probe for 40 min. After incubating at 37 °C for 24 h, fluorescence was measured at 485 nm (excitation) and 527 nm (emission) wavelengths on a microplate reader (Molecular Devices Spectra MAX M2, San José, Califórnia, EUA) [25].
Oxidative Damage
Oxidative damage to proteins was examined by the quantifying carbonylated proteins. A reaction of carbonyl groups with dinitrophenylhydrazine in oxidized proteins, according to the method described by Levine et al. [26] was carried out. The absorbance was evaluated at 340 nm wavelengths on a microplate reader (Molecular Devices Spectra MAX M2, San José, Califórnia, EUA).
The formation of thiobarbituric acid reactive substances (TBARS) during an acid-heating reaction was measured as an index of oxidative stress as described previously [27]. The samples were mixed with 1 ml of trichloroacetic acid 10% and 1 ml of thiobarbituric acid 0.67% (Sigma-Aldrich) and then heated in a boiling water bath for 15 min. Malondialdehyde (MDA) equivalents were determined by measuring the absorbance at 535 nm in SpectraMax Molecular Devices M2 (San José, Califórnia, EUA) using 1,1,3,3-tetramethoxypropane (Sigma-Aldrich) as an external standard. Results were expressed as MDA equivalents per mg of protein.
Nitrosative Damage
Nitrite/nitrate concentration was assayed spectrophotometrically using Griess reagents (1% sulphanilamide in 5% phosphoric acid and 0.1% N‐1‐naphthylethylenediamine dihydrochloride in bi-distilled H2O [NED solution]) and vanadium (III) chloride as previously described by Green et al. [28]. A standard curve was obtained simultaneously with each set of samples, and the optical density at 550 nm (OD550) was measured using an ELISA microplate reader (Molecular Devices Spectra MAX M2, San José, Califórnia, EUA).
Myeloperoxidase Activity
The tissue was homogenized (50 mg/ml) in 0.5% of hexadecyltrimethylammonium bromide (Sigma-Aldrich) and centrifuged (8765×g) for 10 min. The suspension was sonicated and an aliquot of supernatant was mixed with a solution of 1.6 mmol/l 3,3′,5,5′-tetramethylbenzidine (TMB) and 1 mmol/l H2O2. The MPO activity was measured spectrophotometrically at 650 nm at 37 °C. The results were expressed as mU/mg protein [29].
Levels of Cytokines
Concentrations of TNF-α, IL-10, and IL-6 were determined in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA) on microplate reader (Molecular Devices Spectra MAX M2, San José, Califórnia, EUA) using commercial kits (R & D System, Mineápolis, Minnesota, EUA) [30]. Briefly, 96-well plates were sensitized with a specific monoclonal antibody incubated overnight. The plates were blocked with 1% albumin. Samples and/or standards were added to the plate. Specific detection antibodies were added and incubated for 2 h. Then, streptavidin peroxidase was added to the plate and tetramethylbenzidine (TMB) substrate solution was added. The reaction was stopped with the addition of 2 N hydrochloric acid solution (stop solution). At each stage, the plates were washed with wash buffer. Average detection: TNF-α (0.034–2.006); IL-10 (0.055–2.133), and IL-6 (0.046–2.550).
Statistical Analysis
Data collected were analyzed using one-way analysis of variance (ANOVA), followed by the Tukey post hoc method and expressed as mean ± standard deviation in Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 21. Graphs were obtained using GraphPad Prism (San Diego, California, USA) version 7. For all comparisons, P < 0.05 indicated statistical significance.
Results
The effects of paraprobiotics (MAMPs) on cell viability using the RAW-264.7 cell line were first analyzed. In the LPS group, there was a significant reduction in cell viability in relation to the control group. L. gasseri, L. paracasei, and L. casei, (Fig. 1a–c), 0.1, 1, and 2 g/ml improved cell viability when compared to the LPS group. In S. thermophilus (Fig. 1d), a protective effect from 0.01 g/ml dose and from 0.001 g/ml to B. lactis (Fig. 1e) was observed.
Cell viability in RAW-264.7 cells stimulated with LPS and treated with paraprobiotics in different doses (0.0001; 0.001; 0.01; 0.1; 1 and 2 g/ml doses). L. gasseri (a); L. paracasei (b); L. casei (c); S. thermophilus (d); B. lactis (e). Supernatants were collected 24 h after treatment. Data were expressed as mean ± SD. The experiments were performed in triplicate with four wells for each condition. P < 0.05 denoted statistical difference between groups. *Different from DMEM; #Different from LPS
The effect of paraprobiotics (MAMPs) on myeloperoxidase activity was also evaluated (Fig. 2). As expected, The LPS group increased MPO activity compared to the DMEM group, and there was no significant effect of L. gasseri, L. paracasei, and L. casei (Fig. 2a–c). In contrast, S. thermophilus and B. lactis decreased MPO activity from 0.01 g/ml to higher doses (Fig. 2).
Mieloperoxidase activity in RAW-264.7 cells stimulated with LPS and treated with paraprobiotics in different doses (0.0001; 0.001; 0.01; 0.1; 1 and 2 g/ml doses). L. gasseri (a); L. paracasei (b); L. casei (c); S. thermophilus (d); B. lactis (e). Supernatants were collected 24 h after treatment. Data were expressed as mean ± SD. The experiments were performed in triplicate with four wells for each condition. P < 0.05 denoted statistical difference between groups. *Different from DMEM; #Different from LPS
Nitrite/nitrate levels were significantly increased in the LPS group (Fig. 3). There was a decrease in nitrite/nitrate concentrations only in lower doses of L. gasseri, L. paracasei, and L. casei (0.0001; 0.001, and 0.01 g/ml), as opposed to S. thermophilus and B. lactis (Fig. 3).
Nitrite/Nitrate concentrations in RAW-264.7 cells stimulated with LPS and treated with paraprobiotics in different doses (0.0001; 0.001; 0.01; 0.1; 1 and 2 g/ml doses). L. gasseri (a); L. paracasei (b); L. casei (c); S. thermophilus (d); B. lactis (e). Supernatants were collected 24 h after treatment. Data were expressed as mean ± SD. The experiments were performed in triplicate with four wells for each condition. P < 0.05 denoted statistical difference between groups. *Different from DMEM; #Different from LPS
Since these first results demonstrated a protective effect of different paraprobiotics consistently at 0.01 and 0.1 g/ml doses, cytokines (TNF, IL-6, and IL-10) and oxidative stress (DCF-DA, TBA, and Carbonyl) were also analyzed using these doses. Maltodextrin has been included in the next analyses; this compound is the vehicle used to produce MAMPs and we want to make sure that it does not influence the analysis (Fig. S1).
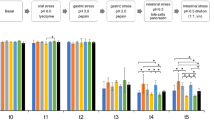
TNF levels increased in the LPS group, but paraprobiotics did not have any significant effect (Fig. 4a, b). IL-6 levels were increased in the LPS group when compared to the DMEM and maltodextrin group (Fig. 4c, d). There was a significant reduction in IL-6 levels at 0.1 g/ml dose in all analyzed paraprobiotics (Fig. 4d). In contrast to the 0.01 g/ml, a significant effect was only observed in L. paracasei, B. lactis, and S. thermophilus (Fig. 4c). Additionally, IL-10 levels decreased in the LPS group and increased only at the 0.1 g/ml dose in all paraprobiotics (Fig. 4e, f).
TNF levels (a and b); IL-6 levels (c and d); and IL-10 levels (e and f) in RAW-264.7 cells stimulated with LPS and treated with paraprobiotics in two different doses (0.01 and 0.1 g/ml doses). Supernatants were collected 24 h after treatment. Data were expressed as mean ± SD. The experiments were performed in triplicate with four wells for each condition. P < 0.05 denoted statistical difference between groups. *Different from DMEM; #Different from LPS; &Different from maltodextrin
The effect of paraprobiotics (MAMPs) on ROS production was analyzed in RAW-264.7 cultures stimulated with LPS, using the fluorescent probe DCF-DA (Fig. 5). The ROS generation increased in the LPS group when compared with DMEM and maltodextrin, and B. lactis decreased ROS only at 0.01 g/ml dose, while L. casei, L. paracasei, L. gasseri, and S. thermophilus were effective at 0.1 g/ml dose (Fig. 5a, b). The DCF-DA results were reinforced by the observed in protein carbonyl and TBARS levels (Figs. 5e, f). The 0.1 g/ml dose was able to decrease protein carbonyl levels compared to LPS groups for all paraprobiotics (Fig. 5c, d).
The levels of intracellular ROS were determined using DCF-DA (a and b), Carbonyl proteins (c and d), and MDA equivalents (e and f) in RAW-264.7 cells stimulated with LPS and treated with paraprobiotics in two different doses (0.01 and 0.1 g/ml doses). All data are presented as mean ± SD. n = 5. *Different of DMEM; #Different of maltodextrin; $Different of LPS. P < 0.05
The results are consistent with the protein damage measured by carbonyl assay. There was an increase in the LPS group, confirming oxidative stress, and a reversion is noted in the dose of 0.01 g/ml except L. casei and B. lactis in this dose and 0.1 g/ml for all paraprobiotics except S. thermophilus, which was less effective (Fig. 5c, d). Besides this, the images indicate that the cells preserved the structure in the control and paraprobiotics groups in LPS-induced RAW-264.7 cells in both doses (Figs. S2 and S3). In the LPS group, the cells had an altered form, indicating cell activation (Figs. S2 and S3).
Discussion
The purpose of this study was to evaluate the effect of different paraprobiotics (MAMPs) on a lineage of RAW-264.7 macrophages stimulated with LPS. It is well known that LPS induces the activation of RAW-264.7 cells [31]; thus we used this paradigm to determine the anti-inflammatory effects of different paraprobiotics. LPS is a potent inducer of cytokines in monocytes, acting via the TLR4 receptor [32]. In addition, it occurs in nitric oxide production and MPO [33], which can induce oxidative stress. Thus, the protective effects of paraprobiotics demonstrated here suggest that they inhibit the LPS-TLR4 pathway, thus decreasing RAW-264.7 activation.
The inflammatory process must be modulated to provide a well-dimensioned defense mechanism. Inflammation is associated with a hemodynamic response [34, 35] and the dysregulation of inflammation plays an important role in different cellular dysfunctions [36, 37]. Studies indicate that paraprobiotics provide benefits such as the modulation of the immune system and secretion of metabolites by non-viable cells, in addition to adherence to intestinal cells that allows the inhibition of pathogens [2]. Experimental studies in neonate rats showed that MAMPs decreased LPS-induced pro-inflammatory and increased anti-inflammatory mediators [5]. In addition, emerging evidence indicates that strains of both L. casei [38,39,40] and B. bifidum [41,42,43] have beneficial effects in their heat-inactivated form through their anti-inflammatory and immunomodulatory effects [2]. A recent study published by Avila et al. [44] showed the beneficial effects of different probiotic strains, including L. casei, in reducing the levels of nitrite/nitrate, MPO, and pro-inflammatory cytokines (IL-1, IL-6) in animals subjected to LPS-induced inflammation. In another study by Cross et al. [45], heat-inactivated L. casei did not induce IL-12 and TNF-α, when compared to viable L. casei, reinforcing the potential anti-inflammatory effect of paraprobiotics (MAMPs). In addition, Del Carmen et al. [46, 47] showed that S. thermophilus has intrinsic immunomodulatory properties, where expressing an antioxidant enzyme enhances its anti-inflammatory activities.
The microorganism inactivation process is fundamental to guaranteeing the immunomodulatory activity since such activity is mediated by all the structural components of the cells, as suggested by Tejada-Simon and Pestka [48]. The RAW-264.7 macrophages were exposed to heat-inactivated paraprobiotics, Bifidobacterium sp., Lactobacillus acidophilus, Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus gasseri, Lactobacillus helveticus, Lactobacillus reuteri, and Streptococcus thermophilus. Non-viable fragments of microbial origin have noticeable advantages over probiotics for industries and consumers in the development of safe and stable products. They have simple handling and can be used in products that go through different processes in the industry, a longer Shelf life, and are advantages in the use of paraprobiotics. These advantages allow the supply of these compounds to different consumers, areas, and industries, reducing the risk of microbial translocation and infection or even improving inflammatory responses in consumers with altered or compromised immune systems [49].
Oxidative stress results in protein denaturation, DNA hydroxylation, apoptosis and lipid peroxidation compromising the cells’ viability [50, 51]. Many authors have reported that probiotics have antioxidant properties [52,53,54,55]. Several mechanisms are speculated, such stimulation of the immune system, neutralization of oxidants in the intestinal tract (by antioxidant enzymes) and the inhibition of intestinal pathogens [56].
The effect of paraprobiotics on intracellular ROS production in RAW-264.7 cultures was analyzed using the peroxide-sensitive DCF-DA probe, and the greatest effectiveness of MAMPs was shown in the dose of 0.1 g/ml for oxidative stress. It was found that ROS has extremely high reactivity, which gradually leads to oxidative damage to biomolecules [57]. In addition, the measurement of nitrate/nitrite concentration or total nitrate and nitrite concentration (NOx) is routinely used as an index of NO production [58] and unregulated production of nitric oxide can cause nitrosative stress, leading to damages of proteins/DNA and cell injury and death [59, 60]. Our results demonstrate that the 0.1 g/ml dose of paraprobiotics is more effective in stress oxidative reduction. L. casei, L. gasseri, and L. paracasei, interestingly, showed efficacy at much lower doses (0.0001, 0.001, and 0.01 g/ml).
It has already been established that Lactobacillus strains possess an antioxidant capacity [61]. Xing et al. [62] found that 13 tested Lactobacillus strains have an antioxidant effect on RAW-264.7 cells. Until now, there are many studies on the antioxidant activities of Lactobacillus still totally relying on the chemical way that do not consider the metabolism and bioavailability of antioxidants. Furthermore, in a recent study, Magistrelli et al., [63] showed that Lactobacillus strains exert promising results in decreasing oxidative stress, pro-inflammatory cytokines and potentially pathogenic bacterial overgrowth in the PBMC cells of patients with Parkinson’s disease.
The differences in the mode of action of MAMPs of paraprobiotics verified in the present study, be it in the concentration or in the type of microorganism used, may be associated with the different structural components of cells that may be part of several bacteria, such as the constituents of the cell wall, which interact with immune cells in a specific way. Peptidoglycan is the main constituent of Gram-positive bacterial cell walls, accounting for up to 90% of its weight, while it constitutes only 15–20% of the cell wall in Gram-negative bacteria [2, 64]. On the walls of Gram-positive bacteria, there are molecules that project to the outer surface of the peptidoglycan layer, known as teicoic acids. These acids, together with proteins present on the cell wall surface, are responsible for the antigenic determination of Gram-positive bacteria because they differ between different species and lineages, where each combination and interaction with specific molecules and receptors can stimulate the body’s immune activity in different ways. One advantage of using paraprobiotics is that this intervention may be safer than probiotics because they reduce the risk of infection, microbial translocation or enhanced inflammatory responses [14].
In general, our results demonstrate that 0.1 g/ml dose of paraprobiotics is more effective in reducing ROS generation and decreasing pro-inflammatory cytokines. Lactobacillus strains exert promising results, which may be due to the mode of action of each paraprobiotic, be it in the concentration or in the type of microorganism used, and may be associated with the different structural components of cells that may be part of several bacteria, such as the constituents of the cell wall, which interact with immune cells in a specific way. Paraprobiotics may contribute to improving intestinal homeostasis, immunomodulation, and host metabolism. Thus, the use of paraprobiotics seems promising.
References
Iacono A, Raso GM, Canani RB, Calignano A, Meli R (2011) Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem 22(8):699–711. https://doi.org/10.1016/j.jnutbio.2010.10.002
Taverniti V, Guglielmetti S (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6:261–274. https://doi.org/10.1007/s12263-011-0218-x
Moro-García MA, Alonso-Arias R, Baltadjieva M, Benítez CF, Barrial MAF, Ruisánchez ED et al (2013) Oral supplementation with Lactobacillus delbrueckii subsp. bulgaricus 8481 enhances systemic immunity in elderly subjects. Age 35:1311–1326. https://doi.org/10.1007/s11357-012-9434-6
Nithya V, Muthukumar SP, Halami PM (2012) Safety assessment of Bacillus licheniformis Me1 isolated from milk for probiotic application. Int J Toxicol 31:228–237. https://doi.org/10.1177/1091581812443388
Li J, Zhang W, Wang C, Yu Q, Dai R, Pei X (2012) Lactococcus lactis expressing food-grade β-galactosidase alleviates lactose intolerance symptoms in post-weaning Balb/c mice. Appl Microbiol Biotechnol 96:1499–1506. https://doi.org/10.1007/s00253-012-3977-4
Sudha MR, Bhonagiri S, Kumar MA (2013) Efficacy of Bacillus clausii strain UBBC-07 in the treatment of patients suffering from acute diarrhea. Benef Microbes 4:211–216. https://doi.org/10.3920/BM2012.0034
Sugimoto S, Ishii Y, Izama N, Masuoka N, Kano M, Sone T et al (2012) Photoprotective effects of Bifidobacterium breve supplementation against skin damage induced by ultraviolet irradiation in hairless mice. Photodermatol Photoimmunol Photomed 28:312–319. https://doi.org/10.1111/phpp.12006
Ducrotte P, Sawant P, Jayanthi V (2012) Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 18:4012–4018. https://doi.org/10.3748/wjg.v18.i30.4012
Amdekar S, Singh V, Singh DD (2011) Probiotic therapy: immunomodulating approach toward urinary tract infection. Curr Microbiol 63:484–490. https://doi.org/10.1007/s00284-011-0006-2
Borges S, Barbosa J, Silva J, Texeira P (2013) Evaluation of characteristics of Pediococcus spp. to be used as a vaginal probiotic. J Appl Microbiol 115:527–538. https://doi.org/10.1111/jam.12232
Teanpaisan R, Piwat S (2014) Lactobacillus paracasei SD1, a novel probiotic, reduces mutans streptococci in human volunteers: a randomized placebo-controlled trial. Clin Oral Investing 18(3):857–862. https://doi.org/10.1007/s00784-013-1057-5
Bordoni A, Amaretti A, Leonardi A, Boschetti E, Danesi F, Matteuzzi D et al (2013) Cholesterol-lowering probiotics: in vitro selection and in vivo testing of bifidobacteria. Appl Microbiol Biotechnol 97:8273–8281. https://doi.org/10.1007/s00253-013-5088-2
Almada CN (2017) Paraprobiotics: impact of inactivation methods on their efficacy, stability in food and health benefits = Paraprobióticos: impacto de métodos de inativação sobre a eficácia, estabilidade em alimentos e efeitos benéficos à saúde. Tese—Universidade Estadual de Campinas (UNICAMP), p 166
Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B et al (2018) Postbiotics: An evolving term within the functional foods field. Trends Food Sci Technol 75:105–114. https://doi.org/10.1016/j.tifs.2018.03.009
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. https://doi.org/10.1016/j.immuni.2011.05.006
Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M et al (1999) The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811–815. https://doi.org/10.1038/44605
Campos MA, Almeida IC, Takeuchi O, Akira S, Valente EP, Procopio DO et al (2001) Activation of toll-like receptor-2 by glycosylphosphatidylinositol anchors from a protozoan parasite. J Immunol 167:416–423. https://doi.org/10.4049/jimmunol.167.1.416
Janeway CA (2001) How the immune system protects the host from infection. Microb Infect 3:1167–1171. https://doi.org/10.1016/s1286-4579(01)01477-0
Janeway CA, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216. https://doi.org/10.1146/annurev.immunol.20.083001.084359
Sellge G, Kufer TA (2015) PRR-signaling pathways: learning from microbial tactics. Semin Immunol 27(2):75–84. https://doi.org/10.1016/j.smim.2015.03.009
Girbes ARJ, Beishuizen A, van Schijndel RJMS (2008) Pharmacological treatment of sepsis. Fund Clin Pharmacol 22:355–361. https://doi.org/10.1111/j.1472-8206.2008.00606.x
Dong D, Zhou NN, Liu RX, Xiong JW, Pan H, Sun SQ et al (2017) Sarsasapogenin-AA13 inhibits LPS-induced inflammatory responses in macrophage cells in vitro and relieves dimethylbenzene-induced ear edema in mice. Acta Pharmacol Sin 38(5):699–709. https://doi.org/10.1038/aps.2016.180
Souza NC, de Oliveira Nascimento EN, de Oliveira IB, Oliveira HML, Santos EGP, Moreira Cavalcanti Mata MER, Gelain DP, Moreira JCF, Dalmolin RJS, de Bittencourt Pasquali MA (2020) Anti-inflammatory and antixidant properties of blend formulated with compounds of Malpighia emarginata DC (acerola) and Camellia sinensis L. (green tea) in lipopolysaccharide-stimulated RAW 2647 macrophages. Biomed Pharmacother 128:110277. https://doi.org/10.1016/j.biopha.2020.110277
Souza NC, de Oliveira JM, Morrone MDS et al (2017) Antioxidant and anti-inflammatory properties of Anacardium occidentale leaf extract. Evid Based Complement Altern Med. https://doi.org/10.1155/2017/2787308
Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell JV et al (2013) Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: a quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol In Vitro 27(2):954–963. https://doi.org/10.1016/j.tiv.2013.01.016
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG et al (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-h
Draper HH, Hadley M (1990) Malondialdehyde determination as índex of lipid peroxidation. Methods Enzymol 186:421–431. https://doi.org/10.1016/0076-6879(90)86135-i
Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite and nitrate in biological fluids. Anal Biochem 126:131–138. https://doi.org/10.1016/0003-2697(82)90118-x
De Young LM, Kheifets JB, Ballaron SJ, Young JM (1989) Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions 26:335–341. https://doi.org/10.1007/BF01967298
Comim CM, Cassol OJ Jr, Constantino LS, Felisberto F, Petronilho F, Rezin GT, Scaini G, Daufenbach JF, Streck EL, Quevedo J, Dal-Pizzol F (2011) Alterations in inflammatory mediators, oxidative stress parameters and energetic metabolism in the brain of sepsis survivor rats. Neurochem Res 36(2):304–311. https://doi.org/10.1007/s11064-010-0320-2
Nitkin CR, Bonfield TL (2017) Balancing anti-inflammatory and anti-oxidant responses in murine bone marrow derived macrophages. PLoS ONE 12(9):e0184469. https://doi.org/10.1371/journal.pone.0184469
Sabroe I, Read RC, Whyte MK, Dockrell DH, Vogel SN, Dower SK (2003) Toll-like receptors in health and disease: complex questions remain. J Immunol 171(4):1630–1635. https://doi.org/10.4049/jimmunol.171.4.1630
Borderie D, Hilliquin P, Hernvann A, Lemarechal H, Kahan A, Menkes CJ et al (2002) Inhibition of inducible NO synthase by TH2 cytokines and TGF beta in rheumatoid arthritic synoviocytes: effects on nitrosothiol production. Nitric Oxide 6(3):271–282. https://doi.org/10.1006/niox.2001.0418
Kaarlola A, Tallgren M, Pettila V (2006) Long-term survival, quality of life, and quality-adjusted life-years among critically ill elderly patients. Crit Care Med 34:2120–2126. https://doi.org/10.1097/01.CCM.0000227656.31911.2E
Falciglia M, Freyberg RW, Almenoff PL, D’Alessio DA, Render ML (2009) Hyperglycemia–related mortality in critically ill patients varies with admission diagnosis. Crit Care Med 37:3001–3009. https://doi.org/10.1097/CCM.0b013e3181b083f7
Nelson WG, De Marzo AM, DeWeese TL, Isaacs WB (2004) The role of inflammation in the pathogenesis of prostate cancer. J Urol 172(5 Pt 2):S6–S12. https://doi.org/10.1097/01.ju.0000142058.99614.ff
Daly BJ, Douglas SL, Kelley CG, O’Toole E, Montenegro H (2005) Trial of a disease management program to reduce hospital readmissions of the chronically critically ill. Chest 128:507–517. https://doi.org/10.1378/chest.128.2.507
Ouwehand AC, Tölkkö S, Kulmala J, Salminen S, Salminen E (2000) Adhesion of inactivated probiotic strains to intestinal mucus. Lett Appl Microbiol 31:82–86. https://doi.org/10.1046/j.1472-765x.2000.00773.x
Donkor ON, Ravikumar M, Proudfoot O, Day SL, Apostolopoulos V, Paukovics G et al (2012) Cytokine profile and induction of T helper type 17 and regulatory T cells by human peripheral mononuclear cells after microbial exposure. Clin Exp Immunol 167:282–295. https://doi.org/10.1111/j.1365-2249.2011.04496.x
Szajewska H, Guarino A, Hojsak I, Indrio F, Kolacek S, Shamir R et al (2014) European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN working group for probiotics and prebiotics. J Pediatr Gastroenterol Nutr 58:531–539. https://doi.org/10.1097/MPG.0000000000000320
Nakamura Y, Terahara M, Iwamoto T, Yamada K, Asano M, Kakuta S et al (2012) Upregulation of polymeric immunoglobulin receptor expression by the heat-inactivated potential probiotic Bifidobacterium bifidum OLB6378 in a mouse intestinal explant model. Scand J Immunol 75:176–183. https://doi.org/10.1111/j.1365-3083.2011.02645.x
Schwendicke F, Horb K, Kneist S, Dörfer C, Paris S (2014) Effects of heat-inactivated Bifidobacterium BB12 on cariogenicity of Streptococcus mutans in vitro. Arch Oral Biol 59:1384–1390. https://doi.org/10.1016/j.archoralbio.2014.08.012
Sugahara H, Yao R, Odamaki T, Xiao JZ (2017) Differences between live and heat-killed Bifidobacteria in the regulation of immune function and the intestinal environment. Benef Microbes 8:463–472. https://doi.org/10.3920/BM2016.0158
Ávila PRM, Michels M, Vuolo F, Bilésimo R, Burger H, Milioli MVM et al (2020) Protective effects of fecal microbiota transplantation in sepsis are independent of the modulation of the intestinal flora. Nutrition 73:110727. https://doi.org/10.1016/j.nut.2020.110727
Cross ML, Ganner A, Teilab D, Fray LM (2004) Patterns of cytokine induction by gram-positive and gram-negative probiotic bacteria. FEMS Immunol Med Microbiol 42(2):173–180. https://doi.org/10.1016/j.femsim.2004.04.001
Del Carmen S, de Moreno de LeBlanc A, Martin R, Chain F, Langella P, Bermúdez-Humarán LG et al (2014) Genetically engineered immunomodulatory Streptococcus thermophilus strains producing antioxidant enzymes exhibit enhanced anti-inflammatory activities. Appl Environ Microbiol 80(3):869–877. https://doi.org/10.1128/AEM.03296-13
del Carmen S, Miyoshi A, Azevedo V, de Moreno de LeBlanc A, LeBlanc JG (2015) Evaluation of a Streptococcus thermophilus strain with innate anti-inflammatory properties as a vehicle for IL-10 cDNA delivery in an acute colitis model. Cytokine 73(2):177–183. https://doi.org/10.1016/j.cyto.2015.02.020
Tejada-Simon MV, Pestka JJ (1999) Proinflammatory cytokine and nitric oxide induction in murine macrophages by cell wall and cytoplasmic extracts of lactic acid bacteria. J Food Protect 62(12):1435–1444. https://doi.org/10.4315/0362-028x-62.12.1435
Adams CA (2010) The probiotic paradox: live and dead cells are biological response modifiers. Nutr Res Rev 23(1):37–46. https://doi.org/10.1017/S0954422410000090
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160:1–40. https://doi.org/10.1016/j.cbi.2005.12.009
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87(7):1157–1180. https://doi.org/10.1007/s00204-013-1034-4
Songisepp E, Kals J, Kullisaar T, Mandar R, Hutt P, Zilmer M, Mikelsaar M (2005) Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutr J 4:22. https://doi.org/10.1186/1475-2891-4-22
Amaretti A, Di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97:809–817. https://doi.org/10.1007/s00253-012-4241-7
Kapila S, Kapila R, Reddi S, Sinha PR (2014) Oral administration of probiotic Lactobacillus casei spp. casei ameliorates oxidative stress in rats. Int J Curr Microbiol Appl Sci. 3:670–684
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem 63:3615–3626. https://doi.org/10.1021/jf506326t
Kleniewska P, Hoffmann A, Pniewska E, Pawliczak R (2016) The influence of probiotic Lactobacillus casei in combination with prebiotic inulin on the antioxidant capacity of human plasma. Oxid Med Cell Longev 2016:1340903. https://doi.org/10.1155/2016/1340903
Wang Y, Wu Y, Wang Y, Fu A, Gong L, Li W et al (2017) Bacillus amyloliquefaciens SC06 alleviates the oxidative stress of IPEC-1 via modulating Nrf2/Keap1 signaling pathway and decreasing ROS production. Appl Microbiol Biotechnol 101(7):3015–3026. https://doi.org/10.1007/s00253-016-8032-4
Moshage H, Kok B, Huizenga JR, Jansen PL (1995) Nitrite and nitrate determination in plasma: a critical evaluation. Clin Chem 41:892–896
Hausladen A, Stamler JS (1999) Nitrosative stress. Methods Enzymol 300:389–395. https://doi.org/10.1016/s0076-6879(99)00143-3
Murphy MP (1999) Nitric oxide and cell death. Biochem Biophys Acta 1411:401–414. https://doi.org/10.1016/s0005-2728(99)00029-8
Zhao BB, Meng J, Zhang QX, Kang TT, Lu RR (2017) Protective effect of surface layer proteins isolated from four Lactobacillus strains on hydrogen-peroxide-induced HT-29 cells oxidative stress. Int J Biol Macromol 102:76–83. https://doi.org/10.1016/j.ijbiomac.2017.03.160
Xing JL, Wang G, Zhang QX, Liu X, Yin B, Fang D et al (2015) Determining antioxidant activities of lactobacilli by cellular antioxidant assay in mammal cells. Funct Foods 19:554–562. https://doi.org/10.1371/journal.pone.0119058
Magistrelli L, Amoruso A, Mogna L, Graziano T, Cantello R, Pane M et al (2019) Probiotics may have beneficial effects in Parkinson’s disease: in vitro evidence. Front Immunol 10:969. https://doi.org/10.3389/fimmu.2019.00969
Warshakoon HJ, Hood JD, Kimbrell MR, Malladi S, Wu WY, Shukla NM (2009) Potential adjuvantic properties of innate immune stimuli. Hum Vaccin 5:381–394. https://doi.org/10.4161/hv.5.6.8175
Acknowledgements
UNESC and Gabbia Biotechnology
Funding
Gabbia Biotechnology and UNESC.
Author information
Authors and Affiliations
Contributions
MM contributed to experimental planning, data collection, data analysis, and writing of paper. GFAJ contributed to experimental planning and data analysis. APLV, MR, and FR contributed to experimental planning. EC and PF contributed to data collection and data analysis. DG and FDP contributed to experimental planning, data analysis, and writing of the paper.
Corresponding author
Ethics declarations
Conflict of interests
Gabbia Biotechnology is developing paraprobiotics for the commercial purposes. Gabriel Jesus, Marina Rosseto, and Ana Paula Voytena are members of Gabbia Biotechnology. The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Consent to Publication
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship, but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Data Availability
Data will be made available on reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Michels, M., Jesus, G.F.A., Voytena, A.P.L. et al. Immunomodulatory Effect of Bifidobacterium, Lactobacillus, and Streptococcus Strains of Paraprobiotics in Lipopolysaccharide-Stimulated Inflammatory Responses in RAW-264.7 Macrophages. Curr Microbiol 79, 9 (2022). https://doi.org/10.1007/s00284-021-02708-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-021-02708-1