Abstract
A Gram-staining-negative, aerobic, flagellated, motile, rod-shaped, halophilic bacterium QX-2T was isolated from the deep-sea sediment of the Southwest Indian Ocean at a depth of 2699 m. Growth of the QX-2T bacteria was observed at 4–50 °C (optimum 30 °C), pH 5.0–12.0 (optimum pH 6.0) and 0%–30% NaCl (w/v) [optimum 4% (w/v)]. 16S rRNA gene sequencing revealed that strain QX-2T has the closest relationship with Halomonas titanicae DSM 22872T (98.2%). Phylogeny analysis classified the strain QX-2T into the genus Halomonas. The average nucleotide identity and DNA–DNA hybridization values between strain QX-2T and related type strains were lower than the currently accepted new species definition standards. Principal fatty acids (> 10%) determined were C16:0 (12.41%), C12:0-3OH (25.15%), summed feature 3 (C16:1 ω7c and/or C16:1 ω6c, 11.55%) and summed feature 8 (C18:1 ω7c and/or C18:1 ω6c, 16.06%). Identified polar lipids in strain QX-2T were diphosphatidylglycerol, phosphatidylglycerol, phosphatidylethanolamine, unidentified phospholipid, unidentified aminophospholipid and five unidentified lipids (L1–L5). The main respiratory quinone was Q-9. The content of DNA G+C was determined to be 54.34 mol%. The results of phylogenetic analysis, phenotypic analysis and chemotaxonomic studies showed that strain QX-2T represents a novel species within the genus Halomonas, for which the name Halomonas sedimenti sp. nov. is proposed, with the type strain QX-2T (MCCC 1A17876T = KCTC 82199T).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Halomonas belongs to the family Halomonadaceae, which was described originally by Vreeland et al. [1]. Currently, the number of validly published Halomonas species is 105 (https://lpsn.dsmz.de/genus/halomonas). The genus Halomonas is usually described as Gram-staining-negative bacilli that tolerate or like salt, and species are isolated from a variety of habitats, e.g., saline-alkali land, saline lakes and marine habitats [2,3,4,5,6,7,8]. Halomonas adopt unique structure and physiological mechanism, displays strong adaptation to the environment and has high research and utilization value.
Among the published species, there are few studies on Halomonas from a deep-sea environment. Results have revealed that Halomonas has a unique adaptive mechanism to survive in the extreme environment of the deep-sea and can tolerate changes in temperature and salinity and heavy metal stress [3]. Heavy metals are stable and cannot be degraded or eliminated easily [9]. Therefore, the mechanism of Halomonas strain resistances to heavy metal stress may provide new concepts to prevent and control heavy metal pollution. In addition, it has been found that many halotolerant and halophilic bacteria can synthesize ectoine, such as Spiribacter salinus [10], Paenibacillus lautus [11], Halomonas elongate [12] and Halomonas boliviensis [13]. Ectoine is an important osmotic pressure regulator, which may explain the tolerance of Halomonas strains to high salt environments. On the basis of its moisturizing and radiation protection properties, ectoine has been used in high-grade cosmetic preparations. There is no chemical synthesis method for ectoine. Thus, exploring the biosynthetic pathway and developing a mass fermentation method of ectoine are important research topics.
During an investigation into the biodiversity of the deep-sea sediment of the Southwest Indian Ocean, Strain QX-2T, which is closely related to the genus Halomonas, was isolated and the exact taxonomic position of this novel strain was determined based on polyphasic taxonomic data. In addition, genes associated with resistance to heavy metal stress and ectoine synthesis in strain QX-2T were analyzed.
Materials and Methods
Isolation and Cultivation
Strain QX-2T was isolated from deep-sea sediment samples collected between May and June 2016 at the SWIR (Southwest Mid-Indian Ridge) area at a depth of 2699 m in the Southwest Indian Ocean (47.43° W, 38.76° S) by the TV grab method. The sample was enriched in marine broth (MB; BD Difco, Franklin Lakes, NJ, USA) containing 10% NaCl (w/v) at 10 °C for 7 days. Cultures were then diluted according to set ratios (i.e., 10–1, 10–2, 10–3, 10–4 and 10–5) and plated onto marine agar (MA, BD Difco) containing 10% NaCl (w/v). Pure cultures were obtained after cultivating for ~ 30 days. Pure bacterial liquid cultures in a 15% glycerol solution were stored at – 80 °C.
Morphological, Physiological and Biochemical
Gram staining was carried out according to the instructions of the Gram Staining Kit (Hangzhou Tianhe microbial reagent). The size and morphology of the cells were observed by transmission electron microscopy (JEM-1230, JEOL). Cell movement was monitored by the hanging drop method [14].
Growth of strain QX-2T was examined at 0, 4, 10, 15, 20, 25, 30, 37, 45, 50, 55 and 60 °C in MB. The pH range for growth was determined in MB at intervals of 1 pH unit with citrate/phosphate (pH 3.0–7.0), Tris/HCl (pH 8.0–9.0) or sodium carbonate/sodium bicarbonate (pH 10.0–12.0) buffers. Growth of strain QX-2T under different salt concentrations was examined by setting the salinity of MB to 0, 0.5%, 1%, 2%, 3%, 4%, 5%, 10%, 15%, 20%, 25%, 30% or 35% (w/v).
API ZYM, API 20NE, API 20E, API 50CH reagent strips (BioMérieux) and the GEN III microplate (Biology) were used to detect enzyme production, hydrolysis and substrate utilization by the QX-2T strain and according to the manufacturer's instructions, with the single modification of adjusting the NaCl concentration to 3.0% in all tests. The IF A solution was used as the inoculum in GEN III microplate experiments. The turbidimeter was calibrated with a standard turbidimetric tube (85% T). The blank of the turbidimeter was adjusted by inoculating the IF A solution in the tube and adjusting the indicator of the turbidimeter to 100%. The bacterial suspension was prepared by scraping bacteria into the IF A inoculum. The light transmittance was controlled to 95% T. One hundred microliters of the bacterial suspension was added to all wells of the GEN III microplate. The microplate was incubated at the optimal growth temperature of QX-2T for 36 h and the results were observed and recorded. Oxidase activity and catalase activity were determined by adding tetramethyl p-phenylenediamine and 3% H2O2 to growing colonies, respectively. The strain was scraped from the fresh plate and smeared onto the oxidase reagent. An immediate change in color to purple or within a short period (i.e., within a minute) showed that the bacteria strain tested positive for oxidase activity. Strains that produced strong bubbles immediately after exposure to hydrogen peroxide indicated that oxidase activity was positive, whereas the presence of weak bubbles indicated a weak positive result. The absence of bubbles indicated that no oxidase activity was present. In addition, 0.2% (w/v) soluble starch, 0.8% (w/v) cellulose or 0.5% (v/v) Tween 20, 40, 60 or 80 were added to MA, respectively. The hydrolysis of these substrates by strain QX-2T was examined [15].
Chemotaxonomic Characterization
After culturing QX-2T on MA at 30 °C for 48 h, fatty acids in the cells were saponified, methylated and extracted according to the standard MIDI (Sherlock microbial identification system, version 6.0B) [16]. The extracted fatty acids were analyzed by gas chromatography (Agilent Technologies 6850), and the TSBA 6.0 database of the microbial identification system was used to identify the test results [17]. The polar lipids of strain QX-2T were extracted by a chloroform/methanol system and analyzed by one-dimensional and two-dimensional thin-layer chromatography (TLC), as described previously [18]. Merck silica gel 60 F254 aluminum back thin-layer plates aluminum-backed thin-layer plates were used in TLC analysis. Two-dimensional development of the dot-shaped sample plates used chloroform–methanol–water (65:25:4, by vol.) as the first solvent and chloroform–methanol–acetic acid–water (85:12:15:4, by vol.) as the second solvent. Subsequently, total lipid amounts were detected with molybdophosphoric acid and specific functional groups were detected using spray reagents for specific functional groups. The quinones were extracted and separated into their different classes by thin-layer chromatography on silica gel, then further analyzed by HPLC (SPD-10AV; Shimadzu), as described by Collins and Jones [19].
Molecular Analysis
The taxonomic position of strain QX-2T was determined by 16S rRNA gene sequencing [20]. The 16S rRNA gene sequence of strain QX-2T was obtained by PCR using bacterial 16S rRNA universal primers to amplify genomic DNA. Genomic DNA was extracted using the bacterial Genomic Extraction Kit (SBS) following the manufacturer’s instructions. In a 50 μL amplification system (SangonBiotech), bacterial universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-TACGGTTACCTTGTTACGACTT-3′) [21] and ExTaq were used to amplify the QX-2T 16S rRNA gene, and gene sequencing was carried out to obtain the 16S rRNA sequence. The GenBank database and EzTaxon (https://www.ezbiocloud.net) software were used to calculate the nucleotide sequence similarity among the 16S rRNA genes from the QX-2T strain and related type strains [22].
According to the results from the GenBank database and EzTaxon web software analysis, type strains with the highest similarity to the QX-2T 16S rRNA gene sequence were selected [23] and used for MEGA Version X phylogenetic analysis [24]. The distance option used was according to the Kimura two-parameter model, and the neighbor-joining (NJ) [25], maximum-likelihood (ML) [26] and minimum evolution (ME) [27] methods were used for clustering. Bootstrap values were calculated based on 1000 repeats.
The reference strains Halomonas titanicae (H. titanicae) DSM 22872T, H. glaciei CGMCC 1.7263T, H. variabilis DSM3051T and H. salicampi NBRC 109914T were obtained from the culture collections and used as controls in phenotypic tests. The draft genome of QX-2T, H. glaciei CGMCC 1.7263T and H. salicampi NBRC 109914T were obtained by Shanghai Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China). The Illumina paired end (500 bp library) sequencing technique was used. The clean data were assembled by SPAdes v3.8.1 using default settings [28]. The contigs longer than 1 kb and similar read coverage were kept for further analysis. The genome sequence data of H. titanicae DSM 22872T and H. variabilis DSM3051T were taken from NCBI with accession numbers AOPO00000000 and BJXV00000000, respectively. gyrB and rpoD gene sequences were extracted from the draft genome of strain QX-2T. BLAST analysis was then performed on gyrB and rpoD gene sequences using the GenBank database. The G+C content of chromosome DNA of the QX-2T strain was determined from the genomic draft sequence. The DNA–DNA hybridization (DDH) calculation was performed using the genome-to-genome calculation tool of the web version of GGDC 2.1 (http//ggdc.dsmz.de/ggdc.php) and the BLAST method [29, 30]. The average nucleotide identity (ANI) value between two genomes was calculated by the EZGenome's web service [31]. The genome annotation tool RAST (https://rast.nmpdr.org/) was used to annotated and analyzed the draft genome of strain QX-2T. Rastk was selected as a comment scheme.
Results and Discussion
Strain QX-2T was observed to be Gram-staining-negative, rod-shaped, flagellated and motile (Supplementary Fig. S1, available in the online Electronic Supplementary Material). Colonies that formed after 1 day incubation on MA at 30 °C were observed to be creamy, circular and 1 mm in diameter. Cells grew between 4 and 50 °C (optimum 30 °C), at pH values of 5.0–12.0 (optimum pH 6.0) and at NaCl concentrations of 0%–30% (w/v) (optimum 4% (w/v)). Catalase and oxidase activities were measured as positive. Starch, cellulose, Tween 20, 40, 60 and 80 were not hydrolyzed. Phenotypic traits that differentiate QX-2T from related type strains of Halomonas species are given in Table 1.
The principal fatty acids (> 10% of the total fatty acids) of strain QX-2T were determined to be C16:0 (12.41%), C12:0-3OH (25.15%), summed feature 3 (C16:1 ω7c and/or C16:1 ω6c, 11.55%) and summed feature 8 (C18:1 ω7c and/or C18:1 ω6c, 16.06%). The percentage of C12:0-3OH differs to that found in other related type strains. Whole-cell fatty acids are presented in Supplementary Table S1. The primary polar lipids of strain QX-2T were identified to be diphosphatidylglycerol (DPG), phosphatidylglycerol (PG), phosphatidylethanolamine (PE), unidentified phospholipid (PL), unidentified aminophospholipid (APL) and five unidentified lipids (L1–L5) (Supplementary Fig. S2). The main component of respiratory quinone for strain QX-2T was Q-9, which is also the main component for species in the genus Halomonas [36].
In this study, a near full-length 16S rRNA gene sequence of QX-2T (1446 nt) was obtained and this sequence was submitted to the NCBI under accession number MT372904. This sequence was consistent with the 16S rRNA gene sequence we extracted from the strain QX-2T draft genome data. Pairwise comparison of the 16S rRNA gene sequences using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and the Ezbiocloud website (https://www.ezbiocloud.net/identify) for the 16S rRNA gene sequence strains QX-2T and H. titanicae DSM 22872T, H. glaciei CGMCC 1.7263T, H. variabilis DSM 3051T and H. salicampi NBRC 109914T gave values of 98.2%, 98.1%, 97.8% and 97.5%, respectively.
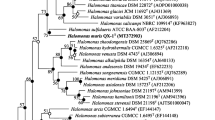
The phylogenetic tree based on the NJ method showed that strain QX-2T formed a clade with H. titanicae DSM 22872T. We used the gyrB (2421 nt, MT672351) and rpoD (1851 nt, MT672350) genes of strain QX-2T to perform phylogenetic tree analysis with other species in the family Halomonadaceae, and the results showed that strain QX-2T belongs to the genus Halomonas (Figs. S3 and S4). The NJ tree based on 16S rRNA gene sequences is shown in Fig. 1. Chromohalobacter canadensis ATCC 43984T (AJ295143) was used as out-group which belongs to the family Halomonadaceae. The ML and ME trees of 16S rRNA gene sequences presented a similar topology to that of the NJ-derived phylogenetic tree. Therefore, these trees were condensed into the NJ phylogenetic tree. The results of the phylogenetic analysis indicated that QX-2T is affiliated to the genus Halomonas.
Neighbor-Joining tree showing the phylogenetic positions of strain QX-2T and related species of the genus Halomonas based on 16S rRNA gene sequences. Bootstrap values (> 50%) based on 1000 replication are shown at branch points. Chromohalobacter canadensis ATCC 43984T (AJ295143) was used as out-group. Filled and open circles at nodes indicate generic branches that were also recovered using the Maximum-Likelihood and Minimum Evolution algorithms, and just the Minimum Evolution algorithm, respectively. Bar, 0.005 substitutions per nucleotide position
A total of 1 Gbp clean data was generated to reach about 200-fold depth of coverage using the Illumina HiSeq 2000 platform. The genome size of strain QX-2T was 4.94 Mb, including 61 contigs. The N50 value of the genome sequence of QX-2T was 214,892, and the L50 value was 9 (Table S2). The accession number for strain QX-2T was JACCGK000000000 at the DDBJ/ENA/GenBank. We also obtained the draft genome of H. glaciei and H. salicampi, under accession numbers JACCDE000000000 and JACCDF000000000, respectively. The G+C content of the genomic DNA of strain QX-2T was 54.34 mol%, which is consistent with the description range of the genus Halomonas [37, 38].
The DDH estimate values of QX-2T were 58.90% with H. titanicae DSM 22872T, 30.10% with H. glaciei CGMCC 1.7263T, 22.90% with H. variabilis DSM 3051T and 21.30% with H. salicampi NBRC 109914T (Table S3). The ANI values of QX-1T were 94.11% with H. titanicae DSM 22872T, 85.96% with H. glaciei CGMCC 1.7263T, 67.26% with H. variabilis DSM 3051T and 75.65% with H. salicampi NBRC 109914T (Table S4). The DDH similarity and ANI values between strain QX-2T and the reference bacteria are within the range of QX-2T being identified as a new species, which requires a DDH similarity lower than the recommended value of ± 70% [39] and ANI values below the standard criteria for species identity (95%–96%) [40].
Annotation results of the draft genome showed that there were 4890 coding genes in the genome of strain QX-2T, including 65 RNA coding genes (Table S2). We found 35 genes encoding for proteins involved in heavy metal resistance for strain QX-2T (Table S5). These genes provided heavy metal resistance to mercury, arsenic, manganese, cobalt, zinc, cadmium, lead and copper. This resistance most likely arises from adaptation of strain QX-2T to the extreme environment of the southwest Indian Ocean hydrothermal region; this is a deep-sea hydrothermal region that is a typical heavy metal rich area [41]. Modern industry continues to produce large quantities of waste-water containing heavy metals that are a major hidden danger to human safety and the environment. In recent years, biosorbents have been used for treating environmental pollution because these products are readily available, economically viable and display fast adsorption properties [42]. Given that strain QX-2T produces proteins that afford heavy metal resistance, this strain has potential use in the development of biosorbents and offers insights into the effective control of heavy metal pollutants.
Microorganisms in extreme environments accumulate compounds to survive in high osmotic environments. These compounds mainly include amino acids and their derivatives, polyols, sugars, betaine and ectoine [43, 44]. Ectoine is the most common compatible solute synthesized by moderately halophilic bacteria. Ectoine is compatible with intracellular metabolism, balances osmotic pressure inside and outside the cell, protects against osmotic stress caused by high salt concentrations and is compatible with the intracellular system without affecting the function of other biological macromolecules [45]. Thus, ectoine protects cells and biomacromolecules under adverse environmental conditions [12, 46]. Therefore, ectoine has important application value in pharmaceutical preparations [47], cell protectants [48], cosmetics and other fields.
Genes associated with the synthesis of ectoine were identified in the genome of strain QX-2T. Previous studies have shown that the biosynthesis of ectoine involves a series of enzymatic reactions. The genes ectA, ectB and ectC of strain QX-2T may encode l-diaminobutyric acid transaminase, l-diaminobutyric acid acetyltransferase and ectoine synthase, respectively, and using aspartate semialdehyde as the precursor substrate these three enzymes can catalyze the synthesis of ectoine in three steps [12]. The four genes doeA, doeB, doeC and doeD of strain QX-2T may encode proteases that degrade ectoine [12]. The TeaABC system belongs to the TRAP-T transporter family, which may participate in the absorption and excretion of ectoine and regulate the biosynthesis of ectoine [49]. In addition, we also found the rpoS regulatory factor in strain QX-2T, which is similar to that found in strain Chromohalobacter salexigens. This gene product may also participate in the biosynthesis of ectoine [50]. However, although there were genes related to the synthesis of ectoine in the genome of strain QX-2T, the specific biosynthetic pathway for producing ectoine requires further studies and evaluation.
Conclusion
Phylogenetic analysis, phenotypic analysis and chemotaxonomic studies confirmed that strain QX-2T represents a novel species within the genus Halomonas, for which the name Halomonas sedimenti sp. nov. is proposed. The genes encoding heavy metal resistance proteins and ectoine synthesis were found in the draft genome of strain QX-2T. Thus, strain Halomonas sedimenti sp. nov. QX-2T offers great potential in various commercial applications.
Taxonomic and Nomenclatural Proposals
Description of Halomonas sedimenti sp. nov.
Halomonas sedimenti (se.di.men’ti L. gen. n. sedimenti, of sediment, referring to the sediment of the Southwest Indian Ocean, where the type strain was isolated).
Cells are Gram-staining-negative, aerobic, halophilic, motile and rod-shaped with dimensions of 0.7–0.9 μm wide and 2.3–3.0 μm long. Colonies on MA are creamy, convex, glossy, smooth, circular with an entire margin and 1 mm in diameter after 1 day of incubation at 30 °C. Growth occurs in MB with 0%–30% NaCl (optimum 4%), at 4–50 °C (optimum 30 °C) and over the pH range of 5.0–12.0 (optimum pH 6.0). Starch, cellulose, gelatin, Tween 20, 40, 60 and 80 were not hydrolyzed. Catalase and oxidase activities were positive. Nitrate was reduced to nitrite. Indole and H2S were not produced. Enzyme activity was observed for alkaline phosphatase, esterase (C4), esterase lipase (C8), leucine arylamidase, valine arylamidase, cystine arylamidase, naphthol-AS-BI-phosphohydrolase, α-glucosidase, urease, arginine hydrolase and phenylalanine deaminase, but not for lipase (C14), trypsin, chymotrypsin, acid phosphatase, α-galactosidase, β-galactosidase, β-glucosidase, β-glucuronidase, α-mannosidase, α-fucosidase lysine decarboxylase or ornithine decarboxylase. Acid was produced from l-arabinose, d-fructose and aesculin, but not from d-ribose, d-xylose, l-xylose, d-galactose, d-glucose, l-rhamnose, cellobiose, d-maltose, d-lactose, melibiose, sucrose, trehalose, raffinose, d-mannitol, d-sorbitol, inositol, 2-ketogluconate or 5-ketogluconate. Strain QX-2T utilized d-maltose, d-trehalose, d-cellobiose, gentiobiose, sucrose, d-turanose, d-lactose, d-melibiose, d-salicin, d-glucose, d-mannose, d-fructose, d-galactose, d-fucose, l-fucose, l-rhamnose, d-sorbitol, d-mannitol, d-arabitol, myo-inositol, d-fructose-6-PO4, d-aspartic acid, l-alanine, l-aspartic acid, l-glutamic acid, d-malic acid, l-malic acid, dextrin, inosine, glycerol, gelatin and citrate, but not arabinose, stachyose, d-raffinose, d-Glucose-6-PO4, d-serine, l-serine, l-arginine, l-histidine or d-saccharic. Principal fatty acids (> 10%) determined were C16:0 (12.41%), C12:0-3OH (25.15%), summed feature 3 (C16:1 ω7c and/or C16:1 ω6c, 11.55%) and summed feature 8 (C18:1 ω7c and/or C18:1 ω6c, 16.06%). The polar lipids were DPG, PG, PE, PL, APL and L1–L5. The main respiratory ubiquinone was Q-9. The DNA G+C content of the type strain was 54.34 mol%.
The type strain, QX-2T (= MCCC 1A17876T = KCTC 82199T), was isolated from deep-sea sediment of the Southwest Indian Ocean at 2699 m. The GenBank accession number of the 16S rRNA gene sequence of strain QX-2T is MT372904, and the draft genome sequence accession number of strain QX-2T is JACCGK000000000.
Abbreviations
- MCCC:
-
Marine Culture Collection of China
- CGMCC:
-
China General Microbiological Culture Collection Center
- KCTC:
-
Korean Collection for Type Cultures
- DSM:
-
Leibniz Institut Deutsche Sammlung von Mikroorganismen und Zellkulturen (German Collection of Microorganisms and Cell Cultures)
- ANI:
-
Average nucleotide identity
- DDH:
-
DNA–DNA hybridization
References
Vreeland RH, Litchfield CD, Martin EL, Elliot E (1980) Halomonas elongata, a new genus and species of extremely salt-tolerant bacteria. Int J Syst Evol Microbiol 30(3):485–495. https://doi.org/10.1099/00207713-30-2-485
Poli A, Nicolaus B, Denizci AA, Yavuzturk B, Kazan D (2013) Halomonas smyrnensis sp. nov., a moderately halophilic, exopolysaccharide-producing bacterium. Int J Syst Evol Microbiol 63(1):10–18. https://doi.org/10.1099/ijs.0.037036-0
Kaye JZ, Marquez MC, Ventosa A, Baross JA (2004) Halomonas neptunia sp. nov., Halomonas sulfidaeris sp. nov., Halomonas axialensis sp. nov. and Halomonas hydrothermalis sp. nov.: halophilic bacteria isolated from deep-sea hydrothermal-vent environments. Int J Syst Evol Microbiol 54(Pt 2):499–511. https://doi.org/10.1099/ijs.0.02799-0
Jiang J, Pan Y, Hu S, Zhang X, Hu B, Huang H, Hong S, Meng J, Li C, Wang K (2014) Halomonas songnenensis sp. nov., a moderately halophilic bacterium isolated from saline and alkaline soils. Int J Syst Evol Microbiol 64(Pt 5):1662–1669. https://doi.org/10.1099/ijs.0.056499-0
Gan L, Long X, Zhang H, Hou Y, Tian J, Zhang Y, Tian Y (2018) Halomonas saliphila sp. nov., a moderately halophilic bacterium isolated from a saline soil. Int J Syst Evol Microbiol 68(4):1153–1159. https://doi.org/10.1099/ijsem.0.002644
Wang T, Wei X, Xin Y, Zhuang J, Shan S, Zhang J (2016) Halomonas lutescens sp. nov., a halophilic bacterium isolated from a lake sediment. Int J Syst Evol Microbiol 66(11):4697–4704. https://doi.org/10.1099/ijsem.0.001413
Ming H, Ji WL, Li M, Zhao ZL, Cheng LJ, Niu MM, Zhang LY, Wang Y, Nie GX (2020) Halomonas lactosivorans sp. nov., isolated from salt-lake sediment. Int J Syst Evol Microbiol 70(5):3504–3512. https://doi.org/10.1099/ijsem.0.004209
Xu L, Xu XW, Meng FX, Huo YY, Oren A, Yang JY, Wang CS (2013) Halomonas zincidurans sp. nov., a heavy-metal-tolerant bacterium isolated from the deep-sea environment. Int J Syst Evol Microbiol 63(Pt 11):4230–4236. https://doi.org/10.1099/ijs.0.051656-0
Twardowska I (2004) Ecotoxicology, environmental safety, and sustainable development–challenges of the third millennium. Ecotoxicol Environ Saf 58(1):3–6. https://doi.org/10.1016/j.ecoenv.2004.03.008
Leon MJ, Hoffmann T, Sanchez-Porro C, Heider J, Ventosa A, Bremer E (2018) Compatible solute synthesis and import by the moderate halophile Spiribacter salinus: physiology and GENOMICS. Front Microbiol 9:108. https://doi.org/10.3389/fmicb.2018.00108
Richter AA, Mais CN, Czech L, Geyer K, Hoeppner A, Smits SHJ, Erb TJ, Bange G, Bremer E (2019) Biosynthesis of the stress-protectant and chemical chaperon ectoine: biochemistry of the transaminase EctB. Front Microbiol 10:2811. https://doi.org/10.3389/fmicb.2019.02811
Schwibbert K, Marin-Sanguino A, Bagyan I, Heidrich G, Lentzen G, Seitz H, Rampp M, Schuster SC, Klenk HP, Pfeiffer F, Oesterhelt D, Kunte HJ (2011) A blueprint of ectoine metabolism from the genome of the industrial producer Halomonas elongata DSM 2581 T. Environ Microbiol 13(8):1973–1994. https://doi.org/10.1111/j.1462-2920.2010.02336.x
Van-Thuoc D, Guzman H, Quillaguaman J, Hatti-Kaul R (2010) High productivity of ectoines by Halomonas boliviensis using a combined two-step fed-batch culture and milking process. J Biotechnol 147(1):46–51. https://doi.org/10.1016/j.jbiotec.2010.03.003
Skerman VBD (1960) A guide to the identification of the genera of Bacteria. Q Rev Biol 36(2):870
Dong X-Z, Cai M-Y (2001) Determinative manual for routine bacteriology. Scientific Press, Beijing (English translation)
Fykse EM, Tjarnhage T, Humppi T, Eggen VS, Ingebretsen A, Skogan G, Olofsson G, Wasterby P, Gradmark PA, Larsson A, Dybwad M, Blatny JM (2015) Identification of airborne bacteria by 16S rDNA sequencing, MALDI-TOF MS and the MIDI microbial identification system. Aerobiologia 31(3):271–281. https://doi.org/10.1007/s10453-015-9363-9
Sasser M (1990) Identification of bacteria by gas chromatography of cellular fatty acids. USFCC Newsl 20:1–6
Kates M (1986) Lipid extraction procedures. Techniques of lipidology. Elsevier, Amsterdam, pp 100–111
Collins MD, Jones D (1981) Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev 45(2):316–354
Olsen GJ, Woese CR (1993) Ribosomal RNA: a key to phylogeny. FASEB J 7:113–123. https://doi.org/10.2307/2420341
Lane DJ (1991) 16S/23S rRNA sequencing. Nucleic Acid Tech. Bacterial Syst. 463:115–175
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62(Pt 3):716–721. https://doi.org/10.1099/ijs.0.038075-0
Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J (2017) Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol 67(5):1613–1617. https://doi.org/10.1099/ijsem.0.001755
Kumar S, Stecher G, Tamura KJMB (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 bigger dataset. Evolution 33(7):1870
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Felsenstein J (1981) Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol 17(6):368–376
Rzhetsky A, Nei M (1992) Statistical properties of the ordinary least-squares, generalized least-squares, and minimum-evolution methods of phylogenetic inference. J Mol Evol 35(4):367–375
Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA (2012) SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19(5):455–477. https://doi.org/10.1089/cmb.2012.0021
Oguntoyinbo FA, Cnockaert M, Cho GS, Kabisch J, Neve H, Bockelmann W, Wenning M, Franz C, Vandamme P (2018) Halomonas nigrificans sp. nov., isolated from cheese. Int J Syst Evol Microbiol 68(1):371–376. https://doi.org/10.1099/ijsem.0.002515
Meier-Kolthoff JP, Auch AF, Klenk HP, Goker M (2013) Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 14:60. https://doi.org/10.1186/1471-2105-14-60
Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM (2007) DNA–NA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57(Pt 1):81–91. https://doi.org/10.1099/ijs.0.64483-0
Sanchez-Porro C, Kaur B, Mann H, Ventosa A (2010) Halomonas titanicae sp. nov., a halophilic bacterium isolated from the RMS Titanic. Int J Syst Evol Microbiol 60(Pt 12):2768–2774. https://doi.org/10.1099/ijs.0.020628-0
Reddy GS, Raghavan PU, Sarita NB, Prakash JS, Nagesh N, Delille D, Shivaji S (2003) Halomonas glaciei sp. nov. isolated from fast ice of Adelie Land, Antarctica. Extremophiles 7(1):55–61. https://doi.org/10.1007/s00792-002-0295-2
Sorokin DY, Tindall BJ (2006) The status of the genus name Halovibrio Fendrich 1989 and the identity of the strains Pseudomonas halophila DSM 3050 and Halomonas variabilis DSM 3051. Request for an opinion. Int J Syst Evol Microbiol 56(Pt 2):487–489. https://doi.org/10.1099/ijs.0.63965-0
Lee JC, Kim YS, Yun BS, Whang KS (2015) Halomonas salicampi sp. nov., a halotolerant and alkalitolerant bacterium isolated from a saltern soil. Int J Syst Evol Microbiol 65(12):4792–4799. https://doi.org/10.1099/ijsem.0.000650
Dobson SJ, Franzmann PD (1996) Unification of the Genera Deleya (Baumann et al. 1983), Halomonas (Vreeland et al. 1980), and Halovibrio (Fendrich 1988) and the Species Paracoccus halodenitrificans (Robinson and Gibbons 1952) into a Single Genus, Halomonas, and Placement of the Genus Zymobacter in the Family Halomonadaceae. Int J Syst Evol Microbiol 46(2):550–558. https://doi.org/10.1099/00207713-46-2-550
Franzmann PD, Wehmeyer U, Stackebrandt E (1988) Halomonadaceae fam. nov., a new family of the class proteobacteria to accommodate the genera halomonas and deleya. Syst Appl Microbiol 11(1):16–19. https://doi.org/10.1016/S0723-2020(88)80043-2
Martinez-Canovas MJ, Quesada E, Llamas I, Bejar V (2004) Halomonas ventosae sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int J Syst Evol Microbiol 54(Pt 3):733–737. https://doi.org/10.1099/ijs.0.02942-0
Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Rooney AP, Yi H, Xu XW, De Meyer S, Trujillo ME (2018) Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol 68(1):461–466. https://doi.org/10.1099/ijsem.0.002516
Richter M, Rossello-Mora R (2009) Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106(45):19126–19131. https://doi.org/10.1073/pnas.0906412106
Edgcomb VP, Molyneaux SJ, Saito MA, Lloyd K, Boer S, Wirsen CO, Atkins MS, Teske A (2004) Sulfide ameliorates metal toxicity for deep-sea hydrothermal vent archaea. Appl Environ Microbiol 70(4):2551–2555. https://doi.org/10.1128/aem.70.4.2551-2555.2004
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92(3):407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Ben-Amotz A, Avron M (1983) Accumulation of metabolites by halotolerant algae and its industrial potential. Annu Rev Microbiol 37:95–119. https://doi.org/10.1146/annurev.mi.37.100183.000523
Oren A (2008) Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst 4:2. https://doi.org/10.1186/1746-1448-4-2
Satyanarayana T (2012) Microorganisms in environmental management. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2229-3
Zaccai G, Bagyan I, Combet J, Cuello GJ, Deme B, Fichou Y, Gallat FX, Galvan Josa VM, von Gronau S, Haertlein M, Martel A, Moulin M, Neumann M, Weik M, Oesterhelt D (2016) Neutrons describe ectoine effects on water H-bonding and hydration around a soluble protein and a cell membrane. Sci Rep 6:31434. https://doi.org/10.1038/srep31434
Salvador M, Argandona M, Naranjo E, Piubeli F, Nieto JJ, Csonka LN, Vargas C (2018) Quantitative RNA-seq analysis unveils osmotic and thermal adaptation mechanisms relevant for ectoine production in Chromohalobacter salexigens. Front Microbiol 9:1845. https://doi.org/10.3389/fmicb.2018.01845
Czech L, Hoppner A, Kobus S, Seubert A, Riclea R, Dickschat JS, Heider J, Smits SHJ, Bremer E (2019) Illuminating the catalytic core of ectoine synthase through structural and biochemical analysis. Sci Rep 9(1):364. https://doi.org/10.1038/s41598-018-36247-w
Grammann K, Volke A, Kunte HJ (2002) New type of osmoregulated solute transporter identified in halophilic members of the bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581(T). J Bacteriol 184(11):3078–3085. https://doi.org/10.1128/jb.184.11.3078-3085.2002
Salvador M, Argandoña M, Pastor JM (2015) Contribution of RpoS to metabolic efficiency and ectoines synthesis during the osmoand heat-stress response in the halophilic bacterium Chromohalobacter salexigens. Environmental Microbiology Reports 7(2):301–311
Funding
This work was supported by the COMRA Project of China (DY135-B2-16), COMRA Project of China (DY135-B2-08) and National Basic Research Program of China (973 Program) (No.2015CB755901).
Author information
Authors and Affiliations
Contributions
XQ performed the technical characterization on strain QX-2 and drafted the manuscript. LY, XC, HW and GX conceived the study and aided to draft the manuscript. XT conceived the study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Qiu, X., Yu, L., Cao, X. et al. Halomonas sedimenti sp. nov., a Halotolerant Bacterium Isolated from Deep-Sea Sediment of the Southwest Indian Ocean. Curr Microbiol 78, 1662–1669 (2021). https://doi.org/10.1007/s00284-021-02425-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02425-9