Abstract
Amoxicillin (AMX) is one of the most widely used antibiotics in the world and its presence in wastewater is of great concern for its potential to bacteria selection. However, there is still a gap about the toxicity effect of AMX in nitrifier biomass from activated sludge (AS). This study is based on the implementation of respirometric tests in batches in order to evaluate the toxic effluent toxicity in the nitrification process of AS. The tests were conducted by comparing respiration rates with effluent containing ammonia nitrogen (NH4+-N) and nitrite nitrogen (NO2−-N) called “reference” and batches containing toxic effluent doped with different concentrations of AMX here called “process.” Results with effluent containing concentrations greater than 100 mg L−1 showed that AMX negatively affected the specific growth rate (μm) of ammonia-oxidizing bacteria (AOB) (from 0.50 d−1 to 0.13 d−1) and nitrite-oxidizing bacteria (NOB) (from 0.64 d−1 to 0.15 d−1). Although there is no total inhibition of populations, these μm values are limiting for a feasible development of the nitrification process in AS systems. The removal of AMX decreased from 99 to 37% (liquid phase) when the concentration of AMX increased (20 mg L−1 to 200 mg L−1). A decrease in the microbial community AOB and NOB was observed through fluorescent in situ hybridization (FISH), corroborating the results of respirometry. In summary, the study showed that the inhibition of the AS nitrification process occurs in the presence of high concentrations of AMX and the most susceptible group are the NOB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The use of antibiotics in the world grew 65% between the years 2005 and 2015, with developing countries being the main responsible for this increase [1, 2], it is also estimated that the consumption of drugs will be 63.5% higher until 2020, and that 20–50% of the use of antibiotics is undue, increasing those of side effects and microbial resistance [3, 4]. Amoxicillin (AMX), which belongs to the penicillin group, is one of the most widely used antibiotics, reaching more than half of the consumption of these drugs in Brazil [2, 5], for its broad action spectrum and few side effects, in addition to its low cost [6]. Despite the verified efficiency, when ingested, only 10–20% of the compound is metabolized by the organism, the rest is excreted in parental and metabolized forms, thus reaching effluent treatment stations [7].
In 2018, the European Union released a new version of the “Watch List,” which contains the presence of several antibiotics, including AMX, which can present ecotoxicity in several species, such as algae, plants, and invertebrates, in concentrations from 0.75 µg L−1, in addition to bioaccumulating in fish muscles and consequently being consumed passively, which can generate health and microbial resistance problems [6, 8, 9]. Table 1 shows the average values found for AMX in wastewater and surface water.
Among the biological treatment processes for wastewater, the process known as activated sludge (AS) is one of the most used in the world. It is recognized for the high performance of removing organic material and, when optimized for nutrients, it is possible to obtain NH4+-N removal efficiencies greater than 95% [10]. Most studies on AS focus on the removal of emerging compounds and few on the effects of inhibiting biochemical activities involved in the process. Studies described by [11] analyzed the removal of different emerging pollutants in AS. Among them some antibiotics, the study showed a 40 to 50% removal efficiency for trimethoprim. Regarding AMX [12], achieved removal results in the order of 80% from concentrations of 100 ng L−1 in AS [13]. The removal of diclofenac in AS was evaluated by means of respirometric tests. The concentration of the anti-inflammatory was between 5 and 50 mg L−1, the removals varied between 18.4 and 57.3%, with the lowest removal rate referring to the highest concentration tested of 50 mg L−1. It is believed that the low removal is due to a toxic effect on the microorganisms present in the sludge, and for better removal results, a previous adaptation of the sludge to diclofenac could be performed [14]. Bacterial sensitivity in AS was evaluated in different antibiotics. The concentrations were 10 mg L−1 for all drugs analyzed, and it was concluded that the antibiotics amoxicillin, roxithromycin, sulfamethoxazole, and tetracycline at these concentrations did not affect the removal of organic matter and nitrogen, requiring further studies with different parameters for better understanding the effects of these antibiotics [15]. The researchers Xing and Jin [15] studied the effect of heavy metals and antibiotics on nitrifying bacteria through respirometry, AS with partial (NO2−-N) nitrogen removal, sulfamethoxazole concentrations ranged from 290–3490 mg L−1, the concentration referring to greater inhibition (91%), was 1890 mg L−1, which also corresponds to the IC50. In addition to the toxic effect that drugs impose on the microbial community of AS, which can impair the removal of organic matter or nitrogen, there is also the possibility of generating resistant microorganisms, especially due to the presence of antibiotics [16], the presence of these resistant microorganisms can be a concern in the contamination of environmental matrices through reuse, as well as the use of sludge as a fertilizer [17]. In addition, it can directly affect the biological nitrogen removal process, as it affects the nitrification process (Ammonia-oxidizing bacteria (AOB) and Nitrite-oxidizing bacteria (NOB)).
Recent studies [18,19,20,21,22] show the toxic effects related to the presence of antibiotics both in the environment and in effluents, however, they are still limited, having studied the effects of few antibiotics, and also few studies address the nitrification process, an essential step in the treatment of effluents for nitrogen removal [23]. The chronic impact of tetracycline on biomass with enriched nitrifying community sustained in a lab-scale activated sludge system was evaluated. Activated sludge modeling results indicated inhibitory impact of tetracycline on the growth of nitrifiers with a significant increase of the half-saturation coefficients in corresponding rate equations. Tetracycline also inactivated biomass components of the enriched culture at a gradually increasing rate with time of exposure, leading to total collapse of nitrification. However, the effect of AMX information in AOB and NOB groups as well as the inhibition of the growth rates of these specific populations are few discussed in the literature. Within this context, the objective of this study was to evaluate the effect of AMX on autotrophic nitrifying bacteria and the effect on kinetic behavior in the nitrification process of AS. The assessment of the metabolism of AOB and NOB were made at different concentrations of antibiotic (AMX). The effect of AMX on the nitrification capacity and the degradation of AMX itself by microorganisms was evaluated. The affected bacterial groups were monitored using the fluorescent in situ hybridization (FISH) technique.
Materials and Methods
Respirometric Test
Respirometric methods are widely used to determine kinetic parameters in modeling activated sludge systems. Its application is recommended by the authors of activated sludge models (ASM), due to the fact that most processes of biological wastewater treatments involve the consumption of dissolved oxygen [24]. The main groups of microorganisms of interest for modeling the AS process are heterotrophic and autotrophic bacteria and phosphorus-accumulating organisms. It is assumed that these microorganisms undergo two main processes: growth (synthesis) and decay (endogenous respiration). These processes can be evidenced by means of respirometric tests, such as those performed by [25]. Respirometric tests can be performed with AS biomass with different types and concentrations of substrate. It is assumed that the growth of the bacteria of interest is limited by the concentration of the available substrate (S). Thus, the equation proposed by Monod (Eq. 1) can be applied to most biological models by AS in order to quantify the growth of nitrifying microorganisms. The application of Monod kinetics requires the estimation of two parameters: the specific maximum growth rate (µmax) and the half-saturation constant (KS).
In the present study, the experimental investigation was carried out by means of respirometry tests. The model described in [25] was used, which is based on the relationship between the oxygen consumption rate (OUR) and the amount of substrate added for a given microbial community. OUR was obtained through data on initial and final dissolved oxygen (DO) concentration in the AS in a given period of time (ΔT). Respirometric tests were performed with domestic wastewater doped with 4 different concentrations of AMX: 20, 50, 100, and 200 mg L−1 (all done in triplicate). These values were chosen to observe some effect of acute toxicity in the target bacteria group.
Tests were carried out in batches and the DO concentrations were measured with an Oakton model DO-6+ oximeter. Each test was performed with 1.0 L of AS, and the DO concentration was controlled to a minimum limit of 1.0 mg O2 L−1 to a maximum of 3.0 mg O2 L−1. The AS was collected in a wastewater treatment plant with nutrient removal (WWTP- Parque Andreense), located at Parque Billings Reservoir, Santo André—São Paulo, Brazil. WWTP is operated at a sludge age of 20 days, maintaining a microorganism food ratio (F/M) of 0.15 ± 0.05 kgBOD kgVSS−1 d−1, VSS concentration of 2500 mg L−1, and average annual temperature of 26 ± 5 ºC in tropical climate conditions. To carry out the tests, the AS was placed in a pilot scale reactor (volume 20 L) and was operated in batches (8 h of cycle and feed flow of 12 L day−1) and fed with synthetic domestic sewage (COD 600 mg L−1, TKN 50 mg L−1 and NH4+-N 40 mg L −1). The controlled oxygen concentration was in the range of 1.0 to 3.0 mg L−1 and VSS of 2122 ± 910 mg L−1 and F/M of 0.15 ± 0.07 kgBOD kgVSS−1 d−1. The tests were carried out after acclimatizing the sludge whose treated effluent presented an average concentration of 55 mg COD L−1, 4.5 mg TKN L−1, 1.5 mg NH4+-N L−1, < 0,2 mg NO2−-N L−1, and NO3−-N L−1. To evaluate the nitrifying bacteria, two substrates were used: Ammonium chloride for AOB and sodium nitrite for NOB. The alkalinity of the medium was initially adjusted before each test. Figure 1 shows the summary of the main stages of respirometric tests.
Kinetics of Nitrification and Experimental Methods for Its Determination
The maximum specific growth rates of nitrifying bacteria (µm), Monod half-saturation constants (Kn), and specific substrate utilization rate (rn) were determined based on the Eqs. 2, 3, 4, 5, 6, and 7. To determine the half-saturation constant (Kn), Monod kinetics was limited. Through the analysis of respirograms, when µ = ½ µmax or OURn = ½ OURn.max, the ratio between the area equivalent to the concentration of the residual substrate (ammonia or nitrite) was calculated by the respective stoichiometric oxygen coefficient − 4.57 for NH4+-N and 1.14 for NO2−-N [26].
Analytical Methods
Laboratory analyses were performed at the Laboratory of Urban Served Water Treatment (LabTAUS) at the Federal University of ABC-UFABC. All parameters were determined by methods in accordance with Standard Methods for the Examination of Water and Wastewater [27]. NH4+-N, NO2−-N, and NO3−-N were quantified by ion chromatography (Dionex-100, AS4A-SC) [28, 29].
AMX was determined by HPLC in reverse phase with DAD and automatic sampler (Agilent 1220). Mobile phase consisted of Acetonitrile and Water (HPLC grade, 1:1, v/v), with reading wavelength at 220 nm and a Zorbax 5B-CN column of 5 µl L−1, 4.6 × 150 mm, particle size of 5 μm, and flow rate at 1 ml min−1, to then read the samples.
Quantification was made by external calibration curve at AMX concentrations 5 mg L−1, 10 mg L−1, 25 mg L−1, 100 mg L−1, and 200 mg L−1. The calibration curve used to determine the concentrations of the collected samples had an R2 value of 0.9999, indicating that the data expressed by means of this equation have a high probability of being similar to the real value.
Characterization of Groups of Microorganisms Active in the Process by Molecular Biology—FISH
To investigate the effect of AMX on the gene expression of AOB and NOB in biological sludge, 2.0 mg of sludge sample was fixed in 4% paraformaldehyde for 16 and 24 h at 4 °C, and then washed twice in sodium phosphate buffer. The samples were dehydrated in 50%, 80%, and 100% ethanol for 10 min each. The ethanol in the biomass was completely replaced by xylene, by means of 3:1 series immersion; 1:1 and 1:3, ethanol:xylene solution, by volume, and finally in 100% xylene, for periods of 10 min at room temperature.
Then, the biomass was incorporated into paraffin (m.p. 56 and 57 ºC) by serial immersion in xylene-paraffin (1:1) for 30 min at 60 ºC, followed by 100% paraffin. After paraffin solidification, 8-mm-thick sections were prepared and placed on microscopic slides coated with gelatin. The paraffin was removed by immersing xylene and ethanol for 30 min each, followed by air drying the slides. Through the FISH technique, it was possible to identify microbial groups present in the biomass with relative simplicity. In this technique, oligonucleotide probes were used to identify specific organisms [30]. Table 2 shows the probes that were used in this study.
Hybridizations were performed at 46 °C for 2 h with a hybridization buffer (0.9 mol L−1 NaCl, formamide, 20 mmol L−1 Tris/HCl, pH 8.0, 0.01% SDS) containing each labeled probe (5.0 ng mL.µL−1). After hybridization, unbound oligonucleotides were removed by a washing step at 48 °C for 15 min in buffer containing the same components as the hybridization buffer, except for the probes. For the detection of all DNA, 4,6-diamidino-2-phenylindole (DAPI) was diluted with methanol to a final concentration of 1.0 ng mL µL−1. The slides were covered with DAPI methanol and incubated for 15 min at 37 °C. The slides were washed once with methanol, rinsed briefly with dd.H2O, and immediately dried in air. The hybridization images were captured using a confocal laser scanning microscope (EVOS™ FL Color Imaging Systems - For Fluorescence and Transmitted Light Applications, Thermo Fisher Scientific Inc.) installed at the Escola Polytechnic of the University of São Paulo (EP-USP, Brazil). A total of 10 images were captured for each probe in each size class. The representative images were selected and the final evaluation of the image was made in Adobe Photoshop.
Results and Discussion
Validation of the Respirometric Test and Laboratory Analysis
Initially, to validate the data obtained, it was verified whether the OUR exerted corresponds to the concentration of the added substrate. Initial tests were performed for each substrate (ammonium chloride and sodium nitrite) to verify if the balance between the area under the OUR curve and the added concentration of substrates was consistent. In the tests, a concentration of a standard solution of 10.0 mg L−1 of NH4+-N and 15.0 mg L−1 of NO2−-N was added for the AOB and NOB. For the curve referring to the AOB, the average area calculated was 44.4 mg O2 L−1, which represents, stoichiometrically, a concentration of (44.4 ÷ 4.57) = 9.72 mg L−1. Likewise, with the curve corresponding to NOB, an average area of 11.2 mg O2 L−1, which represents, stoichiometrically, a concentration of (16.5 ÷ 1.14) = 14.47 mg L−1. Both tests showed a high recovery value (97.2%) NH4+-N and (96.5%) NO2−-N, so it was concluded that oxygen consumption was compatible with the demand for the added substrate, validating the test for the conditions established in this study.
Impact of AMX on AS Performance
After the end of each respirometric test, an effluent sample was collected to determine NH4+-N, NO2−-N, NO3−-N, and AMX concentrations. For the reference AOB test (without the addition of AMX), the NH4+-N concentration in the effluent resulted in 0.8 mg L−1 and for NOB the NO2−-N concentration was 1.0 mg L−1; removal efficiencies were 92.0% and 93.0%, respectively. The values of NO3−-N concentrations in the effluent after the tests were compatible with the nitrification process. For the reference test with NH4+-N, the NO3−-N value was 8.2 mg L−1 and in the nitrite test it was 13.7 mg L−1, which shows that the nitrification was practically complete.
In the AOB tests called processes I, II, III, and IV (with the addition of AMX: 20 mg L−1, 50 mg L−1, 100 mg L−1, and 200 mg L−1), the concentration of NH4+-N in the effluent was 3.5 mg L−1, 4.6 mg L−1, 5.6 mg L−1, and 7.6 mg L−1, with efficiencies of 65.0%, 54.0%, 44.0%, and 24.0%. For NOB, the NO2−-N concentration in the effluent was 4.9 mg L−1, 11.4 mg L−1, 13.7 mg L−1, and 14.0 mg L−1; the removal efficiencies were 67.0%, 24.0%, 9.0%, and 4.0%, respectively. When comparing the results of the processes with those of reference, it was observed a decrease in the efficiency of removal of NH4+-N and NO2−-N, during the increase of AMX concentrations. The decrease in efficiency is more evident in tests with NO2−-N, that is, for the NOB population.
Similar to that observed with the conversion of NH4+-N to NO3−-N, AMX removal was also impacted. Its concentrations in the effluent were < 0.1 mg L−1, 12.9 mg L−1, 56.3 mg L−1, and 126.1 mg L−1, with efficiencies for removing 99.9%, 74.0%, 44.0%, and 37.0%, in cases I, II, III, and IV, respectively. Although the focuses of this study were not to investigate the treatment of the pollutant, but its effect on the nitrification process, the results show that it is possible to remove the compound from the liquid phase at high concentrations, i.e., higher than that found in wastewater. In wastewater treatment systems, the main mechanisms of AMX removal are adsorption and these compounds are hardly biodegradable, the key to the efficiency of the process being the solid retention time (SRT) and the hydraulic retention time (HRT) [22, 34, 35].
Kinetic Behavior of Nitrifying Autotrophic Bacteria
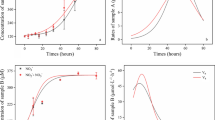
The respirograms show the OUR referring to endogenous and exogenous respiration during the tests (Fig. 2) for processes I, II, III, and IV (20 mg L−1, 50 mg L−1, 100 mg L−1, and 200 mg L−1), respectively. It is noted that there were small differences in oxygen consumption for OURend in relation to the reference tests, this is due to the change of AS during the end of each test. For comparison of results, data were OURend normalized. In general, the AS responded well to experimental conditions, which allowed the determination of the kinetic behavior of AOB and NOB and to evaluate the effect of AMX on nitrifying bacteria population.
OUR can directly reflect microbial activity and the rate of oxygen utilization from the nitrification process during aerobic wastewater treatment. As shown in Fig. 3, the OUR exhibited a gradual decrease with increasing AMX concentration in the AOB tests. There was a 37.5% reduction for OUR at 20 mg L−1 compared to 0 mg L−1 of AMX (p < 0.05). The reduction in OUR in processes II, III, and IV was 31.2%, 62.5%, and 75.0%, respectively. It is observed that the increase in AMX from 20 mg L−1 to 50 mg L−1 did not increase the OUR reduction.
The effect of OUR reduction is most visible in tests with NOB. A 32.0% reduction is observed for OUR at 20 mg L−1 compared to 0 mg L−1 of AMX (p < 0.05). When the AMX concentration was greater than 50 mg L−1, the reduction was 74.0%, which clearly states that AMX inhibited the use of oxygen by NOB from this range. Some antibiotics such as ampicillin, norfloxacin, sulfadiazine, and oxytetracycline have a negative effect on the OUR of activated sludge and biofilms [20, 36,37,38,39].
Yu et al. [20] demonstrates that ampicillin inhibited the activities of AOB and NOB at concentrations greater than 30 mg L−1. In the study, the reduction was 27.5%. [40] pointed out that sulfamethoxazole could also restrict OUR in activated sludge. In the study, there was a decrease of 20.5% for OUR (AOB) and 32.5% for (NOB), for concentrations of 30 mg L−1 of sulfamethoxazole suggesting that the antibiotic could produce more inhibitory impacts on NOB activities than AOB. This statement is in line with the results obtained in the present study, which demonstrates that the effect of AMX is more pronounced in NOBs and with a similar inhibitory potential to other groups of antibiotics. However, in the study by [23] with effluent treatment with activated sludge tetracycline showed that the chronic impact was much more pronounced in the community of ammonia-oxidizing bacteria (AOB). This had a negative impact on the growth kinetics of nitrifying bacteria.
With the OUR values, the maximum specific growth rates of AOB and NOB (µm) and the maximum utilization rates of NH4+-N and NO2−-N (rn.max) were determined, presented in Table 3. The results presented were corrected to a temperature of 20 °C. The determinations followed the model described in [25] and the details of each term are described in the nomenclature list. For example, for the AOB´s test reference, the following values were adopted:
Therefore, it is calculated as follows:
Concentration of autotrophic bacteria:
Concentration of nitrified NH 4 + -N:
Concentration of nitrogen required for sludge production:
Maximum OUR of nitrating bacteria obtained through respirometric tests:
According to the kinetic model used as a reference [25], the maximum rate of nitritation and nitration can be calculated as follows:
rn = OURn.max ÷ 4.57 = 16 ÷ 4.57 = 3.5 mg L−1 h−1 or 84.0 mg L−1 d−1.
Therefore, the maximum growth rate of AOB (μm) is calculated as follows:
µm = (Yn × rn.max ÷ Xn) × 24 = (0.1 × 3.5 × 24.0 ÷ 16.8) = 0.50 d−1.
The average values of μm for the reference test were 0.50 d−1 (AOB) and 0.64 d−1 (NOB). These values are compatible with those found in the literature and in the models (ASM 1, ASM 2, and ASM 3) for AS (µm = 0.20–1.02) described in [24]. The µm constant represents the total growth of microorganisms per unit time. Thus, a µm = 0.50 d−1 value means that the mass of synthesized microorganisms has a rate of 50% per day. As the growth rate is exponential, when there is no substrate limitation, the growth in one day would be N1 ÷ N0 = exp. (0.50 × 1.0) = 1.65, that is, the mass of AOB can increase in a day up to a factor of 1.65 or 65%. When analyzing the µm results for the process tests, a significant decrease in the growth of AOB and NOB populations was observed.
The mean µm values for AOB were 0.30 d−1, 0.34 d−1, 0.20 d−1, and 0.13 d−1 and for NOB they were 0.42 d−1, 0.16 d−1, 0.18 d−1, and 0.15 d−1 (processes I, II, III, and IV), respectively. When considering that there was no limitation of substrate “food” for microorganisms, it is observed that the increase in AMX concentrations had a significant impact on the activities of nitrifying bacteria. As, for example, in process III (addition of 100 mg L−1 of AMX), the AOB population had its growth rate reduced to 22% per day and for NOB process II (with 50 mg L−1) the reduction was 17%.
Generally, for municipal wastewater the NH4+-N concentration available for nitrification is always much higher than the half-saturation constant (Monod model-Eq. 1), in this case the sludge age (Rs) of the AS system can be simplified to Rs = 1 ÷ (µm-bn). This equation expresses that until a minimum sludge age, nitrification does not develop, because AOB removal rate is higher than the maximum rate of net growth. In this sense, it can inferred that in a WWTP with sludge age above the minimum for the development of the nitrification process, its efficiency can be affected by the presence of AMX without acting completely inhibiting the process, as it will depend on the above all on the µm. As observed, the value of µm varies a lot, however, the value of 0.4 is the most recommended in the literature [41], which is higher than that obtained in the tests of processes I, II, III, and IV.
Microscopic FISH Image
To observe the toxicity effect of AMX on the development of nitrifying bacteria during the tests, a combination of oligonucleotide probes specific for Mix (AOB and NOB), shown in Table 2, was used. Images obtained using the FISH technique of samples collected before and after respirometric tests are shown in Fig. 4.
A decrease in the total amount of AOB and NOB is observed in Fig. 4 during respirometric tests, which were subjected to a gradual increase in AMX concentrations. In Fig. 4a, the AOB and NOB populations represent a considerable fraction of the entire microbial population present in the AS reference image. In Fig. 4b, the image showed a small decrease in AOB and NOB populations, which resulted in a 35% reduction in the nitrification process efficiency.
Figure 4c shows the image of AOB and NOB when the concentration of AMX ranged from 20 mg L−1 to 50 mg L−1. The inhibition effect was greater in the NOB population, and the reduction in NO2−-N removal efficiency was 76% and NH4+-N 46%. However, in tests with AMX concentration at 100 mg L−1, the image in Fig. 4d shows a significant decrease in the population of AOB and NOB, which reflected significantly in the removal of NO2−-N. The inhibition effect was 91% for NOB and 56% for AOB.
Microscopy FISH images for process IV, with 200 mg L−1 of AMX, do not show the presence of AOB and NOB. In this test, the inhibition in the AOB population increased significantly, the inhibition in NH4+-N removal was 76% and NO2−-N was 96%. With these results it can be inferred that NOB population is more susceptible to the toxic effects of AMX than the AOB populations. However, all microbial populations are negatively affected, with their greatest effect at concentrations above 100 mg L−1.
Conclusion
The study shows that the respirometry technique can be used as an excellent tool to monitor the metabolic activity of nitrifying bacteria in activated sludge systems and can be incorporated directly into the treatment process. The results showed that the specific growth rate (μm) of AOB is significantly affected at concentrations of AMX in the order of 100 mg L−1 and for NOB, starting at 50 mg L−1. The results also show that the population of NOB was the most sensitive to AMX toxicity. The complete inhibition of the nitrification process in the activated sludge occurred at a concentration of 200 mg L−1. The study shows that the activated sludge system, even if it is old enough for the development of the nitrification process, AMX negatively affects the growth rate of this population.
Although AMX negatively influences nitrification at higher concentrations than found in wastewater, it is an alert to the question of the need for technologies to remove this antibiotic from wastewater, aiming not only to ensure the nitrification process operation, but mainly at protecting aquatic organisms and human health.
Abbreviations
- µm :
-
Maximum specific growth constant for nitrifying bacteria (days)
- AOB:
-
Ammonia-oxidizing bacteria
- bn :
-
Constant decay for the nitrifying organisms (adopted: 0.04 × 1.03(t−20))
- fn :
-
Fraction of AS that remains an endogenous residue
- Kn :
-
Monod half-saturation constants (mg L−1)
- Nc :
-
Nitrification capacity (mg L−1)
- Nl :
-
Nitrogen concentration in excess sludge (mg L−1)
- NOB:
-
Nitrite-oxidizing bacteria
- OUR:
-
Oxygen uptake rate (mgO2 L−1 h−1)
- OURend :
-
Oxygen uptake rate to endogenous respiration (mgO2 L−1 h−1)
- OURexo :
-
Oxygen uptake rate to exogenous respiration (mgO2 L−1 h−1)
- OURn :
-
Oxygen uptake rate to nitrogen compounds (mgO2 L−1 h−1)
- Qeff :
-
Effluent flow (L d−1)
- Qinf :
-
Influent flow (L d−1)
- Rh :
-
Hydraulic retention time (days)
- rn :
-
Constant specific substrate utilization (mgN mgVSS−1 d−1)
- Rs :
-
Sludge age (days)
- S:
-
Substrate ammonia nitrogen (NH4+-N) or nitrite nitrogen (NO2−-N) (mg L−1)
- TKN:
-
Total kjeldahl nitrogen (mg L−1)
- TKNeff :
-
TKN concentration in the effluent (mg L−1)
- TKNinf :
-
TKN concentration in the influent (mg L−1)
- Vr :
-
Reactor volume (L)
- Xn :
-
Concentration of active nitrifying organisms in volatile biomass (mgVSS L−1)
- Xv :
-
Concentration of VSS in the aeration tank (mg L−1)
- Yn :
-
Cell yield coefficient for nitrifying bacteria (adopted: 0.1 kgVSS kgN−1
References
Klein E, Boeckel T, Martinez E, Pant S, Gandra S, Levin S, Goossens H, Laxminarayan R (2018) Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. PNAS 115(15):E3463–E3470. https://doi.org/10.1073/pnas.1717295115
World Health Organization (2018) WHO fact sheet on antimicrobial resistance. World Health Organization, Geneva. https://www.who.int/mediacentre/. Accessed 10 Oct 2020
Pennente K, Lyle J, Gardocki B (2015) Global medicines use in 2020: outlook and implications. IMS Institute for Healthcare Informatics, Parsippany, NJ
Kotwani A, Holloway K (2011) Trends in antibiotic use among outpatients in New Delhi, India. BMC Infect Dis 11(1):99. https://doi.org/10.1186/1471-2334-11-99
Githinji LJM, Musey MK, Ankumah RO (2010) Evaluation of the fate of ciprofloxacin and AMX in domestic wastewater. Water Air Soil Pollut 219(1–4):191–201. https://doi.org/10.1007/s11270-010-0697-1
Elizalde-Velázquez A, Gómez-Oliván LM, Galar-Martínez M, Islas-Flores H, Dublán-García O, SanJuan-Reyes N (2016) AMX in the aquatic environment, Its fate and environmental risk. Environmental health risk - Hazardous factors to living species. https://doi.org/https://doi.org/10.5772/62049.
Bound JP, Voulvoulis N (2004) Pharmaceuticals in the aquatic environment––a comparison of risk assessment strategies. Chemosphere 56(11):1143–1155. https://doi.org/10.1016/j.chemosphere.2004.05.010
Loos R, Marinov D, Sanseverino I, Napierska D, Lettieri T (2018) Review of the 1st watch list under the water framework directive and recommendations for the 2nd watch List. Publications office of the European union, Luxembourg. https://doi.org/https://doi.org/10.2760/614367
World Health Organization (2019) Executive summary: the selection and use of essential medicines 2019: report of the 22nd WHO expert committee on the selection and use of essential medicines: WHO Headquarters, Geneva, 1–5 April 2019 (No. WHO/MVP/EMP/IAU/2019.05). World Health Organization.
Ribeiro RP, Bueno RF, Piveli RP, Kligerman DC, de Mello WZ, Oliveira JL (2017) The response of nitrous oxide emissions to different operating conditions in activated sludge wastewater treatment plants in southeastern Brazil. Water Sci Technol 76(9):2337–2349. https://doi.org/10.2166/wst.2017.399
Deblonde T, Cossu-Leguille C, Hartemann P (2011) Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health 214(6):442–448. https://doi.org/10.1016/j.ijheh.2011.08.002
Matsuo H, Sakamoto H, Arizono K, Shinohara R (2011) Behavior of pharmaceuticals in waste water treatment plant in Japan. Bull Environ Contam Toxicol 87(1):31–35. https://doi.org/10.1007/s00128-011-0299-7
Rubiano JC, Perdomo, MP, Pramparo L (2017) Estudio respirométrico de la biodegradación de Diclofenaco. Entre Ciencia e Ingeniería. 11(21):9–13 https://doi.org/https://doi.org/10.31908/19098367.3268
Avella AC, Essendoubi M, Louvet JN, Görner T, Sockalingum GD, Pons MN, Manfait M, de Donato P (2010) Activated sludge behaviour in a batch reactor in the presence of antibiotics: study of extracellular polymeric substances. Water Sci Technol 61(12):3147–3155. https://doi.org/10.2166/wst.2010.924
Xing BS, Jin RC (2018) Inhibitory effects of heavy metals and antibiotics on nitrifying bacterial activities in mature partial nitritation. Chemosphere 200:437–445. https://doi.org/10.1016/j.chemosphere.2018.02.132
Xia Y, Wen X, Zhang B, Yang Y (2018) Diversity and assembly patterns of activated sludge microbial communities: a review. Biotechnol Adv 36(4):1038–1047. https://doi.org/10.1016/j.biotechadv.2018.03.005
Mao D, Yu S, Rysz M, Luo Y, Yang F, Li F, Hou J, Mu Q, Alvarez PJJ (2015) Prevalence and proliferation of antibiotic resistance genes in two municipal wastewater treatment plants. Water Res 85:458–466. https://doi.org/10.1016/j.watres.2015.09.010
Meng L, Li X, Wang X, Ma K, Liu G, Zhang J (2017) Amoxicillin effects on functional microbial community and spread of antibiotic resistance genes in amoxicillin manufacture wastewater treatment system. J Environ Sci 61:110–117. https://doi.org/10.1016/j.jes.2017.09.020
Liu N, Hou T, Yin H, Han L, Huang G (2019) Effects of amoxicillin on nitrogen transformation and bacterial community succession during aerobic composting. J Hazard Mater 362:258–265. https://doi.org/10.1016/j.jhazmat.2018.09.028
Yu N, Zhao C, Ma B, Li S, She Z, Guo L, Zhang Q, Zhao Y, Jin C, Gao M (2019) Impacto da ampicilina na remoção de nitrogênio, comunidade microbiana e atividade enzimática do lodo ativado. Biores Technol 272:337–345. https://doi.org/10.1016/j.biortech.2018.10.048
Busto RV, Roberts J, Hunter C, Escudero A, Helwig K, Coelho LHG (2020) Mechanistic and ecotoxicological studies of AMX removal through anaerobic degradation systems. Ecotoxicol Environ Saf 192:110207. https://doi.org/10.1016/j.ecoenv.2020.110207
Matsubara ME, Helwig K, Hunter C, Roberts J, Subtil EL, Coelho LHG (2020) AMX removal by pre-denitrification membrane bioreactor (A/O-MBR): performance evaluation, degradation by-products, and antibiotic resistant bacteria. Ecotoxicol Environ Saf 192:110258. https://doi.org/10.1016/j.ecoenv.2020.110258
Katipoglu-Yazan T, Merlin C, Pons M, Ubay-Cokgor E (2015) Chronic impact of tetracycline on nitrification kinetics and the activity of enriched nitrifying microbial culture. Water Res 72:1–12. https://doi.org/10.1016/j.watres.2014.12.041
Bueno RF, Piveli RP, Campos F (2019) Extended aeration activated sludge process operated under low dissolved oxygen concentration: Kinetic behavior of nitrifying heterotrophic and autotrophic bacteria. Lodo ativado com aeração prolongada operado sob baixa concentração de oxigênio dissolvido: comportamento cinético das bactérias heterotróficas e autotróficas nitrificantes. Engenharia Sanitária e Ambiental, 24(5), 939–947. https://doi.org/https://doi.org/10.1590/s1413-41522019134260.
van Haandel AC, Marais GVR (1999) The behavior of the activated sludge system: theory and applications for projects and operations. Campina Grade.
Bueno RF, Piveli RP, Campos F, Sobrinho PA (2018) Simultaneous nitrification and denitrification in the activated sludge systems of continuous flow. Environ Technol 39(20):2641–2652. https://doi.org/10.1080/09593330.2017.1363820
APHA (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association, Washington, DC
APHA (1998) Standard methods for examination of water and wastewaters (sections 2541B-E, 5310B, 4500-NH3 H, 4500-NB and 4500-PH). American Public Health Association, Washington, DC
APHA (2005) Standard methods for the examination of water and wastewater. Method 4110 C. Determination of anions by ion chromatography. American Public Health Association, Washington, DC
Winkler MKH, Bassin JP, Kleerebezem R, Sorokin DY, van Loosdrecht MCM (2012) Unravelling the reasons for disproportion in the ratio of AOB and NOB in aerobic granular sludge. Appl Microbiol Biotechnol 94:1657–1666. https://doi.org/10.1007/s00253-012-4126-9
Daims H, Nielsen JL, Nielsen PH, Schleifer KH, Wagner M (2001) In situ characterization of Nitrospira-like nitrite oxidizing bacteria active in wastewater treatment plants. Appl Environ Microb 67:5273–5284. https://doi.org/10.1128/AEM.67.11.5273-5284.2001
Wagner M, Rath G, Koops HP, Flood J, Amann R (1996) In situ analysis of nitrifying bacteria in sewage treatment plants. Water Sci Technol 34(1–2):237–244
Mobarry BK, Wagner M, Urbain V, Rittmann BE, Stahl DA (1996) Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microb 62:2156–2162. https://doi.org/10.1128/AEM.62.6.2156-2162.1996
Akashdeep SO, Yanyan J, Huiqun Z, Samir KK, Hui L (2019) Insights into the fate and removal of antibiotics in engineered biological treatment systems: a critical review. Environ Sci Technol 53(13):7234–7264. https://doi.org/10.1021/acs.est.9b01131
Kim S, Eichhorn P, Jensen JN, Weber AS, Aga DS (2005) Removal of antibiotics in wastewater: effect of hydraulic and solid retention times on the fate of tetracycline in the activated sludge process. Environ Sci Technol 39(15):5816–5823. https://doi.org/10.1021/es050006u
Yi K, Wang D, Yang Q, Li X, Chen H, Sun J, An H, Wang L, Deng Y, Liu J, Zeng G (2017) Effect of ciprofloxacin on biological nitrogen and phosphorus removal from wastewater. Sci Total Environ 605–606:368–375. https://doi.org/10.1016/j.scitotenv.2017.06.215
Zheng D, Chang Q, Li Z, Gao M, She Z, Wang X, Guo L, Zhao Y, Jin C, Gao F (2016) Performance and microbial community of a sequencing biofilm reactor treating synthetic mariculture wastewater under long-term exposure to norfloxacin. Bioresour Technol 222(2016):139–147. https://doi.org/10.1016/j.biortech.2016.09.114
Li Z, Chang Q, Li S, Gao M, She Z, Guo L, Zhao Y, Jin C, Zheng D, Xu Q (2017) Impact of sulfadiazine on performance and microbial community of a sequencing batch biofilm reactor treating synthetic mariculture wastewater. Bioresour Technol 235:122–130. https://doi.org/10.1016/j.biortech.2017.03.113
Wang S, Gao M, Wang Z, She Z, Jin C, Zhao Y, Guo L, Chang O (2015) Effects of oxytetracycline on performance and microbial community of an anoxic-aerobic sequencing batch reactor treating mariculture wastewater. RSC Adv 5:53893–53904. https://doi.org/10.1039/C5RA06302G
Kassotaki E, Buttiglieri G, Ferrando-Climent L, Rodriguez-Roda I, Pijuan M (2016) Enhanced sulfamethoxazole degradation through ammonia oxidizing bacteria co-metabolism and fate of transformation products. Water Res 94:111–119. https://doi.org/10.1016/j.watres.2016.02.022
Metcalf, Eddy (2013) Wastewater engineering: treatment and resource recovery. 5th ed. Mcgraw-Hill Education.
Acknowledgements
We would like to thank the Basic Sanitation Company of the State of São Paulo-SABESP for authorizing the use of activated sludge from WWTP-Parque Andreese to carry out the study.
Author information
Authors and Affiliations
Contributions
JKF: Investigation, Writing—original draft. ACSC: Investigation, Writing—original draft. MYK: Investigation, Writing—original draft. ABO: Methodology, Writing—review & editing. LHC: Supervision, Methodology. ELS: Supervision, Methodology. RFB: Conceptualization, Supervision, Writing—review & editing. The authors declare that there is no conflict of interest in the published information.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faria, J.K., Conceição, A.C.S., Kohatsu, M.Y. et al. Effect of Amoxicillin on Nitrogen Oxidation Bacteria Present in Activated Sludge: Respirometry Investigation. Curr Microbiol 78, 167–178 (2021). https://doi.org/10.1007/s00284-020-02287-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02287-7