Abstract
Microbial assemblages in terrestrial environments, such as soils, utilize hydrolytic enzymes to function biologically in various environments including for the degradation of organic carbon compounds and cycling of nutrients that eventually contributes to the ecological and agricultural productivity of such environments. In this study, 3 soil types (i.e., sandy, loamy and clayey) with varying characteristics were collected within the premises of Albion College in Michigan, with the goal of comparing the occurrences of indigenous bacterial populations and their respective hydrolytic enzyme activities in the soils. The soils were examined for their organic matter content (% OM), while bacterial abundance was determined by combinations of viable counts and nucleic acid staining, and enzymatic activities measured using fluorescein diacetate (FDA) analysis. Results from the study showed loamy soil to have a significantly higher % OM at 30% on average as compared to 2.5% and 6.6% recorded in the sandy and clayey soils. Comparatively, bacterial numbers (both viable and total counts) were also significantly higher in loamy soils than the other two soils. The same trend was observed for FDA analysis with higher fluorescein released in the loamy soil relative to the two other soils. Overall, clear differences were observed in the relationships between % OM and bacterial numbers and hydrolytic enzyme activities among the three soil types and between the two seasons examined. The results suggest that % OM strongly influences both bacterial abundance and hydrolytic enzyme activities in loamy soil and less so in both sandy and clayey soils examined in the study. This study in conclusion revealed potential strong relationships between soil organic carbon and indigenous bacterial populations as well as their FDA activities in various soil types.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microbial activity in terrestrial environments, such as soils and sediments, results mostly from the actions of various hydrolytic enzymes frequently produced by complex aggregates of heterotrophic bacterial assemblages for their energy generation through the decomposition of various organic carbon materials and nutrient cycling [e.g., 1]. The ecological importance of several of these enzymes in the hydrolysis of fluorescein diacetate (3′,6′-diacetylfluorescein [FDA]) for estimating total microbial activity in various environments have been previously recognized [2,3,4,5,6]. This is due to the incontrovertible fact that total microbial activity is generally believed to provide an accurate measure of organic matter turnover in natural systems, given that majority of energy in soil passes through microbial decomposition [1].
FDA is widely believed to be widely hydrolyzed by various enzymes, such as proteases, lipases and esterases [e.g., 2, 5] producing fluorescein that can be easily and rapidly quantified using fluorometric or spectrophotometric approaches [5,6,7]. For instance, a controlled study by [7] reported the development and effective utilization of the simple but sensitive spectrophotometric method to rapidly quantify total microbial activity using FDA in a range of soils in their study.
Therefore, based on a similar approach that has been well utilized [e.g., 5–7], we have examined in this study, total microbial occurrences and activities in three different soil types, that were targeted because of their varying characteristics, especially in their organic matter content and anthropogenic influences. The main objectives of the study were to determine the relationship between the organic matter availability among the soil types and total bacterial occurrences as well as their enzymatic activities. Also, to compare these factors measured among the three soils between sampling seasons during the study period.
Materials and Methods
Soil Type Description and Collection
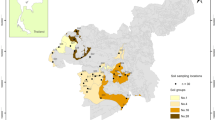
The three different types of soil samples (i.e., sandy, loamy and clayey) used for this study to obtain wide ranging differences in soil characteristics were collected within the premises of Albion College, Albion (42.24445°N 84.7434°W) in Michigan, USA. Fresh top soils (~ 1 m deep) were collected in triplicates during the summer (May–June) and fall (October–November) seasons at the same exact locations for the three soil types and quickly stored in clean Ziplock bags before subsamples were immediately used for various laboratory assays. The sandy soils used were tan to brownish in coloration, comprised of coarse particles and were collected from beneath freshly mowed grasses close to the botanical garden on the campus quad. The loamy soils were dark brown and dark, comprising very smooth particle sizes and were collected within the forested premises of the 25 acres Whitehouse Research Nature Center (WHNC) comprising mostly of oak-hickory, flood-plain trees and extensive marsh and swamp lands. Also collected within the forested premises of the WHNC at Albion College, were the clayey soils used with red and dark brown coloration that consisted of interspersed small particle sizes.
Determination of Soil Organic Matter Contents
The loss of weight on ignition (LOI 360°) procedure was used in this study to determine soil organic matter as previously described by [8] and [9]. Briefly, 5 g of each soil samples were weighed into tarred 20 mL beakers and dried in a muffle furnace for 2 h at 105 °C. The weight of the dried soil samples was recorded and placed back into the furnace at 360 °C for another 2 h incubation, then cooled down to less than 105 °C and weighed again in a daft-free environment under the hood. The percent weight loss-on-ignition (LOI) was then calculated based on the differences in recorded weights before estimating the percent organic matter content from the LOI using regression analysis [9].
Determination of Bacterial Abundance
Viable bacterial numbers in the soil samples were determined using standard microbiological approach as previously described [10]. Briefly, soil samples of 0.1 g were weighed and serially diluted in 9 mL sterile dilution blanks before transferring 100 µL of diluent aseptically into sterile nutrient agar plates in triplicates and incubated at 37 °C for 24 h. Distinct bacterial colonies formed were then counted in each plate using a colony counter and total viable bacterial numbers (CFU) calculated with respect to the dilution factor and the volume plated. Also, total bacterial numbers in the soils were determined by measuring 0.1 g of each soils into 1 mL of sterile deionized water, serially diluted, vortexed and then concentrating onto 0.2 µm pore-size black polycarbonate filters (Poretics, Livermore, CA) before staining with 200 µL of 15 µg/µL of DAPI (4′,6 diamidino-2-phenylindole) solution for between 3 and 5 min. Filters were rinsed with sterile water and then mounted onto glass slides with Type FF immersion oil [11]. Between 300 and 500 of the stained bacterial cells in 10 separate fields were then counted under an epifluorescence microscope.
Measurement of Fluorescein Enzyme Activity
Activities of Fluorescein diacetate [3′,6′-diacetylfluorescein (FDA)] in the soil samples were determined using the approach described by [5] and [6]. First, a standard curve was constructed to calculate the concentration of fluorescein released using a standard stock solution 10 mg fluorescein (C20H12O5, Sigma-Aldrich) in 10 mL of reagent-grade acetone and final volume was then adjusted to 50 mL with sodium phosphate buffer (pH 7.6) for a 602 µM solution. This fluorescein stock was then diluted down in triplicate to 0.0 mg/mL, 0.03, 0.1, 0.3, 0.5 and 1.0 mg/L in 50 mL volumetric flasks and brought to volume with sodium phosphate buffer (pH 7.6), before adding 2.5 mL acetone. The optical densities of the standard solutions were then measured at 490 nm using a spectrophotometer.
FDA activity was measured in the samples by weighing out 1 g of previously air-dried soil in a 125 mL flask and adding 50 mL of 60 mM sodium phosphate buffer (pH 7.6) and 0.5 mL of 5 mM FDA substrate solution (previously prepared by adding 20 mg FDA lipase substrate [(C24H16O7, Sigma-Aldrich] in 10 mL acetone). Blanks with no soils, but just the reagents and controls with each soil type containing 0.5 mL acetone instead of the FDA lipase substrate solution were also set up in order to measure color not derived from the hydrolysis of FDA. The triplicate flasks were then arranged on a shaking incubator at 24 h. At varying time intervals, i.e., 0, 4, 6, 8 and 24 h, subsamples of 30 mL of soil slurries from each flask were collected in 50 mL centrifuge tubes and spun down at maximum speed (8000 rpm) after adding 3 mL acetone to stop FDA activity for 5 min at 4 °C. The supernatant was then measured for absorbance at a wavelength of 490 nm. Concentrations of fluorescein released were then calculated in reference to the equation (y = 1.4789x + 0.0058) obtained from standard curve prepared prior between the diluted fluorescein standard and their respective OD @490 nm (R2 = 0.880).
Statistical Analysis
The Student’s t tests and ANOVA analyses were performed to analyze the differences in bacterial abundance and fluorescein diacetate (FDA) hydrolytic activities between different soil types. Post hoc tests were also carried out for pairwise comparison using Bonferroni correction test. Relationships between organic matter contents and bacterial populations a s well as FDA enzyme activity were further examined using linear regression and Pearson Correlation analysis. Statistical significance was set at p ≤ 0.05.
Results
Soil Characteristics and Organic Matter Content
There were significant differences among the soil types used for the study in their characteristics. Particularly, the organic matter content measured in the loamy soil were found to be much higher than in the two other soil types examined (p < 0.05); however, the amounts of % OM were much similar between the clayey and the sandy soil (Table 1). The same trend was observed in % OM when compared among the soils between the two seasons examined (Table 2), with the loamy soil having more significant OM content than measured in the other two soils (Fig. 1).
Bacterial Occurrences
Numbers of viable bacteria enumerated varied significantly in the three soils (p < 0.05), with the loamy soil having the highest counts and lowest numbers found in the clayey soil in both the summer and the fall season (Fig. 2). The CFU numbers found among the soil types used were more varied during the fall as compared to the summer (Table 2). Total bacterial numbers based on DAPI-stained cells followed the same trend as recorded for the viable numbers among the three soils, with the highest numbers found in the loamy soil and the lowest in the clayey soil in both seasons examined (Fig. 3).
Fluorescein Enzyme Activity
The amount of fluorescein released over the 24 h incubation period was significantly higher in the loamy soil and lowest in the clayey soil (Fig. 4). The results of ANOVA showed significant variations among the soils in both the summer (F = 5.726, p < 0.0001) as well as in the fall (F = 3.758, p < 0.0001). The results of post hoc tests using Bonferroni correction revealed significant differences between the sampling times among the three soil types examined (p < 0.05). Also, significant variations were recorded in fluorescein release after 8 h incubation among the three soils in both summer and fall seasons examined in this study.
Relationships Between OM Content, Bacterial Occurrences and FDA Activity
A strong linear relationship (R2 = 0.7285) was observed between % OM content and viable bacterial counts in the sandy soil, but not such relationship was recorded in both the loamy and sandy soil (Fig. 5a). Similarly, the relationship between % OM content and DAPI-stained bacterial cells in the sandy soil was also strong (R2 = 0.6621), but less so in loamy (R2 = 0.1753) and clayey (R2 = 0.484) soils (Fig. 5b).
When the relationship between % OM content and total bacterial counts were examined among the soils and compared between seasons, the relationship was found in the summer to be stronger (R2 = 0.6621) only in sandy soil (Fig. 6a), but stronger in the fall in both loamy (R2 = 0.5517) and sandy (R2 = 0.7167) soils, while not in clayey soil (Fig. 6b).
The relationships found between the % OM content and fluorescein released in both the loamy and sandy soils were very strong in the summer R2 = 0.6747 and 0.7311 (Fig. 7a); as well as in the fall R2 = 0.6339 and 6902 (Fig. 7b), but comparatively weaker in the clayey soil in both seasons. To determine whether total bacterial counts correlated with FDA activity, the relationship between the two were examined in the three soils from both summer and fall, and a very strong linear relationship was found only in the sandy soil in the summer (R2 = 0.8946, Fig. 8a) and fall (R2 = 0.710, Fig. 8), but not in the other two soil types examined during the study.
Discussion
The comparatively high bacterial occurrences as well as FDA activity observed in the loamy soil in this study relative to the other two sources is not at all surprising. Loamy soils are characteristically loaded with complex organic compounds consisting of mostly recalcitrant humic and/or detrital materials [12, 13]. The result obtained from this study corroborates strongly with those observed by Adam and Dunkan [7], who also reported relatively lower FDA activity in both sandy and clayey soils they examined. Contradictory results have been observed in previous studies on FDA activity in different soil types. While many soil enzyme activities have been generally observed to increase with increasing organic matter presence in soils, as typically found in high microbially active loamy soils [e.g., 14, 15], however, a study by Green et al. [5] reported that high clay soils with also high organic matter contents exhibited relatively higher FDA hydrolytic activities among the soils examined in their study.
The strong linear relationships observed in the sandy soil between % OM and microbial occurrences and FDA activity in this study further validates earlier observations that soils that are typically disturbed by human activities such as mowing and fertilization have enzymatic activities per unit soil organic carbon (SOC) higher than those found in forested soils (such as the clayey and loamy soils used in our study [16,17,18]. This FDA trend was also corroborated by Medeiros et al. [16] in their study that examined enzyme activities per unit SOC in sandy soils with various crop coverage. Similarly, a study by Tokuda and Hayatsu [19] reported higher FDA hydrolytic activity in acidic tea field soils than those in neutral arable soils and also showed a negative relationship between FDA and soil pH, an indication that other characteristics may also be potentially driving microbial activities soils.
FDA hydrolysis is a non-specific, but sensitive test capable of accurately reflecting the hydrolytic activity of soil microbes in a wide range of soil types as generally acceptable measure of total microbial activity [5,6,7, 20]. FDA is hydrolyzed by all the major enzymes involved in organic carbon decomposition in soils, such as proteases, lipases and esterases [5]. In this study, FDA activity was significantly influenced by % OM in both the loamy and sandy soils, but not clayey, during the two seasons examined. Also, FDA showed strong correlations with bacterial occurrences in sandy soil during the summer (0.89) and fall (0.75) seasons. This strongly indicates that enzyme activities were associated with active microbes in the soils and these microbial cells are the major sources of these soil enzymes. The strong relationship between bacterial numbers and FDA activity as observed in this study further corroborate reported results from earlier similar studies [e.g., 21, 22]. For instance, the study by Gadja et al. [22] also found significant correlation between microbial biomass and enzyme activities with organic carbon content in their study that examined silt and sandy loamy soils. They found all biological activities measured to be generally higher in the silt soil as compared to the sandy loamy soils in their experimental soils.
In this study, we demonstrated that bacterial occurrences and their hydrolytic enzymatic activities in different soil types located within a temperate nature center were affected differently by SOC contents. However, the effects of SOC bacterial populations and FDA activities between the soils were found to be insignificant among the two different seasons examined. Therefore, a follow-up study on the soil types over a complete seasonal cycle utilizing multivariate analyses may probably reveal differences associated with various environmental factors that may also change temporally among the soils.
References
Heal OW, McClean SF (1975) Comparative productivity in ecosystems-secondary productivity. In: Dobben WH, Lowe-McConnell RH (eds) Unifying concepts in ecology. W. Junk BV Publishers, The Hague, Holland, pp 89–108
Battin TJ (1997) Assessment of fluorescein diacetate hydrolysis as a measure of total esterase activity in natural stream sediment biofilms. Sci Tot Environ 198:51–60
Chrzanoski TH, Crotty RD, Hubbard JG, Wech RP (1984) Applicability of the fluorescein diacetate method of detecting active bacteria in freshwater. Microb Ecol 10:179–185
Fontvieille DA, Outaguerouine A, Thevenot D (1992) Fluorescein diacetate hydrolysis as a measure of microbial activity in aquatic systems: application to activated sludges. Environ Technol 13:531–540
Green VS, Stott DE, Diack M (2006) Assay for fluorescein diacetate hydrolytic activity: optimization for soil samples. Soil Biol Biochem 38:693–701
Schnurer J, Rosswall T (1982) Fluorescein diacetate hydrolysis as a measure of total microbial activity in soil and litter. Appl Environ Microb 43:1256–1261
Adam G, Duncan H (2001) Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol Biochem 33:943–951
Combs SM, Nathan MV (1998) Soil organic matter. Brown JR (ed) Recommended chemical soil test procedure for the north central region. NCR Publ #221 (revised). Missouri Agri Exp Sta SB 1001. Columbus, Missouri
Schulte EE, Hopkins BG (1996) Estimation of soil organic matter by weight loss-on-ignition. In: Magdoff FR, et al. (eds) Soil organic matter analysis and interpretation. Soil Science Society of America, Madison, Wisconin, pp 21–31
Leboffe MJ, Pierce BE (2006) Microbiology laboratory theory and application, 2nd edn. Morton Publishing Company, Englewood, p 629
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Maier RM, Pepper IL, Gerba CP (2000) Environmental microbiology. Academic Press, Cambridge, p 585
United States Department of Agriculture, USDA (1999) Soil taxonomy: A basic system of foil classification for making and interpreting soil surveys. USDA, Natural Resources Conservation Service. 2nd Edition, pp 886.
Dick RP (1994) Soil enzyme activities as indicators of soil quality. In: Doran JW, Jones AJ (eds), Defining soil quality for a sustainable environment. Soil Sc Soc Amer J 44: 282–287.
Trasar-Cepeda C, Leiros C, Gil-Sotres F, Seoane S (1998) Towards a biochemical quality index for soils: an expression relating several biological and biochemical properties. Biol Fertil Soil 26:100–106
Medeiros EV, Notaro KA, Barros JA, Moraes WS, Silva AS, Moreira KA (2015) Absolute and specific enzymatic activities of sandy Entisol from tropical dry forest, monoculture and intercropping areas. Soil Tillage Res 145:208–215
Notaro KA, Medeiros EV, Duda GP, Moreira KA, Barros JA, Santos UJ, Lima JR, Moraes W (2018) Enzymatic activity, microbial biomass and organic carbon of Entisol from Brazilian tropical dry forest and annual and perennial crops. Chilean J Agric Res 78:1–12
Trasar-Cepeda Leiros MC, Gil-Sotres F (2008) Hydrolytic enzyme activities in agricultural and forest soils. Some implications for their use as indicators of soil quality. Soil Biol Biochem 40:2146–2155
Tokuda S, Hayatsu M (2002) Soil microbial biomass and fluorescein diacetate hydrolytic activity in Japanese acidic tea field soils. Soil Sc Plant Natr 48(5):865–869
Elbl J, Makova J, Javorekova S, Medo J, Kintl A, Losak T, Lukas V (2019) Response of microbial activities in soil to various organic and mineral amendements as an indicator of soil quality. Agronomy 9(285):1–19
Baloja EL, Machineski O, Truber VP, Auler PMA (2011) Effect of tillage systems and permanent plantcover intercropped with orange trees on soil enzyme activities. Braz Archiv Biol Technol 54:221–228
Gadja AM, Przewloka B, Gawryjolek K (2013) Changes in soil quality associated with tillage system applied. Inter Agrophys 27:133–141
Acknowledgements
We appreciate the comment and suggestions by colleagues and members of the Arietta Lab at the University of Calgary that was used in improving the quality of the study. This study was supported in part by the Foundation for Undergraduate Research, Scholarship, and Creative Activity (FURSCA) fund that was awarded to AW and the Hewlett-Mellon Fund for Faculty Development grant awarded to OAO by Albion College, Albion, Michigan, USA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declares that there are no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wilkerson, A., Olapade, O.A. Relationships Between Organic Matter Contents and Bacterial Hydrolytic Enzyme Activities in Soils: Comparisons Between Seasons. Curr Microbiol 77, 3937–3944 (2020). https://doi.org/10.1007/s00284-020-02223-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02223-9