Abstract
This study aimed to screen alpha-amylase producing microorganisms from honey as a low water activity medium, a suitable source for selecting stable and cost-beneficial bacterial enzyme production systems. Plackett–Burman method was used to select twelve effective factors including pH, inoculum size, temperature, time, corn starch, KH2PO4, peptone, MgSO4, CaCl2, NaCl, glycerin, and yeast extract concentrations on bacterial alpha-amylases production yield. The Box–Behnken method was utilized to optimize the level of selected significant factors. The stability of bacterial alpha-amylases was also determined in low pH and high-temperature conditions. In addition, in silico study was used to create the alpha-amylase structure and study the stability in high-temperature and low water available condition. Among all isolated and characterized microorganisms, Bacillus megaterium produced the highest amount of alpha-amylases. The in silico data showed the enzyme 3D structure similarity to alpha-amylase from Halothermothrix orenii and highly negative charge amino acids on its surface caused the enzyme activity and stability in low water conditions. Based on Box–Behnken results, the temperature 35 °C, pH 6 and starch 40 g/l were determined as the optimum level of significant factors to achieve the highest alpha-amylases unit (101.44 U/ml). This bacterial alpha-amylases enzyme showed stability at pH 5 and a range of temperatures from 40 to 60 °C that indicates this enzyme may possess the potential for using in industrial processes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Honey plays an important role as a reservoir for a wide range of sugar-tolerant microorganisms, which come from pollen, nectar, digestive tracts of honey bees, air, and other microbial sources [1, 2]. Honey contains water, sugar, several minerals, and about 54 chemical elements such as Na, K, Ca, Mg, P, Cu, Pb, Zn and Mn that provide many nutritions' factors (such as carbon source and macro/microelements) to keep on microorganism [2]. Due to low moisture contents, honey has low water activity (aw) that makes it a selective culture medium for existing of some hydrolytic enzymes (i.e., alpha-amylase) producing microorganisms (spore-forming bacteria and of sugar-tolerant microorganisms) in the honey environment condition such as Bacillus spp. and Aspergillus spp. [1, 3]. Alpha-amylase hydrolyzes the branched/unbranched starch through breakage of the alpha-1,4 bonds between glucose subunits. Among all alpha-amylases, microbial alpha-amylases have commonly used in industry because of some beneficial properties such as the best stability in harsh conditions. Easy genetic manipulation and high inexpensive production procedure are advantages of microbial alpha-amylases production [4,5,6]. Alpha-amylases, produced by Escherichia sp., Bacillus amyloliquefaciens, Bacillus subtilis, Aspergillus oryzae and Rhizopus sp., are used in food, paper, textile, pharmaceuticals, and detergents production industries [7, 8]. The processed-food industry has widely utilized alpha-amylases in a variety of applications such as brewing, baking, production of cakes, preparation of digestive aids, starch syrups and fruit juices [9].
The starch industry is one of the most important of alpha-amylase applications in the food industry. Alpha-amylases used in the starch processing must be active and stable in a high-temperature and acidic condition which shows the importance of screening of suitable microbial alpha-amylases [10]. Enormous microbial types were isolated from hot water springs environments where a unique suitable place for thermophilic microorganisms that produce active and stable alpha-amylases in high-temperature conditions. For instance, the Geobacillus bacterium has been isolated previously from Manikaran hot springs with the alpha-amylases optimum temperature activity at 80 °C [11].
Starch processing possesses the acidic pH (pH 4.5) condition, and thus the screening of microorganisms producing low pH stable alpha-amylase like Lactobacillus manihotivorans can be considered a crucial issue in alpha-amylases production [12, 13].
For the optimization of microbial alpha-amylases production, genetic/media engineering methodologies have been utilized in industry scale [4]. The instability of recombinants occurs in genetically engineered microorganisms through losing their vectors during fermentation due to structural instability of genetically safe expression vectors caused the reduction of the produced recombinant protein [14]. One of the media engineering methods (response surface methodology, RSM) has been considered a practical approach for enzyme overproduction because of reducing the numbers of experimental trials to find the optimized amount of factors and their interactions [4, 15]. The RSM method has been previously exploited to improve alpha-amylase overproduction by B. amyloliquefaciens [16], A. oryzae S2 [8], A. oryzae CBS 819.72 [17] and A. oryzae CBS 819.72 [17].
According to increase industrial applications of the alpha-amylase enzyme, finding good and novel microbial sources can play an important role to progress the related industries. During the production process, the harsh condition such as acidity and high-temperature condition needs to use the active and stable enzyme [18]. Honey possesses some microorganisms that are stable in acidic and low water activity conditions because of honey low water content and acidic pH [19]. Therefore, several studies have been required to find suitable microbial sources with alpha-amylase production properties from honey. Also, the structure of alpha-amylase, specificity, and catalytic mechanism are essential to carry out possible applications [20]. To study the protein structures, prediction tools look to be appreciated than nuclear magnetic resonance (NMR) and X-ray crystallography due to these methods' time-consuming and difficult processes [21].
This study aimed to screen alpha-amylase producing microorganisms from Iranian collected traditional honey, in silico studies of the alpha-amylase structure, and to screen the most effective factors related to alpha-amylases producing optimization.
Material and Method
Materials
Glucose, MgSO4, CaCl2, KI, agar, KH2PO4, NaCl, HCl, iodine and trichloroacetic acid were purchased from Merck (Merck, Darmstadt, Germany).Yeast, beef, malt extract, and agar were provided from Liofilchem (Liofilchem, Roseto d.Abruzzi, Italy) and Biolife (Biolife, Milano, Italy), respectively. Peptone and tryptone were bought from Himedia (Himedia, Mumbai, India). Alpha-amylase and glycerin were purchased from Sigma (Sigma, Steinheim, USA) and Duksan (Duksan, Kyungkido, South Korea), respectively. Corn starch was bought from Sigma (Sigma, microbial grade, Steinheim, USA).
Sample Collection, Isolation, and Screening of alpha-Amylase Producing Microorganisms
Honey samples were collected using sterile plastic flasks from the west of Iran. Enrichment cultures were performed separately using three common culture medium such as nutrient broth, Luria–Bertani broth, and one medium for fungi (contain malt 20 g/l, peptone 1 g/l, glucose 20 g/l, agar 20 g/l; pH 7) that were incubated in 150 rpm at 30 °C for 24 h into shaker incubator (Vision, Cheonan, South Korea). After incubation, the microorganisms were streak cultured in the same enrichment culture medium containing 15 g/l agar for isolation of single colon of enriched microorganisms. For amylolytic activity determination, every single colon separately was streak cultured on starch agar medium (contain corn 10 g/l, beef extract 3 g/l, and agar 15 g/l; pH 7) then plates were incubated at 30 °C for 24 h (Paat-Ariya Co. Sh 2006 model, Mashhad, Iran). The plates were flooded by Gram’s iodine solution (0.3% I2 and 0.6% KI) and the clear zone around the lines of grown microorganisms was considered as starch hydrolysis activity.
Genomic DNA was extracted from the producing alpha-amylase bacterial isolates by CTAB, SDS, and proteinase K method [22]. The PCR amplification was conducted using a thermal cycler Eppendorf (Eppendorf AG Mastercycler gradient, Hamburg, Germany) on extracted genomic DNA of each Bacillus bacteria by applying the amplification sets of primers forward 5′-AGA GTT TGA TCT GGC TCAG-3′ and reverse 5′-TACCTTGTTAGGACTTCACC-3′ [23] and for coccus bacteria by applying the amplification sets of primers forward 5′-GTAGCCGTATCGGAAGGTGC-3′ and reverse 5′-GTCGTGCTGGGGATAGAGCATT-3′. The gel recovered PCR products were sequenced by a Korean sequencing company (Macrogen). For identification and characterization of bacteria, the sequences were analyzed using the BLAST program with the sequences deposited in NCBI and GenBank (https://www.ncbi.nlm.nih.gov/BLAST) and the bacteria with at least 98% homology were considered to the same species.
Alpha-amylase Assay
The selected microorganisms with amylolytic activity were subjected to alpha-amylase enzyme assay. Firstly, the microorganisms were cultured in a broth medium containing the following: starch 15, peptone 2.5, NaCl 1.5, yeast extract 2, KH2PO4 0.5, MgSO4 0.5, CaCl2 0.1 (all g/l) then were incubated into shaker incubator (150 rpm) at 30 °C for 24 h. Following, the cultures were centrifuged at 8000 rpm for 20 min (Beckman Coulter Allegra X-22R model, Krefeld, Germany). 250 μl of each supernatant was added to 0.5 ml acetate buffer (pH 5), along with 1.25 ml of 1% (w/v) soluble starch solution, then was incubated at 50 °C for 10 min. The reaction was stopped by adding of 1 ml dinitrosallicylic acid solution (1 g of 3, 5 dinitrosalicylic acid in 20 ml NaOH (2 M) containing 30 g sodium potassium tartrate by diluting to 100 ml with distilled water) and immediately the absorbance was measured at 575 nm (UV-1800 Shimadzu model, Kyoto, Japan). For calculating the alpha-amylase unit each sample, a standard curve was prepared based on 1–6 different units of alpha-amylase (10–50 unit) and the regression equation was calculated based on the standard curve.
One unit of the alpha-amylase enzyme was defined as the amount of enzyme equivalent to 1 mol glucose release per minute under the different standard assay conditions. The most alpha-amylase producing microorganism was subjected to optimization of alpha-amylase production.
For the study of pH stability, alpha-amylase solutions were tested at several pHs from 4 to 10 at 30 °C for 60 min. The effects of different temperatures on alpha-amylase stability were performed at the range of 20–60 °C for 60 min.
In Silico Study of Alpha-amylase from Bacillus megaterium
The alpha-amylase amino acid sequences were achieved from UniProt KB at https://www.uniprot.org [24], and multiple sequence alignment was performed with Clustal X version 2.0 then the results were evaluated by Jalview [25]. Protein Calculator v3.4 web tools (https://protcalc.sourceforge.net/) and Expasy’s ProtParam (https://web.expasy.org/protparam/) were utilized to achieve physicochemical properties of the alpha-amylase protein including molecular weight, pI, charge at pH 7, charged residues composition, instability index, and the aliphatic index. HHpred and NCBI-Protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins) were used to achieve the appropriated PDB structure from the alpha-amylase [26] and Modeller v9.19 program software for modeling [27]. The best-designed models were obtained by discrete optimized potential energy score (DOPE scores) and the high-quality alpha-amylase structure images were designed by PyMOL molecular graphics system (Version1 Del and Scientific LLC) and were validated using Procheck, Errata, Verify 3D, MolProbity, Process, and ProSA web tools.
Optimization of Alpha-amylase Production
Screening of effective medium components was fulfilled using Plackett–Burman design for selected factors such as pH, inoculum size, temperature, time, corn starch, KH2PO4, peptone, MgSO4, CaCl2, NaCl, glycerin, and yeast extract concentration. Two levels of effective factors selected by Plackett–Burman screening were shown in Table S1.
Three selected significant factors from Plackett–Burman screening were subjected to the Box–Behnken design with 30 trials at the three different levels (− 1, 0 and + 1), shown in Table S2.
Statistical Analysis
All data were analyzed and experimental matrixes were designed using the Minitab® software version 16.2.4 (Minitab Inc., State College, PA, USA). A one-way analysis of variance [28] was employed for analysis of data and the statistical significance of the factors was determined using the P-values (P ≤ 0.05).
Results and Discussion
Isolation and Screening of Microorganisms Producing Alpha-amylase
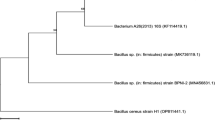
Thirty microorganisms were isolated from the honey samples and nine of them were able to produce alpha-amylase, all nine strains were Gram-positive and eight of them were rod-shaped and one was spherical-shaped. Bacillus megaterium kp7 (Accession numbers for 16S rRNA gene sequences; MG976764) produced the most alpha-amylases enzyme (Accession numbers for amylase gene sequences; X07261.1) compared to all isolated strains (Table 1). Some studies show similar results about microbial sources for the production of alpha-amylase. The ability to produce a novel group of glycoside hydrolase family 13 alpha-amylases has been previously reported for Bacillus megaterium [29]. Many studies' findings have been previously shown the potential/ability of some bacteria regarding alpha-amylase production such as Bacillus subtilis [30], Bacillus amyloliquefaciens [7, 31], and Arthrobacter crystallopoietes N-08 with the ability to produce glucoamylase enzyme. Based on our knowledge, we cannot find any report for the production of alpha-amylase by Kocuria palustris, Acinetobacter lwoffii, and Bacillus endophyticus; thus this is the first time that these bacteria abilities are introduced for alpha-amylase production.
In Silico Studies
The alpha-amylase from the Bacillus megaterum sequence (UniProt KB, P20845) showed 51.11% similarity with alpha-amylase A from Halothermothrix orenii (PDB: 1WZA_A). Figure 1 illustrates the concluding refined model of the Bacillus megaterium alpha-amylase enzyme.
The physicochemical characterization of Bacillus megaterium alpha-amylase showed the calculated pI 6.94, the instability index 31.87, and the aliphatic index 72.74 that indicating the thermostability of protein and the enzyme stability under physiological conditions more than 40 h, respectively [32, 33]. The secondary structure of Bacillus megaterium alpha-amylase illustrated 22% tern, 38% coil, 27% beta-sheet, and 33% helix. The amount of the alpha-amylase helix, beta-sheet, and coil similarity is comparable with alpha-amylase from Halothermothrix orenii (pdb = 1WZA) 31%, 29%, and 38%, respectively, while the tern is similar to alpha-amylase from Bacillus licheniformis (pdb = 1BLI). As seen in Fig. 2, the PRoSA Z-score plot from total determined protein chains in the PDB database indicated the alpha-amylase model locates in a typical range of experimentally determined structures [34]. Ramachandran plot showed the acceptable stereochemical quality for the protein created model with MolProbity clash score (11.15) for the model [35].Verify3D resulted in the 3D structure of alpha-amylase possesses good compatibly with its primary sequence because more than 80 percent of the amino acids owns ≥ 0.2 in the 3D/1D profile [36]. The high ERRAT score (99%) also illustrated the high quality of the protein 3D structure in low water availability condition [37].
Solvent exposed regions of the proteins play an important role in protein stability. Halophilic proteins are stable in low water availability conditions because of containing acidic residues on the surface of proteins caused to increase water-binding capacity [38,39,40] and decrease hydrophobicity at the protein surface [41]. The surface of the alpha-amylase from Bacillus megaterum riches of negative charges residues thus it is stable and active in low water availability condition (i.e., the high concentration of starch). Figure 3 displays the electrostatic surface of the alpha-amylase from Bacillus megaterum.
Finding the Significant Factors by Plackett–Burman Design
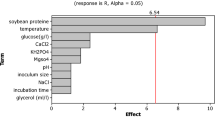
Twelve factors (pH, size inoculum, temperature, time, yeast extract, peptone, KH2PO4, MgSO4, CaCl2, NaCl, and glycerin) were selected for the screening of significant factors by Plackett–Burman design with 20 runs (Table S3). The three factors including pH, corn starch, and temperature were significant (P ≤ 0.05) (Fig. 4).
For the optimization of alpha-amylase production by Aspergillus oryzae, the inoculum size has shown a significant effect on the alpha-amylase production while in our study it did not show a significant effect on enzyme yield.
Owing to the depletion of nutrients in culture medium and production of toxic material by the microorganisms, the optimum fermentation time is considered as an important factor to produce the maximum yield of alpha-amylase [6]. Based on our findings, the fermentation time did not show a significant effect on enzyme production but time point 24 h was somewhat better than 48, whereas Streptomyces erumpens MTCC 7317 has shown the maximum yield of alpha-amylase for 60 h incubation time. These results show the importance of short or long fermentation time effects on bacterial enzyme production yield based on the type of bacteria.
Organic nitrogen sources like yeast extract, soybean proteins and peptone showed a better effect on alpha-amylase production yield than inorganic nitrogen sources such as ammonium chloride, ammonium sulfate and ammonium hydrogen phosphate [6]. The culture medium of Bacillus sp. supplemented by soybean meal enhanced the alpha-amylase production yield [4], whereas this factor did not show a significant impact on enzyme production in this study.
Some crucial salts required for microorganisms growth such as NaCl, MgSO4, KH2PO4, and CaCl2 can increase the alpha-amylase production by enhancing the growth rate of microorganisms through increasing the count of producing bacteria [10]. Besides, some enzymes are a metal enzyme and their structures possess metal ion that directly affects enzyme activity. Among these enzymes, alpha-amylase is a metal enzyme with possessing calcium ion on its structure and this ion required for enzyme activity [6]. Bacillus amyloliquefaciens culture medium supplemented with CaCl2 has shown a significant increase in alpha-amylase production [16], while Bacillus sp. Ps-7 culture medium supplemented by MgSO4 caused to increase significantly alpha-amylase yield [42]. These findings show the importance of these kinds of salts on metal contain enzymes production yield and their activity, but this factor did not affect significantly alpha-amylase production in this study. Glycerin, as a polyol, can play a great role in the formation of metabolic energy through converting to dihydroxyacetone via the glycolytic pathway [4], thus can increase the production of Bacillus sp. alpha-amylase [9]. In addition, glycerol improves the stability of alpha-amylase by extending the half-life of alpha-amylase by stabilization against ionic interactions and thermal denaturation [10]. However, the glycerin did not show a significant effect on enzyme production in this research.
Among the screened factors in this investigation, pH showed a major impact on increasing the alpha-amylase production and selected for Box–Behnken design. Optimum pH is directly related to microbial stability against hydrogen ions concentration, thus can help microorganisms to synthesize and secrete alpha-amylase and increase the enzyme production yield [10].
Corn starch, as a carbon source, causes to increase the population of alpha-amylase producing bacteria and thus can improve enzyme production [43]. Some studies have reported the great role of starch to produce of alpha-amylase by Bacillus sp. and Bacillus amyloliquefaciens [4, 31], which string along with our results and also selected as the main factor for Box–Behnken design.
Temperature, as a physical factor, can affect directly the growing range of microbial source and production of alpha-amylase [6]. Other investigation results showed the significant impact of temperature on bacterial alpha-amylase production and selected as the main factor through Plackett–Burman design to increase alpha-amylase production by microorganisms [17]. The variance analysis of Plackett–Burman design is represented in Table 2.
Box–Behnken Design
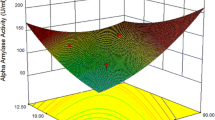
As shown in the Box–Behnken design outcomes represented in Table S4 and surface plots represented in Fig. 5, the optimal levels for alpha-amylase production were achieved at a pH 5, corn starch at 40 g/l, and temperature at 35 °C. The results for Box–Behnken design was significant due to Lack of fit and P-Value were 0.089 and 0.001, respectively. The calculated regression equation represented as:
In the Bacillus family including Bacillus amyloliquifaciens SH8, Bacillus sp. SJC B03, and Bacillus SY134D, pH 5 has been previously reported as the best pH for the production of alpha-amylase that similar to this study results [44,45,46] while pH 6.13 was reported as an optimum pH for Kluyveromyces marxianus IF0 0288 to produce this enzyme.
Some amylase producing strains of Bacillus subtilis (Bacillus sp. I-3, Bacillus sp. PS-7, and Bacillus subtilis IMG22) [4, 42, 47], and Bacillus stearothermophilus can consume corn starch, and soluble starch as a carbon source by producing alpha-amylase enzyme [48]. Upon our findings, Bacillus megaterium kp7 possesses the ability to produce a maximum yield of alpha-amylase in high starch concentration (40 g/l). Similar to our results, Aspergillus niger WLB42 can produce the optimum amount of alpha-amylase at 40 g/l starch concentration in culture medium content [30] whereas the best concentration of corn starch for Bacillus sp. CFR-67 has been previously reported 30 g/ml.
Several types of Bacillus family, including Bacillus aquimaris VITP4 and Bacillus sp. MB6 produced optimum levels of alpha-amylase at 37 °C [49,50,51] whereas the optimum temperature for our isolated Bacillus was achieved 35 °C by Box–Behnken design while Bacillus licheniformis ATCC 9945a showed 40 °C as an optimum temperature [52]. These findings show different bacteria having a diverse optimum temperature for growth and enzyme production.
Effect of Temperature and pH on Amylase Enzyme Stability
The alpha-amylase produced by the isolated Bacillus megaterium was stable at pH 5 and temperature range 40–60 °C. Moreover, the result for temperature stability showed a good correlation to the in silico results (AI = 72.74).
Bacillus sp. I-3 and Bacillus sp. PS-7 produced alpha-amylase stable at pH 5 [42, 47] whereas alpha-amylase from Bacillus sp. ANT-6 was stable at a pH range of 9–13 [53]. Thermobifida fusca NTU22 alpha-amylase was stable at a temperature range of 50–60 °C similar to our findings [54] while Bacillus sp. I-3 alpha-amylase was stable at 65–100 °C [47].
Conclusion
Some bacteria isolated from the honey were able to produce alpha-amylase and the results showed that the isolated Bacillus megaterium produce alpha-amylase more than other isolated bacteria. In addition, the best temperature for the production of alpha-amylase by Bacillus megaterium was 35 °C, starch concentration 40 g/l, and pH of 5. The isolated alpha-amylase was stable at acidic pH and high temperature (60 °C). Based on the findings, the amount of alpha-amylase production was dependent on several effective factors and enzyme-producing bacteria potential. Besides, the in silico results indicate a good correlation to experimental thermostability results from the Bacillus megaterium alpha-amylase. Due to highly negative surface charges of the alpha-amylase, the enzyme is active and stable in low water availability conditions.
References
Snowdon JA, Cliver DO (1996) Microorganisms in honey. Int J Food Microbiol 31(1–3):1–26. https://doi.org/10.1016/0168-1605(96)00970-1
Mohammed F, Abdulwali N, Guillaume D, Bchitou R (2018) Element content of Yemeni honeys as a long-time marker to ascertain honey botanical origin and quality. LWT-Food Sci Technol 88:43–46
Chirife J, Zamora MC, Motto A (2006) The correlation between water activity and moisture in honey: fundamental aspects and application to Argentine honeys. J Food Eng 72(3):287–292
Tanyildizi MS, Özer D, Elibol M (2005) Optimization of α-amylase production by Bacillus sp. using response surface methodology. Process Biochem 40(7):2291–2296
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases: a biotechnological perspective. Process Biochem 38(11):1599–1616
Sundarram A, Murthy TPK (2014) α-Amylase production and applications: a review. J Appl Environ Microbiol 2(4):166–175
Tanyildizi MS, Elibol M, Özer D (2006) Optimization of growth medium for the production of α-amylase from Bacillus amyloliquefaciens using response surface methodology. J Chem Technol Biotechnol 81(4):618–622
Naili B, Sahnoun M, Bejar S, Kammoun R (2016) Optimization of submerged Aspergillus oryzae S2 alpha-amylase production. Food Sci Biotechnol 25(1):185–192. https://doi.org/10.1007/s10068-016-0028-4
de Souza PM, de Oliveira MP (2010) Application of microbial alpha-amylase in industry—a review. Braz J Microbiol 41(4):850–861. https://doi.org/10.1590/S1517-83822010000400004
Sivaramakrishnan S, Gangadharan D, Nampoothiri KM, Soccol CR, Pandey A (2006) a-Amylases from microbial sources—an overview on recent developments. Food Technol Biotechnol 44(2):173–184
Sudan SK, Kumar N, Kaur I, Sahni G (2018) Production, purification and characterization of raw starch hydrolyzing thermostable acidic alpha-amylase from hot springs, India. Int J Biol Macromol 117:831–839. https://doi.org/10.1016/j.ijbiomac.2018.05.231
Silvestri EE, Yund C, Taft S, Bowling CY, Chappie D, Garrahan K, Brady-Roberts E, Stone H, Nichols TL (2017) Considerations for estimating microbial environmental data concentrations collected from a field setting. J Expo Sci Environ Epidemiol 27(2):141–151. https://doi.org/10.1038/jes.2016.3
Aguilar G, Morlon-Guyot J, Trejo-Aguilar B, Guyot J (2000) Purification and characterization of an extracellular α-amylase produced by Lactobacillus manihotivorans LMG 18010T, an amylolytic lactic acid bacterium. Enz Microb Technol 27(6):406–413
Fiedler M, Skerra A (2001) proBA complementation of an auxotrophic E. coli strain improves plasmid stability and expression yield during fermenter production of a recombinant antibody fragment. Gene 274(1–2):111–118. https://doi.org/10.1016/s0378-1119(01)00629-1
Sharma S, Malik A, Satya S (2009) Application of response surface methodology (RSM) for optimization of nutrient supplementation for Cr (VI) removal by Aspergillus lentulus AML05. J Hazard Mater 164(2–3):1198–1204. https://doi.org/10.1016/j.jhazmat.2008.09.030
Gangadharan D, Sivaramakrishnan S, Nampoothiri KM, Sukumaran RK, Pandey A (2008) Response surface methodology for the optimization of alpha amylase production by Bacillus amyloliquefaciens. Bioresour Technol 99(11):4597–4602. https://doi.org/10.1016/j.biortech.2007.07.028
Kammoun R, Naili B, Bejar S (2008) Application of a statistical design to the optimization of parameters and culture medium for alpha-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresour Technol 99(13):5602–5609. https://doi.org/10.1016/j.biortech.2007.10.045
Vyas G, Sharma N (2015) Production and optimization of α-amylase from a novel thermoalkalophilic Bacillus sonorensis GV2 isolated from mushroom compost. Proc Indian Natl Sci Acad 81:1207–1221
Kahraman T, Buyukunal SK, Vural A, Altunatmaz SS (2010) Physico-chemical properties in honey from different regions of Turkey. Food Chem 123(1):41–44
Morya VK, Yadav S, Kim E-K, Yadav D (2012) In silico characterization of alkaline proteases from different species of Aspergillus. Appl Biochem Biotechnol 166(1):243–257
Marks DS, Hopf TA, Sander C (2012) Protein structure prediction from sequence variation. Nature Biotechnol 30(11):1072
Akhi MT, Ghotaslou R, Alizadeh N, Yekani M, Beheshtirouy S, Asgharzadeh M, Pirzadeh T, Memar MY (2017) Nim gene-independent metronidazole-resistant Bacteroides fragilis in surgical site infections. GMS Hyg Infect Control. https://doi.org/10.3205/dgkh000298
Tarhriz V, Mohammadzadeh F, Hejazi MS, Nematzadeh G, Rahimi E (2011) Isolation and characterization of some aquatic bacteria from Qurugol Lake in Azerbaijan under aerobic conditions. Adv Environ Biol 4:3173–3179
Larkin MA, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R (2007) Clustal W and Clustal X version 20. Bioinformatics 23(21):2947–2948
Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ (2009) Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25(9):1189–1191
Söding J, Biegert A, Lupas AN (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucl Acids Res 33(suppl 2):W244–W248
Webb B, Sali A (2014) Comparative protein structure modeling using MODELLER. Curr Protoc Bioinform 47(1):5.6.1–5.6.32
Hlinomaz O, Kaifoszova Z, Beranova M, Machulka T, Belaskova S, Abreu A, Pereira H, Kanakakis J, Kletsiou E, Mauri F (2018) P6259 Improving STEMI patients outcome by implementing nurse assisted patient education program at discharge from primary PCI hospital. Eur Heart J 39(Suppl 1):ehy566
Sarian FD, Janecek S, Pijning T, Ihsanawati NZ, Radjasa OK, Dijkhuizen L, Natalia D, van der Maarel MJ (2017) A new group of glycoside hydrolase family 13 alpha-amylases with an aberrant catalytic triad. Sci Rep 7:44230. https://doi.org/10.1038/srep44230
Wang C-H, Liu X-L, Huang R-B, He B-F, Zhao M-M (2018) Enhanced acidic adaptation of Bacillus subtilis Ca-independent alpha-amylase by rational engineering of pKa values. Biochem Eng J 139:146–153
Zhao W, Zheng J, Wang Y-g, Zhou H-b (2011) A marked enhancement in production of amylase by Bacillus amyloliquefaciens in flask fermentation using statistical methods. J Cent South Univ Technol 18(4):1054–1062
Rogers S, Wells R, Rechsteiner M (1986) Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science 234(4774):364–368
Ikai A (1980) Thermostability and aliphatic index of globular proteins. J Biochem 88(6):1895–1898
Wiederstein M, Sippl MJ (2007) ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucl Acids Res 35(suppl 2):W407–W410
Chen VB, Arendall WB, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr Sect D Biol Crystallogr 66(1):12–21
Eisenberg D, Lüthy R, Bowie JU (1997) VERIFY3D: assessment of protein models with three-dimensional profiles. Meth Enzymol 277:396–404
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of nonbonded atomic interactions. Protein Sci 2(9):1511–1519
Madern D, Ebel C, Zaccai G (2000) Halophilic adaptation of enzymes. Extremophiles 4(2):91–98
Fukuchi S, Yoshimune K, Wakayama M, Moriguchi M, Nishikawa K (2003) Unique amino acid composition of proteins in halophilic bacteria. J Mol Biol 327(2):347–357
Bieger B, Essen L-O, Oesterhelt D (2003) Crystal structure of halophilic dodecin: a novel, dodecameric flavin binding protein from Halobacterium salinarum. Structure 11(4):375–385
Karan R, Capes MD, DasSarma S (2012) Function and biotechnology of extremophilic enzymes in low water activity. Aquat Biosyst 8(1):4
Sodhi HK, Sharma K, Gupta JK, Soni SK (2005) Production of a thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem 40(2):525–534
Ahmed SA, Mostafa FA, Helmy WA, Abdel-Naby MA (2017) Improvement of bacterial α-amylase production and application using two steps statistical factorial design. Biocatal Agric Biotechnol 10:224–233
Pathania S, Sharma N, Handa S (2017) Optimization of culture conditions for production of amylase by Bacillus amyloliquifaciens SH8 using response surface methodology. Proc Indian Natl Sci Acad 1:203–210
Daniel JC, Sudantharadevi V, Sowmiya S (2017) Influence of media components and pH on the production of alpha amylase from Bacillus sp. SJC B03. Indian J Appl Microbiol 20(1):55–62
Bakri Y, Ammouneh H, El-Khouri S, Harba M, Thonart P (2012) Isolation and identification of a new Bacillus strain for amylase production. Res Biotechnol 3(6):51–58
Goyal N, Gupta J, Soni S (2005) A novel raw starch digesting thermostable α-amylase from Bacillus sp. I-3 and its use in the direct hydrolysis of raw potato starch. Enz Microb Technol 37(7):723–734
Srivastava RA, Baruah JN (1986) Culture conditions for production of thermostable amylase by Bacillus stearothermophilus. Appl Environ Microbiol 52(1):179–184
Anupama A, Jayaraman G (2011) Detergent stable, halotolerantɑ-amylase from Bacillus aquimaris vitp4 exhibits reversible unfolding. Int J Appl Biol Pharm Technol 2(2):336–376
Deb P, Talukdar SA, Mohsina K, Sarker PK, Sayem SA (2013) Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. Springerplus 2(1):154. https://doi.org/10.1186/2193-1801-2-154
Paul JS, Lall B, Jadhav S, Tiwari K (2017) Parameter’s optimization and kinetics study of α-amylase enzyme of Bacillus sp. MB6 isolated from vegetable waste. Pro Biochem 52:123–129
Božić N, Ruiz J, López-Santín J, Vujčić Z (9945a) Production and properties of the highly efficient raw starch digesting α-amylase from a Bacillus licheniformis ATCC 9945a. Biochem Eng J 53(2):203–209
Burhan A, Nisa U, Gökhan C, Ömer C, Ashabil A, Osman G (2003) Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem 38(10):1397–1403
Yang C-H, Liu W-H (2004) Purification and properties of a maltotriose-producing α-amylase from Thermobifida fusca. Enz Microb Technol 35(2–3):254–260
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The financial support of the Tabriz University of Medical Sciences is gratefully acknowledged. The results of this article are derived from the Ph.D. thesis of Babak Elyasi Far registered in Tabriz University of Medical Sciences, Tabriz, Iran. No ethical issues to be promulgated.
Author information
Authors and Affiliations
Contributions
BEF performed the study and drafted the manuscript. AD revised the manuscript. AYK designed the study, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. All authors designed the experiment, conducted and wrote the manuscript. All authors have approved the final article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elyasi Far, B., Dilmaghani, A. & Yari Khosroushahi, A. In Silico Study and Optimization of Bacillus megaterium alpha-Amylases Production Obtained from Honey Sources. Curr Microbiol 77, 2593–2601 (2020). https://doi.org/10.1007/s00284-020-02019-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02019-x